Abstract
Successful interaction with the world depends on accurate perception of the timing of external events. Neurons at early stages of the primate visual system represent time-varying stimuli with high precision. However, it is unknown whether this temporal fidelity is maintained in the prefrontal cortex, where changes in neuronal activity generally correlate with changes in perception. One reason to suspect that it is not maintained is that humans experience surprisingly large fluctuations in the perception of time. To investigate the neuronal correlates of time perception, we recorded from neurons in the prefrontal cortex and midbrain of monkeys performing a temporal-discrimination task. Visual time intervals were presented at a timescale relevant to natural behavior (<500 ms). At this brief timescale, neuronal adaptation—time-dependent changes in the size of successive responses—occurs. We found that visual activity fluctuated with timing judgments in the prefrontal cortex but not in comparable midbrain areas. Surprisingly, only response strength, not timing, predicted task performance. Intervals perceived as longer were associated with larger visual responses and shorter intervals with smaller responses, matching the dynamics of adaptation. These results suggest that the magnitude of prefrontal activity may be read out to provide temporal information that contributes to judging the passage of time.
Keywords: vision, frontal eye field, superior colliculus, macaque, latency
Systems neuroscience research has focused on the spatial aspects of vision, including form, orientation, and size (1, 2). However, the role of time in vision is no less important. Research on the temporal aspects of primate vision has largely consisted of studies of the temporal dynamics of neural activity in the early stages of visual processing, where neurons are exquisitely sensitive to changes in spatial and temporal frequency (3, 4). Neuronal correlates of visual timing in higher-order visual areas, and potential associations between that activity and the perception of timing, remain unexplored.
A major consideration when studying primate vision is that it is interrupted by saccadic eye movements. Consequently, each “snapshot” of the visual world, during an intersaccadic interval, is less than a half-second long (5). The accurate perception of time intervals at such brief timescales is important in itself (e.g., for estimating the speed of briefly appearing objects) and is elemental for longer timing judgments that span multiple saccades. However, despite a growing interest in time estimation in the visual system (6), few experiments have studied it at subsecond timescales.
Our overall goal was to determine the relationship between neuronal activity and the perception of visual timing at subsecond timescales. The simplest possibility is that the latency of sensory responses dictates subsecond temporal perception. However, this straightforward hypothesis is complicated by the fact that visual time perception can vary on the order of tens to hundreds of milliseconds (7), even though stimulus-evoked visual activity is timed precisely across brain regions (<10 ms) (8, 9). Therefore, additional factors, such as the strength of responses, may influence time perception.
The encoding of time by response strength is supported by psychophysical studies showing that stimulus changes that typically lead to increased firing rates also lead to longer time judgments, but those eliciting relatively lower firing rates lead to shorter judgments (for review, see ref. 10). Furthermore, at subsecond timescales, changes in response strength predominate; a ubiquitous property of sensory neurons is adaptation, in which responses to sequential stimuli increase with the time between stimuli (11). Inherent to brief sensory adaptation is an intrinsic strength code of time (12–14). Thus, adaptation-like changes in response strength could contribute to the perception of brief time intervals in multiple sensory modalities.
We tested whether the timing or the strength of visual activity covaried with changes in time perception. Monkeys reported the relative amount of time between two flashes of light presented at intervals <500 ms while we recorded the activity of single neurons in the prefrontal cortex or, for comparison, the midbrain. Surprisingly, we found that the strength of prefrontal responses, not their timing, correlated with the animals’ perception of time. Strength changes matched those naturally present in adaptation: just as longer time intervals elicit larger second responses and shorter intervals elicit smaller responses, intervals perceived as longer and shorter were associated with larger and smaller second responses, respectively. This effect was not evident in midbrain neurons despite otherwise similar response properties. Our results suggest that time-interval perception is not dictated by neuronal response timing but is influenced strongly by systematic changes in neuronal response magnitude in the prefrontal cortex, and likely in other visual areas.
Results
Monkeys Are Capable of Fine Temporal Discrimination.
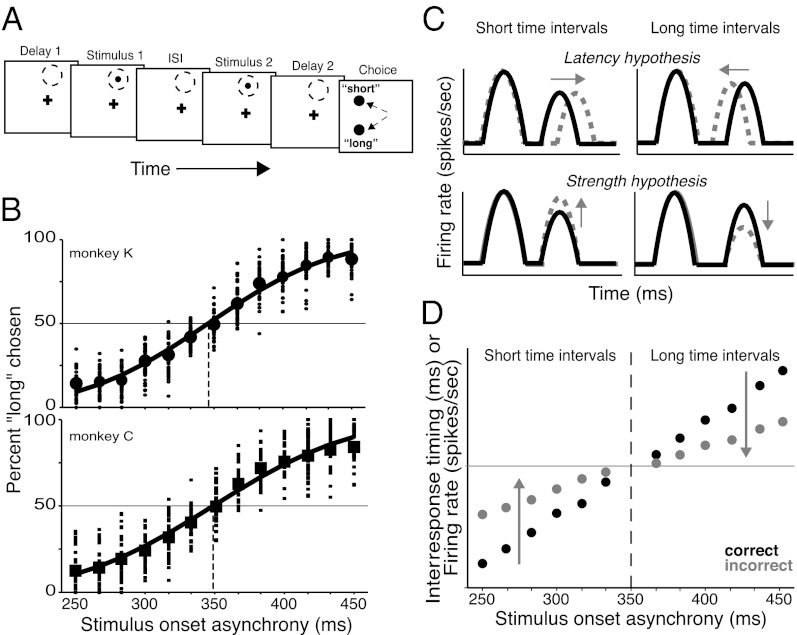
Monkeys were trained to perform a time-interval discrimination task (Fig. 1A). On each trial, we varied the amount of time between two flashes of light [stimulus onset asynchrony (SOA): 250–450 ms at 16.7-ms intervals) presented during fixation. This range of times was chosen to approximate timescales regularly encountered by the visual system during natural behavior, and to elicit adapted yet easily detectable neuronal responses (15, 16). Monkeys were rewarded for making a saccade to one of two choice targets, “short” or “long,” based on that trial’s SOA relative to a learned reference interval of 350 ms. Although SOAs incremented by only ∼17 ms and varied within a total range of 200 ms, monkeys readily discriminated the time intervals such that performance scaled with task difficulty (i.e., proximity to the reference interval) (Fig. 1B). Mean Weber fractions across SOAs were 0.11 and 0.14 for monkeys K and C, respectively, nearly equivalent to those of human subjects in comparable tasks (17, 18). These results extend previous findings of visual time discrimination in monkeys (6, 19) and demonstrate that they can perform fine temporal discrimination at brief timescales.
Fig. 1.
Temporal-discrimination task, performance, and predictions. (A) Monkeys fixated a spot (cross) followed by a randomized delay period (Delay 1). Two identical stimuli (Stimulus 1 and 2) flashed successively in the receptive field (dashed circle), separated by an ISI. ISI plus the duration of stimulus 1 is equivalent to the SOA. Monkeys reported whether the SOA was shorter or longer than 350 ms by making a saccade to one of two choice targets in the ipsilateral hemifield. (B) Percentage of “long” choices as a function of presented time interval for monkeys K (Upper; circles) and C (Lower; squares) over all recording sessions. Task difficulty increases as SOAs approach 350 ms. Small symbols indicate performance in individual sessions. Cumulative Gaussian curve was fit to the average performance (large symbols) across SOAs. Vertical dashed line indicates point of subjective equality. (C) Predicted responses of latency (Upper) and strength (Lower) hypotheses are shown for short (Left) and long (Right) time intervals. Schematics of average activity are shown for correct trials (black traces) and incorrect (dashed gray traces) trials. Arrows indicate the direction of change predicted for incorrect trials. (D) Predicted changes in the inter-response timing or strength of the population’s second visual response (both on ordinate) at each SOA (abscissa).
Predicted Changes in the Timing and Strength of Neuronal Activity.
We recorded from visually responsive neurons in the frontal eye field (FEF) in the caudal prefrontal cortex. The FEF contains visuomotor and cognitive activity (20, 21) that has been shown to correlate with visual perception (22–24), suggesting that it may play a central role in perisaccadic distortions of time (17). The FEF also receives inputs from the basal ganglia and cerebellum (25), two structures thought to play key roles in time keeping (26, 27). We compared neuronal activity in trials in which the time interval (i.e., SOA) was identical but the monkey’s reported percept (i.e., “short” or “long” relative to the reference interval) differed.
If the latency hypothesis is true and the perceived time between stimuli is represented by the time between visual responses, then we would expect to see changes in the interresponse times that correspond to the monkey’s behavioral report. Thus, when short time intervals (<350 ms) are presented, the interresponse time would be longer on incorrect trials, corresponding to the erroneous “long” choice, compared with that of correct trials (Fig. 1C, Upper Left). For long time intervals (>350 ms), the opposite result is expected; the interresponse time on incorrect trials would be shorter than that of correct trials (Fig. 1C, Upper Right). Across time intervals, we would then expect to see contrasting directions of change in interresponse timing as a function of task performance on either side of the 350-ms reference interval (Fig. 1D).
The strength hypothesis predicts that the magnitude, not the timing, of the visual response should correspond with the behavioral report. Specifically, the second visual response should vary in size but the first response remains relatively constant, matching the strength changes seen during adaptation (15). Thus, when presented with a short interval, the monkey should erroneously deem it “long” if the second visual response is relatively large compared with responses on correct trials (Fig. 1C, Lower Left). Conversely, when presented with a long interval, erroneous “short” judgments should correlate with relatively smaller second visual responses (Fig. 1C, Lower Right). Again, we would expect to see an inflection point around the reference interval, indicating differential strength changes in the second visual response as a function of task performance (Fig. 1D).
Response Strength in the Prefrontal Cortex Correlates with Time Perception.
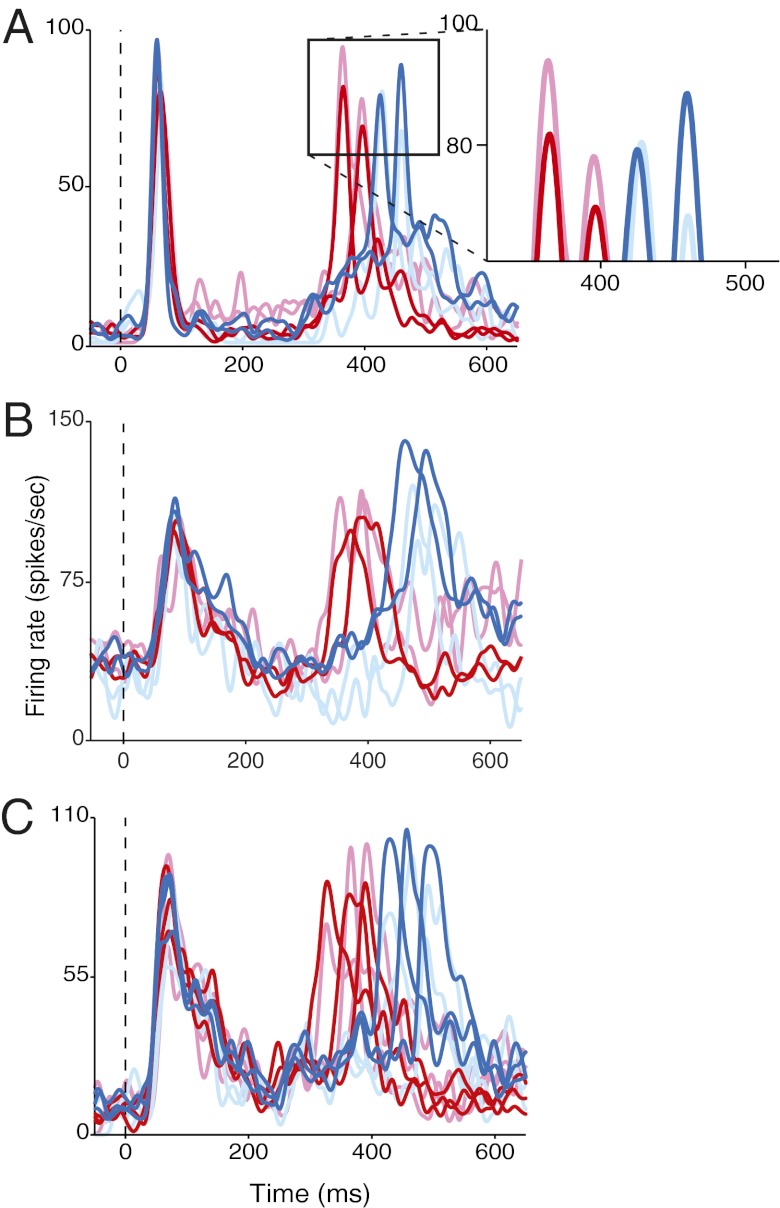
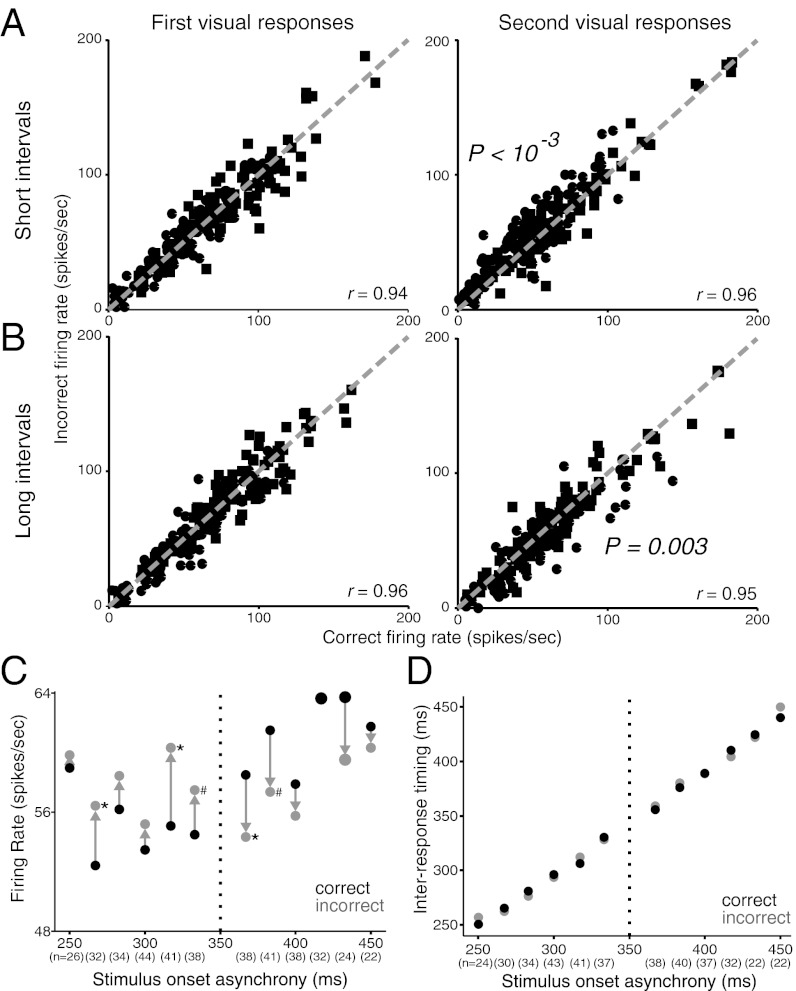
Fig. 2 shows the responses of three individual FEF neurons to various time intervals. An effect common to all three neurons, for most time intervals, was a systematic difference in the strength of second visual responses in correct vs. incorrect trials. In short-interval trials (red), second visual responses were generally larger when the monkeys incorrectly judged the interval as “long” (lighter traces) versus when they correctly judged them as “short” (darker traces). In long-interval trials (blue), the effect was reversed. The same changes in response strength with time estimation performance were present at the FEF population level as well (Fig. 3). On short-interval trials, second visual responses were larger in incorrect trials compared with correct trials using identical stimuli (P < 0.001) (Fig. 3A, Right). Conversely, second visual responses were smaller in incorrect long trials compared with their correct counterparts (P = 0.003) (Fig. 3B, Right). First visual responses showed no significant changes between correct and incorrect trials (P > 0.05) (Fig. 3 A and B, Left). Relative measures of the visual responses (second – first) were subject to uncorrelated variability between responses, yet they still yielded a significant effect for short trials (P = 0.002) and a trend for long trials (P = 0.08). In sum, larger second responses were associated with longer time percepts and smaller responses with shorter percepts, consistent with the strength hypothesis.
Fig. 2.
Responses of three single FEF neurons to short (red) and long (blue) intervals during temporal-discrimination task. (A) SOAs: 300, 333, 367, 400 ms. Lighter colors indicate activity during incorrect trials. Activity traces are aligned to first stimulus onset (dashed vertical line). (Inset) Close-up of the second visual responses. (B) SOAs: 283, 317, 383, 417 ms. (C) SOAs: 267, 300, 333, 367, 400, 433 ms.
Fig. 3.
Changes in FEF population activity as a function of behavioral performance. (A) Visual responses to short time-interval trials. First responses (Left) and second responses (Right). Each symbol represents responses at a single time interval for a given neuron; a single neuron contributes up to three points per plot. Circles, monkey K; squares, monkey C. Dashed diagonal lines are unity lines. P values shown are for signed-rank test; r values are correlation coefficients. Seven outlying datapoints were omitted from the second visual responses for display purposes, but included for all statistics and analyses. (B) Same as A, for long time-interval trials. Four (first responses) and two (second responses) outlying datapoints were omitted for display purposes, but included for all statistics and analyses. See Fig. S1 for SC data. (C) Mean population strength of second visual responses at each time interval. Sample sizes are listed below each interval. Same format as Fig. 1D. Symbols to right of incorrect responses indicate significance level of difference between correct and incorrect firing rates at that time interval (*P < 0.05, #P < 0.06; signed-rank tests). Datapoints at 417 ms overlap. (D) Mean interresponse time across population at each time interval. Time between responses onsets (response onset asynchrony, the neuronal analog of SOA) using Peak metric. Same conventions as in C.
The strength hypothesis also was supported at the level of individual time intervals across FEF neurons. Second visual responses were larger on average in incorrect trials for all short time intervals (Fig. 3C, Left half) and smaller, as predicted, for nearly all long time intervals (Fig. 3C, Right half). Pooled differences in second response strength grouped by short and long choices were each significantly different from zero in the directions predicted by the strength hypothesis (P = 0.002; short median = −2.62 ± 0.82 spikes per second, long median = 3.15 ± 0.99 spikes per second, n = 6, ± SE of median). In contrast, time judgments were unrelated to changes in the first visual responses (P > 0.05) or in the interresponse timings (P > 0.05) (Fig. 3D; see also Response Timing Does Not Account for Changes in Time Perception, below).
When the reference interval of 350 ms was presented, there was no correct answer and reward was randomly dispensed at a 50% rate. Average performance at the reference interval was near chance (Fig. 1B), and trial-by-trial performance was unrelated to neuronal activity (no difference in second visual responses as a function of time judgment; signed-rank test, P = 0.99). This dissociation between neuronal activity and performance at the reference interval supports the conclusion of Swaminathan and Freedman (28), who reported similar results for the prefrontal cortex. It would have been informative to use variable-reference tests that could have isolated cognitive signals associated with perceptual ambiguity (29), but we found that we needed a constant reference interval for successful training and maintenance of long-term performance. Because the reference interval data were atypical, we excluded them from further analysis.
To assess whether the strength code was specific to the prefrontal cortex, we also recorded from neurons in the midbrain superior colliculus (SC). We sampled both the retinal-recipient, purely visual superficial layers (supSC) and the visuomotor intermediate layers (intSC). intSC and FEF neurons have similar visual and oculomotor activity patterns (30, 31). However, unlike the robust strength changes found in the FEF, we found few, if any, correlations between the strength or latency of population visual activity and behavioral performance in either the intSC or supSC (Fig. S1).
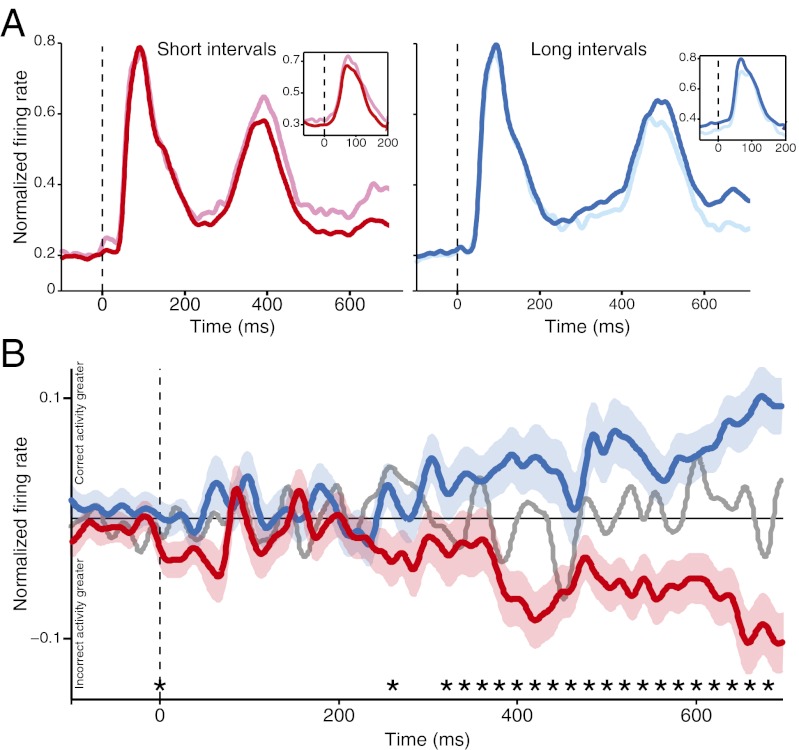
Population activity profiles illustrate the time course of strength changes in each brain region. In the FEF, first visual responses showed no relationship with the monkey’s choice (Fig. 4A). Differences in the activity profiles emerged around the time of the second visual response, continued throughout the response, and were sustained after the second stimulus was turned off. To highlight the difference between incorrect and correct trials, we subtracted incorrect activity from correct activity grouped by short and long intervals (difference signals) (Fig. 4B). A sliding-window analysis confirmed that FEF signals diverged and remained separated from each other at ∼330 ms. In contrast, activity in the intSC and supSC exhibited only brief, transient fluctuations around the time of visual responses before returning to zero (Fig. S2).
Fig. 4.
FEF population response profiles. (A) Responses aligned to first stimulus onset (dashed vertical line). Activity during short (Left, red) and long (Right, blue) time intervals. Lighter colors indicate activity on incorrect trials. (Insets) Second visual responses aligned to second stimulus onset. (B) Difference population signals for grouped short (red), long (blue), and reference (gray; mean only) intervals aligned to the first stimulus onset (dashed vertical line). Shading represents ± 1 SEM. For reference trace, positive values on the y axis indicate greater activity for “short” choices. Asterisks are centered on bins for which the “short” and “long” difference signals significantly diverged from each other (P < 0.05). See Fig. S2 for SC data.
Response Timing Does Not Account for Changes in Time Perception.
Unlike second visual response strength, interresponse timing did not vary systematically with time judgments as the latency hypothesis predicted (Fig. 1C, Upper). After validating our latency metrics (SI Text and Tables S1 and S2), we analyzed the time between visual responses in the FEF and SC using six analytical approaches. None of the measures yielded results that conformed to the latency hypothesis (P > 0.05) (see Fig. 3D for representative results). Although we occasionally found changes for either short or long time intervals consistent with the latency hypothesis, we also found changes in the opposite direction (Tables S3 and S4). Although it is possible that the neurons use a more complex temporal code (32, 33), our analyses suggest that time perception is not strictly governed by the time between visual responses.
Trial-by-Trial Variability in the FEF Response Strength Correlates with Temporal Judgments.
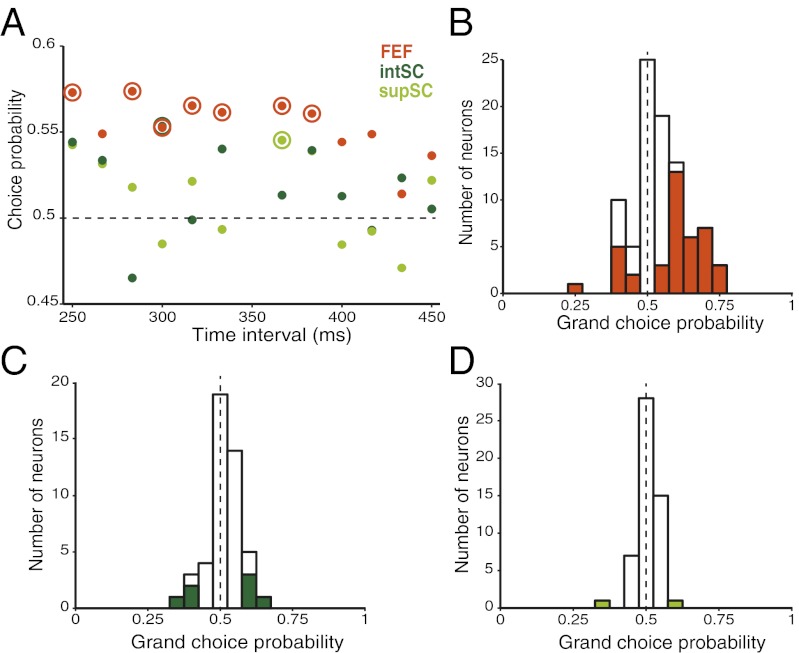
Correlations between response strength and time judgments in the population data could be caused by a few strongly modulated neurons in an otherwise task-irrelevant population. To determine the number of neurons with informative time-related activity and to account for neuronal response variability, we calculated the choice probability (CP) for each neuron in our FEF and SC datasets (34, 35). CP values greater than 0.5 corresponded to firing rate changes consistent with the strength hypothesis (larger responses associated with longer percepts).
On average, FEF neurons fired more during longer time percepts than shorter percepts for all time intervals (Fig. 5A, orange dots >0.5). Significant time-related activity in FEF neurons was present at 7 of 12 individual time intervals (P < 0.05, one-sample t test) (Fig. 5A, orange circles), compared with sporadic and few significant values in both intSC and supSC (P > 0.05) (Fig. 5A, light and dark green symbols). Mean CPs did not significantly differ across time intervals in any brain region (P > 0.05, one-way ANOVA), justifying a calculation of grand choice probability (gCP) for each neuron across time intervals.
Fig. 5.
FEF neuronal discrimination is stronger and more prevalent than in the SC. (A) Choice probability as a function of time interval (SOA) for FEF (orange), intSC (dark green), and supSC (light green) neurons. Values significantly different from 0.5 are circled (P < 0.05). (B) Distribution of grand choice probabilities in FEF. Filled bars indicate individual neurons with values significantly different from 0.5 (n = 40 significant at P < 0.05; 26 from monkey K). (C) Same format for intSC data (n = 7 significant, 4 from monkey K). (D) Same format for supSC data (n = 2 significant, 1 from monkey K).
Forty-four percent (40 of 90) of FEF neurons showed significant gCPs, in comparison with 15% (7 of 47) of neurons in intSC and 4% (2 of 52) in supSC (P < 0.05; permutation test). Mean gCP in the FEF was significantly greater than chance (P < 0.001, mean = 0.54) (Fig. 5B) and significantly greater than the gCPs of intSC and supSC (P < 0.01). Grand CPs in intSC and supSC did not significantly differ from chance or one another (P > 0.05, intSC mean = 0.52, supSC mean = 0.51) (Fig. 5 C and D). Similar results were obtained for individual trial durations truncated by saccade onset instead of a fixed epoch (mean gCPs: FEF = 0.53, intSC = 0.50, supSC = 0.49; ± 0.01 SEM for each area).
Hence, compared with the intSC and supSC, the FEF contained more choice-related neurons and showed a greater capacity to represent the monkeys’ perceptual choices across time intervals. The fact that over 40% of FEF neurons showed significant trial-by-trial correlations with the monkeys’ performance, but only ∼10% of SC neurons showed comparable effects, suggests that the coding of perceived time by response strength is not a general property of the oculomotor system, and among the three regions tested, meaningful time judgment activity was unique to the FEF.
Motor-Related Activity Does Not Account for Strength Encoding of Time Perception.
Choice target locations were fixed across recording sessions in our task (SI Methods). Consequently, differences in activity during short- or long-choice trials were confounded with selectivity for the impending movement to the associated choice target. It was therefore possible that changes in visual response strength were related to saccade generation. We took several precautions, however, to minimize the effects of premotor activity. First, choice targets were placed ∼15° away from the neuron’s receptive field and in the visual hemifield ipsilateral to physiological recordings. Eye movements into the ipsilateral visual space are known to elicit negligible presaccadic activity from neurons in the FEF and SC (36, 37). Second, we reversed the response mapping between monkeys. Third, we recorded from visually responsive neurons, biasing our population sample away from purely saccade-related cells (SI Results). Finally, we recorded from the FEF and intSC, two closely linked visuomotor structures with comparable response properties (30, 31). If saccade-related artifacts were found in one structure, it seemed likely that we would find similar effects in the other structure.
We then carried out three additional tests to ensure that our results were not because of motor-related activity (SI Results). First, we found that FEF neurons showed little to no modulation in firing based on the location of the impending choice target. Second, we found that significant choice probabilities were distributed across a wide range of FEF neurons with both visually dominant and motor-dominant activity (Fig. S3). Finally, our results could not be explained by differences in the rates of microsaccades between choice targets. Although we cannot entirely rule out the influence of spatially independent, anticipatory motor activity specific to the FEF, it is likely that motor activity had little influence on visual responses during our time-perception task. This finding also implies that our results are effector-independent, but further experiments with other motor responses (e.g., reaches) are needed for verification.
Strength Coding and Other Cognitive Signals.
Changes in response strength might occur before the second visual response, during “delay activity” that can occur in the interstimulus interval (ISI) (Fig. 4A). To investigate this possibility, we quantified the amount of delay activity in each FEF neuron. We measured activity 100 ms before second stimulus onset and compared it to the baseline firing rate (signed-rank test, P < 0.05). We only measured time intervals longer than 300 ms to safely exclude the offset of the preceding first visual response in shorter SOAs (Table S2), and to allow for a full 100-ms delay epoch for all included responses. We found that 39% (35 of 90) of FEF neurons had consistent delay activity (i.e., significant in 50% or more of a neuron’s tested time intervals).
It appeared that delay activity facilitated visual activity but failed to convey a temporal code; only the second visual responses coded for time intervals. First, we focused on the 35 “delay neurons” (defined above) as presenting the best opportunity to find an influence of delay activity on overall CP, our index for identifying time-interval coding. For each of these neurons, we pooled time intervals with significant delay activity, yielding 98 datapoints. We defined “delay strength” as the P value obtained from the initial delay activity versus baseline activity comparison (signed-rank test), and compared this with the neuron’s overall CP for the same time interval. We found no significant correlation (P = 0.33; r2 = 0.10). Second, in all 90 FEF neurons, we studied the relationship between delay activity and second visual responses. We found that CPs of delay and second visual response epochs were correlated (P < 0.001; r2 = 0.20) and that the second visual epoch gCP (mean 0.52) was not significantly larger than the delay epoch gCP (mean 0.51; P = 0.09; permutation test). However, only the second visual epoch’s gCP was significantly greater than 0.5 (P < 0.001); the delay epoch’s gCP was not (P = 0.09; signed-rank tests). The same results were found using two alternative approaches: by including only the subsets of neurons with individually significant gCPs (as opposed to raw firing rates above) within each epoch (visual epoch, 17 of 90 neurons: delay epoch, 19 of 90 neurons) and by limiting the analysis to longer intervals (SOA > 250 ms) for all neurons (n = 90), so that any lingering effects of first visual responses were avoided. For both approaches, mean gCPs were not significantly different between second visual responses and delay activity (subset approach: 0.58 vs. 0.53, respectively; longer-intervals approach: 0.52 vs. 0.51, respectively; P = 0.07 for both comparisons; permutation tests). However, for both approaches the mean gCP of second visual responses significantly exceeded 0.5 (subset approach: P = 0.02; longer-intervals approach: P = 0.006; signed-rank tests), but the mean gCP of delay activity did not (subset approach: P = 0.26; longer-intervals approach: P = 0.21; signed-rank tests). In a final analysis, we explicitly isolated the second visual responses by subtracting from them the activity levels in the delay epoch for all 90 neurons. The resulting gCPs (mean = 0.51) did not significantly differ from those of the original second visual responses (mean = 0.52 as noted above; P = 0.07; permutation test), and remained significantly greater than 0.5 (P = 0.04; signed-rank test). These results suggest that, relative to delay period activity, the visual response provided the greater contribution to the strength code for timing.
Discussion
Our findings provide direct neuronal evidence that trial-by-trial fluctuations in visual response strength can account for time-related changes in visual perception. This result was true in a frontal cortical area, the FEF, but not in the superficial and intermediate layers of the SC. Time intervals were represented veridically by response latencies in all areas tested, but variations in the perception of time were associated only with changes in FEF response strength. Thus, fluctuations in the timing of neuronal activity are an unlikely source of perceptual changes, even in the context of an explicit timing task.
This is not to say that the timing of visual (or other sensory) responses is irrelevant for determining the timing of events in the external world. To the contrary, FEF visual responses represented temporal intervals in a highly linear manner (Fig. 3D). Certainly this latency code provides information about ISIs. However, we found that natural variation in the latencies of single neurons (temporal noise) has little or no effect on temporal perception. This temporal noise might be uncorrelated across neurons, so that mean latency at the population level remains accurate. Our critical positive result was that variation in the strength of neuronal response does affect temporal perception. This finding implies that magnitudes are correlated across neurons to influence the mean population response. Put simply, monkeys behave as if they are “tricked” by aggregate noise in the visual response strength, even though it contradicts the accurate mean response latency.
At the <500-ms timescale that we tested, such a magnitude code may be provided intrinsically by adaptation. Three lines of evidence support a role for adaptation in time perception. First, FEF neurons showed a “directionality” of strength changes that correlated with perception and matched those seen during adaptation (e.g., larger responses led to longer percepts and smaller responses led to shorter percepts). Second, we found fewer strength-related effects at the longest intervals tested (>400 ms) (Fig. 5A), consistent with a ceiling effect when adaptation is minimal (15). Finally, brief adaptation is ubiquitous throughout sensory cortices (15, 38), and therefore ideally suited for mediating time perception in single neurons. It remains unclear, however, whether time perception strictly depends upon adapted cortical responses at brief timescales. FEF neurons are capable of representing a broad range of cognitive signals (20, 21, 24), including interval duration (39). Additional work is needed to determine if this mixture of FEF signals acts in concert with or as an alternative to adaptation to modulate visual response strength. Moreover, adaptation is unlikely to play a role in the perception of cross-modal timing. When stimuli of differing modalities are presented successively, the suppressive effects are minimal (40, 41).
FEF neurons showed clear changes in firing rate as a function of interval category (short and long categories) (Fig. 3C) and ambiguous activity at the reference interval (Fig. 4B). Unlike the category codes found in previous work (28, 29, 42), most of our FEF neurons, as well as the population average, increased their firing when a given interval was perceived as lasting longer. If the neurons represented temporal categories, the “long” category vastly outnumbered the “short” category. Such a pronounced asymmetry in category representations was not found in previous work (28, 29, 42). However, this asymmetric, directional modulation conforms to the temporal code in adaptation. Therefore, if the monkeys (and neurons) were performing categorization, adaptation would appear to provide the neural scaffolding for representations in the temporal domain. This difference between the representations of purely temporal and spatially dependent stimuli (29) challenges the notion of a common magnitude system for space, time, and number in the prefrontal cortex (43).
The ubiquity of neuronal adaptation, along with the coarse category-like response pattern in the FEF, makes it difficult to establish firmly the causal role that FEF may play in time perception. Strength coding could occur either before or after the categorical decision. We think that the most parsimonious scenario is that adaptation in earlier stages of visual processing performs preprocessing of timing information so that finer interval discriminations are more readily discriminable at the level of single neurons in later areas. Further work is needed to establish the position of FEF in this putative functional pathway from sensory encoding to categorization.
Behavioral studies that presumably manipulate the size of neuronal visual responses elicit strong time illusions. For example, the time between stimuli presented just before a saccadic eye movement is perceptually compressed relative to baseline measures (17). Our results suggest that presaccadic suppression of visual activity (44) leads to a relatively diminished second visual response associated with a shorter percept of time. A similar understanding of the relationship between stimulus changes and response dynamics can also explain contrast-dependent compression of time judgments, as well as elongation of time perception during rapid serial visual processing (45).
Visual response magnitudes must be read out to affect temporal judgments and provide an estimate of time. Because the nervous system’s ability to integrate spike rates is well-established, one attractive feature of the strength hypothesis is that it does not invoke specialized neural circuitry. In this way, magnitude-encoding of temporal intervals in cortical neurons may be one of many simultaneous, overlapping timing processes in the brain (46), thus providing a physiologically plausible alternative to dominant “central clock” models of time perception (47).
Methods
Two adult male monkeys (Macaca mulatta) were surgically prepared for neuronal recordings in the FEF and SC. All experiments were conducted under the supervision of the University of Pittsburgh Institutional Animal Care and Use Committee and complied with the guidelines of the United States Public Health Service Guide for the Care and Use of Laboratory Animals. The FEF and SC were identified through stereotaxic coordinates, structural MRI, and established properties of neuronal activity and stimulation-evoked saccades. Receptive field centers were mapped with memory-guided or visually guided saccades using stimuli presented at eight target directions and at least eight amplitudes (1–35°). Eye position was monitored using a scleral search coil and timings of stimuli were documented with a photodiode.
On each trial, monkeys viewed two sequential stimuli presented in the receptive field center. The monkeys then made a two-alternative forced-choice temporal discrimination (Fig. 1A) by reporting whether the SOA (250–450 ms) was shorter or longer than a learned reference interval of 350 ms. To ensure that the monkeys judged the time between stimuli and no other temporal parameter, delay periods (randomized duration, range of 800 ms) preceded and followed presentation of the paired stimuli. Monkeys reported their percept (“short” or “long”) by making a saccade to one of two targets in the ipsilateral visual hemifield. Liquid reward was dispensed for correct judgments. Action potential times, eye-position samples, photodiode output, and task-event timings were stored at 1-ms resolution for offline analysis.
Supplementary Material
Acknowledgments
We thank Matthew Smith, Amanda Clause, and Chris Henry for helpful comments; Ryan Kelly for programming assistance; and Karen McCracken for technical support. This work was supported by National Institutes of Health Grants R01 EY017592 and P41 EB001977, and an Andrew Mellon Fellowship from the University of Pittsburgh (to J.P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217177110/-/DCSupplemental.
References
- 1.Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. In: Bechtel W, Stufflebeam RS, Mundale J, Mandik P, editors. Philosophy and the Neurosciences: A Reader. Malden, MA: Wiley-Blackwell; 2001. pp. 199–208. [Google Scholar]
- 2.Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- 3.Bair W, Koch C, Newsome W, Britten K. Power spectrum analysis of bursting cells in area MT in the behaving monkey. J Neurosci. 1994;14(5 Pt 1):2870–2892. doi: 10.1523/JNEUROSCI.14-05-02870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredfeldt CE, Ringach DL. Dynamics of spatial frequency tuning in macaque V1. J Neurosci. 2002;22(5):1976–1984. doi: 10.1523/JNEUROSCI.22-05-01976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg DJ, Boehnke SE, Marino RA, Munoz DP, Itti L. Free viewing of dynamic stimuli by humans and monkeys. J Vis. 2009;9(5):19.1–19.15. doi: 10.1167/9.5.19. [DOI] [PubMed] [Google Scholar]
- 6.Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol. 2006;95(5):3281–3285. doi: 10.1152/jn.01011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eagleman DM. Human time perception and its illusions. Curr Opin Neurobiol. 2008;18(2):131–136. doi: 10.1016/j.conb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsálek P, Koch C, Maunsell J. On the relationship between synaptic input and spike output jitter in individual neurons. Proc Natl Acad Sci USA. 1997;94(2):735–740. doi: 10.1073/pnas.94.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shadlen MN, Newsome WT. The variable discharge of cortical neurons: Implications for connectivity, computation, and information coding. J Neurosci. 1998;18(10):3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eagleman DM, Pariyadath V. Is subjective duration a signature of coding efficiency? Philos Trans R Soc Lond B Biol Sci. 2009;364(1525):1841–1851. doi: 10.1098/rstb.2009.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohn A. Visual adaptation: Physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97(5):3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 12.Grande LA, Spain WJ. Synaptic depression as a timing device. Physiology (Bethesda) 2005;20:201–210. doi: 10.1152/physiol.00006.2005. [DOI] [PubMed] [Google Scholar]
- 13.Mayo JP, Sommer MA. Neuronal adaptation: Delay compensation at the level of single neurons? Behav Brain Sci. 2008;31(2):210–212. [Google Scholar]
- 14.Buonomano DV. Decoding temporal information: A model based on short-term synaptic plasticity. J Neurosci. 2000;20(3):1129–1141. doi: 10.1523/JNEUROSCI.20-03-01129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo JP, Sommer MA. Neuronal adaptation caused by sequential visual stimulation in the frontal eye field. J Neurophysiol. 2008;100(4):1923–1935. doi: 10.1152/jn.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehnke SE, et al. Visual adaptation and novelty responses in the superior colliculus. Eur J Neurosci. 2011;34(5):766–779. doi: 10.1111/j.1460-9568.2011.07805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrone MC, Ross J, Burr D. Saccadic eye movements cause compression of time as well as space. Nat Neurosci. 2005;8(7):950–954. doi: 10.1038/nn1488. [DOI] [PubMed] [Google Scholar]
- 18.Westheimer G. Discrimination of short time intervals by the human observer. Exp Brain Res. 1999;129(1):121–126. doi: 10.1007/s002210050942. [DOI] [PubMed] [Google Scholar]
- 19.Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38(2):317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- 20.Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore T. The neurobiology of visual attention: Finding sources. Curr Opin Neurobiol. 2006;16(2):159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 22.O’Shea J, Walsh V. Visual awareness: The eye fields have it? Curr Biol. 2004;14(7):R279–R281. doi: 10.1016/j.cub.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Thompson KG, Schall JD. Antecedents and correlates of visual detection and awareness in macaque prefrontal cortex. Vision Res. 2000;40(10–12):1523–1538. doi: 10.1016/s0042-6989(99)00250-3. [DOI] [PubMed] [Google Scholar]
- 24.Kim J-N, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2(2):176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 25.Lynch JC, Hoover JE, Strick PL. Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res. 1994;100(1):181–186. doi: 10.1007/BF00227293. [DOI] [PubMed] [Google Scholar]
- 26.Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36(1):3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diedrichsen J, Ivry R, Pressing J. Cerebellar and basal ganglia contributions to interval timing. In: Meck WH, editor. Functional and Neural Mechanisms of Interval Timing. Boca Raton: CRC; 2003. pp. 457–483. [Google Scholar]
- 28.Swaminathan SK, Freedman DJ. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat Neurosci. 2012;15(2):315–320. doi: 10.1038/nn.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrera VP, Yanike M, Cassanello C. Frontal eye field neurons signal changes in decision criteria. Nat Neurosci. 2009;12(11):1458–1462. doi: 10.1038/nn.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohler CW, Wurtz RH. Role of striate cortex and superior colliculus in visual guidance of saccadic eye movements in monkeys. J Neurophysiol. 1977;40(1):74–94. doi: 10.1152/jn.1977.40.1.74. [DOI] [PubMed] [Google Scholar]
- 31.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004;91(3):1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- 32.Singer W. Time as coding space? Curr Opin Neurobiol. 1999;9(2):189–194. doi: 10.1016/s0959-4388(99)80026-9. [DOI] [PubMed] [Google Scholar]
- 33.VanRullen R, Guyonneau R, Thorpe SJ. Spike times make sense. Trends Neurosci. 2005;28(1):1–4. doi: 10.1016/j.tins.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13(1):87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 35.Parker AJ, Newsome WT. Sense and the single neuron: Probing the physiology of perception. Annu Rev Neurosci. 1998;21(1):227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 36.Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53(3):603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 37.Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci. 1999;19(7):2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henson RNA. Neuroimaging studies of priming. Prog Neurobiol. 2003;70(1):53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 39.Barborica A, Ferrera VP. Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat Neurosci. 2003;6(1):66–74. doi: 10.1038/nn990. [DOI] [PubMed] [Google Scholar]
- 40.Kayser C, Petkov CI, Logothetis NK. Visual modulation of neurons in auditory cortex. Cereb Cortex. 2008;18(7):1560–1574. doi: 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- 41.Avillac M, Ben Hamed S, Duhamel J-R. Multisensory integration in the ventral intraparietal area of the macaque monkey. J Neurosci. 2007;27(8):1922–1932. doi: 10.1523/JNEUROSCI.2646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291(5502):312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 43.Walsh V. A theory of magnitude: Common cortical metrics of time, space and quantity. Trends Cogn Sci. 2003;7(11):483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Ibbotson M, Krekelberg B. Visual perception and saccadic eye movements. Curr Opin Neurobiol. 2011;21(4):553–558. doi: 10.1016/j.conb.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose D, Summers J. Duration illusions in a train of visual stimuli. Perception. 1995;24(10):1177–1187. doi: 10.1068/p241177. [DOI] [PubMed] [Google Scholar]
- 46.Gorea A. Ticks per thought or thoughts per tick? A selective review of time perception with hints on future research. Jf Physiol Paris. 2011;105(4–6):153–163. doi: 10.1016/j.jphysparis.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev. 1977;84(3):279–325. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.