Abstract
UBXD8 is a membrane-embedded recruitment factor for the p97/VCP segregase that has been previously linked to endoplasmic reticulum (ER)-associated degradation and to the control of triacylglycerol synthesis in the ER. UBXD8 also has been identified as a component of cytoplasmic lipid droplets (LDs), but neither the mechanisms that control its trafficking between the ER and LDs nor its functions in the latter organelle have been investigated previously. Here we report that association of UBXD8 with the ER-resident rhomboid pseudoprotease UBAC2 specifically restricts trafficking of UBXD8 to LDs, and that the steady-state partitioning of UBXD8 between the ER and LDs can be experimentally manipulated by controlling the relative expression of these two proteins. We exploit this interaction to show that UBXD8-mediated recruitment of p97/VCP to LDs increases LD size by inhibiting the activity of adipose triglyceride lipase (ATGL), the rate-limiting enzyme in triacylglycerol hydrolysis. Our findings show that UBXD8 binds directly to ATGL and promotes dissociation of its endogenous coactivator, CGI-58. These data indicate that UBXD8 and p97/VCP play central integrative roles in cellular energy homeostasis.
Keywords: biogenesis, lipolysis, ubiquitin, ERAD
Lipid droplets (LDs) are dynamic cytoplasmic organelles composed of a core of neutral lipids [triacylglycerol (TAG) and cholesterol esters] surrounded by a phospholipid monolayer decorated with integral and peripherally associated proteins that mediate essential LD functions, including trafficking and turnover (1, 2). The bounding phospholipid monolayer precludes incorporation into the LD surface of classical bitopic or polytopic integral membrane proteins that normally span a lipid bilayer separating two aqueous compartments, and integral proteins are anchored into the LD surface by the presence of either a hydrophobic hairpin or an amphipathic helix (1–4). Although it is widely accepted that LDs are biosynthetically elaborated from the endoplasmic reticulum (ER), the site of neutral lipid synthesis, how proteins are delivered from their site of synthesis to LDs, how these processes are coupled to metabolic stimuli, and the mechanisms by which LDs are formed and turned over have been largely unexplored.
UBXD8 (also known as ETEA or FAF2) is a hairpin-anchored UBA-UBX domain protein that partitions between the ER and LDs (4, 5). UBXD8 has been identified in the ER membrane as part of a protein complex linked to ER-associated degradation (ERAD) of folding or assembly-defective proteins in the early secretory pathway (6), and also has been independently identified, through proteomic analysis, as a component of LDs (7–9). Depletion of UBXD8 stabilizes ERAD substrates (5, 6, 10–12), supporting a role for UBXD8 in protein degradation. UBX domains bind p97/VCP, a hexameric, ring-shaped ATPase “segregase” that couples ATP hydrolysis to the generation of mechanical force used in a wide variety of ubiquitin (Ub)-dependent cellular processes, including endocytosis, DNA repair, and ERAD (13, 14). The UBX domain of UBXD8 is required for p97/VCP recruitment (5, 15) and for the degradation of at least one ERAD substrate (12). Although the precise role of UBXD8 in ERAD is unclear, it is widely thought that recruitment of p97/VCP is essential to facilitate the extraction or “dislocation” of substrates from the ER membrane (13).
Although neither the mechanism by which UBXD8 partitions between ER and LDs nor the role that this protein plays in LD biology is known, the presence of p97/VCP in LD-enriched biochemical fractions (7) suggests that one function of UBXD8 may be to recruit p97/VCP to LDs. In the present study, we have identified UBAC2, a recently discovered ER-resident rhomboid pseudoprotease (10, 16), as an ER receptor for UBXD8. Our data show that partitioning of UBXD8 between ER and LDs depends on the relative expression of UBAC2 and UBXD8. We exploit this finding to identify a nonproteolytic role for LD-localized UBXD8 and p97/VCP in controlling cellular fat storage by regulating the activity of adipose triglyceride lipase (ATGL), the rate-limiting enzyme in lipolysis.
Results
ER Rhomboid Pseudoprotease UBAC2 Restricts UBXD8 Trafficking to LDs.
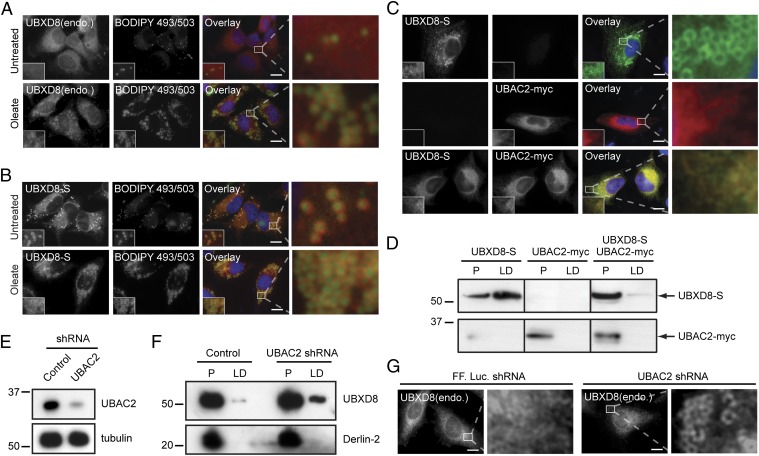
In unstimulated HeLa cells, endogenous UBXD8 was largely restricted to the ER, but was redistributed to LDs after treatment with the lipogenic unsaturated fatty acid oleate (Fig. 1A). In contrast, overexpressed S-peptide–tagged UBXD8 (UBXD8-S) was strongly localized to LDs irrespective of the presence of oleate (Fig. 1B). These findings indicate that the ER membrane has a limited capacity to retain UBXD8, possibly owing to a limiting ER-retention factor, and that excess UBXD8 partitions by default to LDs. We considered the possibility that UBAC2, an ER-localized protein that we recently identified as a UBXD8 interactor (10), could serve such a role in restricting UBXD8 trafficking. UBAC2 is an ER-localized protein with six potential transmembrane domains that identify it as a member of the rhomboid pseudoprotease family (10, 16). Unlike UBXD8, UBAC2 partitioned with dense fractions on sucrose gradient fractionation of cell lysates (Fig. S1A) and remained strictly localized to the ER after oleate treatment (Fig. S1 B and C).
Fig. 1.
UBAC2 regulates the partitioning of UBXD8 between the ER and LD. (A and B) Immunolocalization of endogenous UBXD8 (A; red) or exogenously expressed UBXD8-S (B; red) and LDs (BODIPY 493/503; green) in untreated or oleate-treated HeLa cells. (C) UBXD8-S (green) immunolocalization is restricted to the ER in HeLa cells coexpressing UBAC2-myc (red). (D) UBXD8-S partitioning into LD fractions is suppressed by coexpression of UBAC2-myc. Immunoblot analysis of oleate-treated HEK293 cells expressing the indicated constructs fractionated into ER-enriched (P) and LD-enriched (LD) fractions. Equivalent percentages of each fraction were analyzed. (E) Validation of shRNA depletion of endogenous UBAC2. (F and G) Depletion of endogenous UBAC2 promotes trafficking of endogenous UBXD8 to LDs, as evaluated by immunoblot analysis of equivalent percentages of P and LD fractions (F) and by immunofluorescence microscopy (G). In the micrographs, white boxes indicate the magnified regions. (Scale bars: 10 μm.)
If UBAC2 functions as a limiting ER-retention factor for UBXD8, then it should be possible to reverse the predominant LD localization of overexpressed UBXD8 by increasing the abundance of UBAC2. Indeed, we found that overexpression of UBAC2-myc completely suppressed both the localization (Fig. 1C) and biochemical partitioning (Fig. 1D) of overexpressed UBXD8 to LDs. This effect was specific for UBXD8, in that UBAC2 overexpression did not affect the trafficking of other integral membrane LD-associated proteins (Fig. S2A), and that overexpression of other ER-resident integral membrane proteins found in complex with UBXD8 (10) did not influence the partitioning of UBXD8 (Fig. S2B). Knockdown of endogenous UBAC2 (Fig. 1E) caused a fraction of endogenous UBXD8 to redistribute into LD-enriched fractions (Fig. 1F) and to LDs (Fig. 1G), indicating that the effects of UBAC2 expression on UBXD8 trafficking are not simply artifacts of overexpression, but reflect a bona fide interaction that contributes to the trafficking of UBXD8. Similar partitioning of endogenous UBXD8 between the ER and LDs and ER retention by UBAC2 was observed in Huh7 hepatocytes (Fig. S3 A–E). In contrast, UBXD8 was not detected in LDs in 3T3-L1 adipocytes (Fig. S3F) (17), and its expression was not induced on differentiation (Fig. S3G). These data demonstrate that UBAC2 levels can be experimentally manipulated to selectively control the UBXD8 levels in LDs irrespective of cellular metabolic status.
UBXD8 Increases LD Size and Abundance Through Recruitment of p97/VCP to LDs.
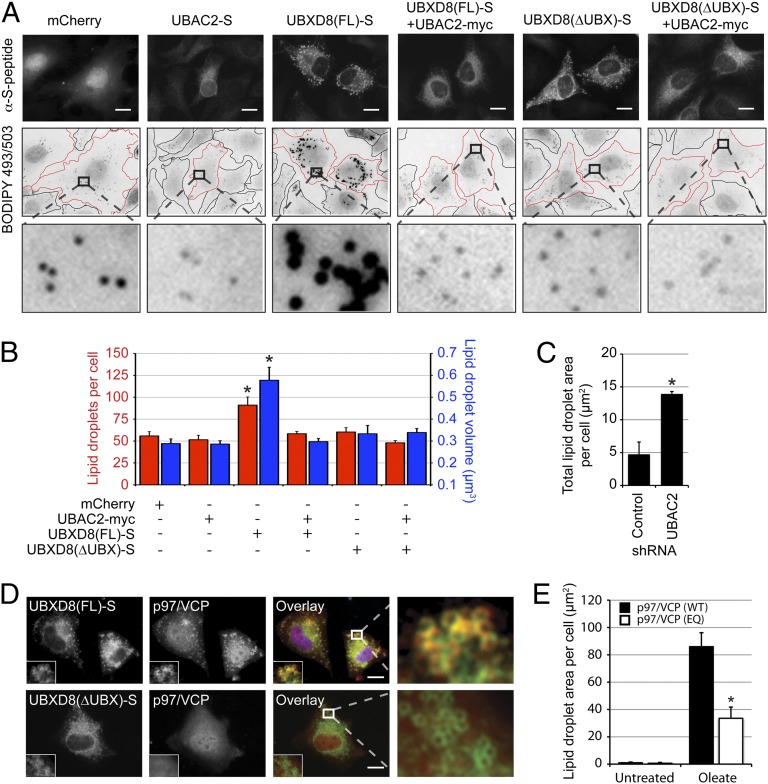
Overexpression of UBXD8-S led to striking increases in both the size and number of LDs (Fig. 2 A and B), an effect that could be related to elevated UBXD8 levels in the ER, LDs, or both compartments. However, our findings that coexpression of UBAC2 with UBXD8 completely suppressed LD enhancement (Fig. 2 A and B) and that knockdown of endogenous UBAC2 also increased cellular LD content (Fig. 2C) argue strongly that the observed effects on LD stores are due to LD-localized UBXD8. An increase in LD content was not observed on overexpression of UBXD8(∆UBX)-S (Fig. 2 A and B), indicating that UBXD8’s effects on LD size and abundance require its UBX domain. Indeed, we found that recruitment of endogenous p97/VCP to LDs was dependent on the UBX domain of UBXD8 (Fig. 2D), and that this recruitment was suppressed by coexpression of UBAC2 (Fig. S4A), suggesting that UBXD8 is the principal p97/VCP binding site on LDs. A role for p97/VCP in LD regulation is also supported by the observation that expression of a dominant-negative p97/VCP variant (18) strongly suppressed the ability of oleate to increase LD content (Fig. 2E). These data indicate that UBXD8 increases LD size and abundance through recruitment of p97/VCP ATPase activity to the LD surface.
Fig. 2.
UBXD8-mediated recruitment of p97/VCP to LDs inhibits LD turnover. (A and B) HeLa cells transfected with the indicated constructs were analyzed by immunofluorescence microscopy and BODIPY 493/503 staining (A), and the size and number of LDs were quantified (B). Cells are outlined in black (untransfected) or red (transfected). (C) UBAC2 depletion increases total LD area under basal conditions. LD content in HeLa cells expressing control or UBAC2 shRNA was analyzed by BODIPY 493/503 staining, and the area per cell was quantified. (D) UBXD8 recruits p97/VCP to LDs via its UBX domain. The distribution of endogenous p97/VCP (red) was analyzed in the presence of the indicated UBXD8-S constructs (green) in HeLa cells. Nuclei were stained with DAPI (blue). (E) p97/VCP ATPase function is required for LD homeostasis. U2OS cells expressing inducible p97/VCP(WT) or p97/VCP(EQ) were incubated in the presence or absence of 200 µM oleate and doxycycline, BODIPY 493/503-stained LDs were analyzed by immunofluorescence microscopy, and the area per cell was quantified. Representative images are shown in Fig. S4B. All graphical data are quantified as mean ± SEM. An asterisk indicates a significant difference (P < 0.05, t test) from the control based on n = 500–600 droplets from each of three independent biological replicates. In the micrographs, white boxes indicate the magnified regions. (Scale bars: 10 μm.)
UBXD8 Inhibits Lipolytic Degradation of LDs.
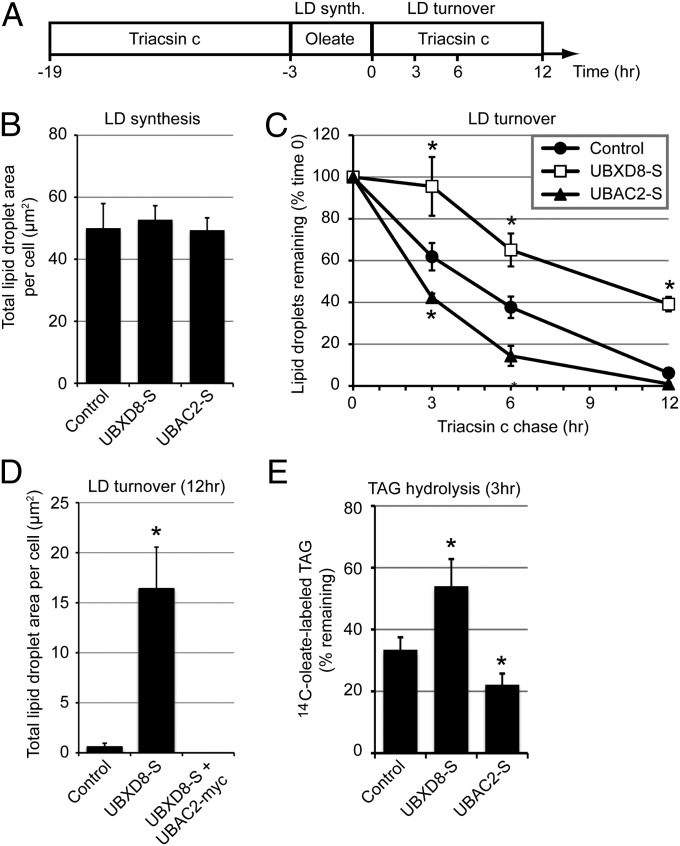
The increases in LD size and abundance induced by UBXD8 recruitment of p97/VCP to LDs could be related to either the promotion of LD biogenesis or the suppression of LD turnover. To evaluate these two possibilities, we used a “pulse-chase” protocol in which cells were initially depleted of LDs with triacsin C, a drug that blocks TAG synthesis by inhibiting fatty acyl-CoA synthetase (19), and then treated briefly with oleate in the absence of drug to induce synchronous LD production (Fig. 3 A and B). Neither UBXD8-S overexpression nor UBAC2-S overexpression affected LD area (Fig. 3B), indicating that oleate-induced LD biogenesis is unaffected by UBXD8 abundance. To assess the role of UBXD8 in LD degradation, we exposed cells treated as above to a second round of triacsin C to synchronously promote LD degradation (Fig. 3 A and C). Cells overexpressing UBXD8-S displayed a significantly reduced rate of LD turnover (Fig. 3C), whereas cells depleted of endogenous UBXD8 from LDs by overexpression of UBAC2-S demonstrated an increased rate of LD turnover (Fig. 3C). The inhibitory effect of UBXD8-S on LD turnover is not simply related to UBXD8 overexpression, but requires the physical presence of UBXD8 on LDs, as demonstrated by the suppression of the effect of UBXD8 overexpession on LD turnover by simultaneous UBAC2 overexpression (Fig. 3D).
Fig. 3.
LD-localized UBXD8 inhibits lipolytic degradation of LDs. (A) Timeline for oleate (200 µM) and triacsin C treatments (10 µM) for the experiments shown in B–E. (B–D) LD area per cell was quantified at time 0 to measure LD synthesis (B) or at the indicated time points to measure LD turnover (C and D) in transfected HeLa cells stained with BODIPY 493/503. (E) HeLa cells expressing the indicated constructs were treated according to the timeline shown in A, except that 14C-oleate (0.3 µCi) was included during the oleate pulse. Cellular lipids from the 0 and 3 h time points were extracted and separated by TLC, and the amount of 14C-oleate–labeled TAG was determined by scintillation counting. All graphical data are quantified as mean ± SEM. An asterisk indicates a significant difference (P < 0.05, t test) from the control based on three independent biological replicates. (Scale bars: 10 μm.)
Given that LD volume is composed almost entirely of neutral lipids, the most direct way for overexpressed UBXD8 to slow the decrease in LD content after inhibition of de novo TAG synthesis with triacsin C is by inhibiting the rate of TAG hydrolysis. Indeed, we found that UBXD8 overexpression significantly inhibited the decrease in 14C-TAG after triacsin C treatment of cells pulse-labeled with 14C oleate, whereas overexpression of UBAC2 accelerated TAG hydrolysis (Fig. 3E). We conclude that UBXD8 acts in LDs to inhibit TAG hydrolysis.
UBXD8 Binds and Inhibits ATGL, but Does Not Affect Its Stability.
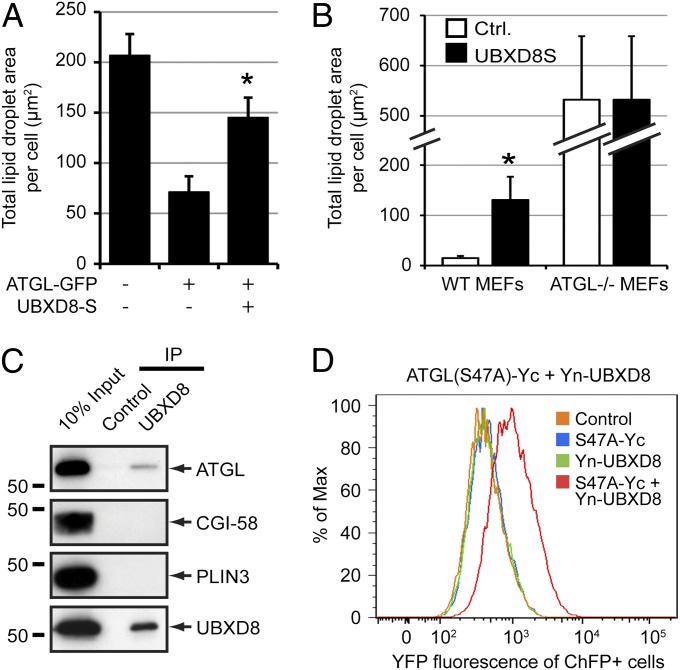
The most direct mechanism through which UBXD8 can inhibit lipolysis is by inhibiting the activity of ATGL, the lipase that catalyzes the rate-limiting step in TAG hydrolysis (20–22). In support of this, we found that LD content was reduced between threefold and fourfold in oleate-treated cells overexpressing ATGL-GFP compared with GFP-overexpressing controls, and that this effect was largely abrogated by simultaneous overexpression of UBXD8-S (Fig. 4A), suggesting that UBXD8 antagonizes the effect of ATGL in LDs. To determine whether UBXD8 requires ATGL to influence cellular LD content, we analyzed the effect of UBXD8 expression in WT or ATGL−/− mouse embryonic fibroblasts (MEFs). As observed in HeLa cells, expression of UBXD8 in WT MEFs increased LD content (Fig. 4B). In contrast, UBXD8 expression had no effect on LD content in ATGL−/− MEFs (Fig. 4B), despite the fact that MEFs lacking this lipase retain the ability to synthesize LDs in response to oleate (Fig. S5). These data support the conclusion that UBXD8 inhibits LD turnover by negatively modulating ATGL.
Fig. 4.
UBXD8 binds ATGL and impairs ATGL-dependent LD turnover. (A) UBXD8 suppressed LD degradation induced by ATGL overexpression. Total LD area per cell was quantified in HeLa cells expressing the indicated constructs. (B) ATGL is required for LD up-regulation by UBXD8. LD area per cell was quantified in WT or ATGL−/− MEFs expressing a control plasmid or UBXD8-S. (C) UBXD8 interacts with ATGL. Immunoblot analysis of UBXD8 complexes immunopurified from detergent-solubilized LD-enriched fractions isolated from oleate-treated HEK293 cells. (D) Assessment of the ATGL–UBXD8 interaction by bimolecular fluorescence complementation. HEK293 cells transiently transfected with the indicated C-terminal (Yc) or N-terminal (Yn) “split YFP” constructs were treated with 200 µM oleate for 24 h and analyzed by flow cytometry.
Immunoprecipitation of UBXD8 complexes from detergent-solubilized LDs coprecipitated ATGL, but not the LD-associated proteins CGI-58 or PLIN3 (also known as TIP47) (Fig. 4C), indicating that endogenous UBXD8 and ATGL interact with each other either directly or indirectly. To confirm this interaction, we used a bimolecular fluorescence complementation assay in which UBXD8 fused to the N terminus of YFP (Yn-UBXD8) was coexpressed with a catalytically inactive ATGL variant (so as not to induce LD degradation) fused to the C terminus of YFP [ATGL(S47A)-Yc]. YFP fluorescence was observed only on coexpression of the two constructs (Fig. 4D), confirming an interaction between UBXD8 and ATGL in intact cells and indicating that the interaction between these two proteins is likely direct.
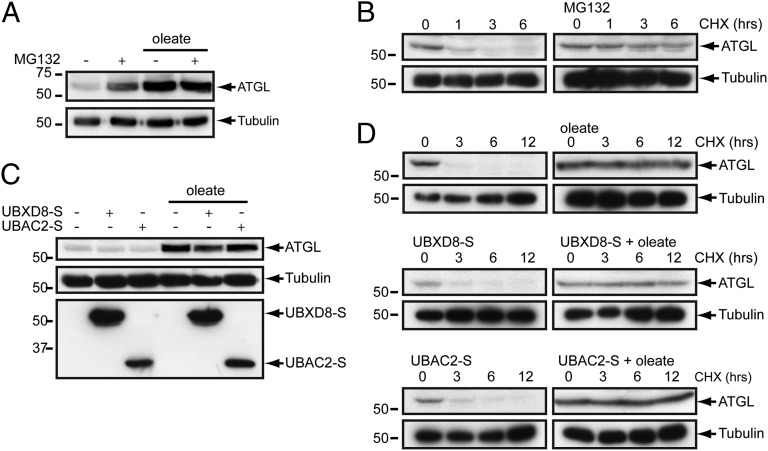
Considering that in the ER, UBXD8 is thought to recruit p97/VCP to facilitate the extraction and degradation of ERAD substrates, a plausible mechanism by which UBXD8 can negatively regulate ATGL activity is to promote its extraction from LDs and its degradation by the Ub-proteasome system (UPS) (5, 6, 10–12). Indeed, we found that ATGL is a short-lived (t1/2 ∼45 min) protein that it is strongly stabilized (t1/2 >12 h) by the proteasome inhibitor MG132 (Fig. 5 A and B) and, surprisingly, by oleate (Fig. 5 C and D). Stabilization of ATGL by oleate is not related to a general impairment of the UPS, given that degradation of the UPS substrates GFPu (cytosolic) and TCRα-GFP (TCR subunit α, ER) were unaffected by the presence of oleate (Fig. S6 A and B). Surprisingly, the steady-state abundance (Fig. 5C) and half-life of ATGL (Fig. 5D), as well as the association of ATGL with LDs (Fig. S6C), were unaffected by overexpression of UBXD8 or UBAC2, arguing against a role for UBXD8 in controlling the extraction, turnover, or abundance of ATGL.
Fig. 5.
UBXD8 levels do not impact the proteasomal degradation of ATGL. (A) Oleate suppresses ATGL proteasomal degradation. Steady-state levels of endogenous ATGL in HEK293 cells incubated in the presence or absence of 200 μM oleate for 24 h were evaluated by immunoblot analysis. Where indicated, 10 µM MG132 was included for the final 12 h. (B) ATGL is a constitutive proteasome substrate. Cycloheximide (CHX) shutoff analysis of endogenous ATGL turnover in HEK293 cells in the presence or absence of 10 µM MG132. (C) UBXD8 does not influence ATGL levels. Steady-state levels of endogenous ATGL in HEK293 cells or HEK293 cells stably expressing UBXD8-S or UBAC2-S incubated in the presence or absence of 200 μM oleate for 24 h were evaluated by immunoblot analysis. (D) UBXD8 does not influence ATGL turnover. CHX shutoff analysis of endogenous ATGL turnover in control HEK293 cells or HEK293 cells stably expressing UBXD8-S or UBAC2-S.
UBXD8 Negatively Regulates ATGL by Promoting Dissociation of Its Endogenous Activator CGI-58.
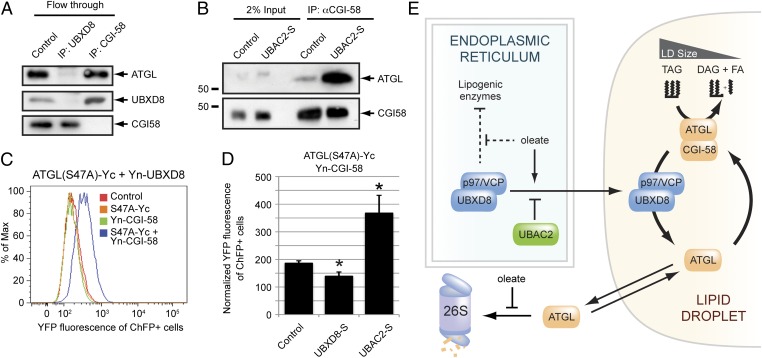
An alternative mechanism by which UBXD8 might inhibit ATGL is that, through its association with p97/VCP, UBXD8 could promote the dissociation of ATGL from its endogenous activator CGI-58 (23), a mechanism consistent with the known segregase function of p97/VCP (13). Antibodies against endogenous UBXD8, but not CGI-58, largely depleted ATGL from detergent-solubilized LDs (Fig. 6A), indicating that the majority of ATGL in LDs isolated from oleate-treated cells is associated with UBXD8, not with CGI-58. In contrast, the amount of endogenous ATGL captured with CGI-58 antibodies was substantially increased by overexpression of UBAC2-S (Fig. 6B), a condition that restricts all detectable endogenous UBXD8 to the ER (Fig. S6D).
Fig. 6.
UBXD8 regulates the association of ATGL with its activator CGI-58. (A) Endogenous ATGL is preferentially associated with UBXD8 over CGI-58. Immunoblot analysis of endogenous ATGL, UBXD8, or CGI-58 in LD-enriched fractions from oleate-treated HEK293 cells immunodepleted with the indicated antibodies. (B) Depletion of endogenous UBXD8 from LDs promotes the interaction of CGI-58 and ATGL. Immunoblot analysis of endogenous CGI-58 complexes from LD-enriched fractions isolated from oleate-treated control or UBAC2-S–expressing HEK293 cells. (C) Assessment of the ATGL–CGI-58 interaction by bimolecular fluorescence complementation. HEK293 cells transiently transfected with the indicated C-terminal (Yc) or N-terminal (Yn) “split YFP” constructs were treated with 200 µM oleate for 24 h and analyzed by flow cytometry. (D) LD-associated UBXD8 modulates the interaction between ATGL and CGI-58. Quantification of flow cytometry data of ATGL(S47A)-Yc and Yn-CGI-58 bimolecular fluorescence complementation in oleate-treated HEK293 cells expressing the indicated constructs. Normalized YFP fluorescence levels (from four independent experiments) are presented as mean ± SEM. An asterisk indicates a significant difference (P < 0.05, t test) from the control cells. (E) Model illustrating the role of UBXD8 and p97/VCP as regulators of cellular fat storage. The UBXD8–UBAC2 interaction establishes a dominant tethering mechanism that restricts a pool of UBXD8 from trafficking to LDs. In the ER, UBXD8 mediates the oleate-regulated inhibition of lipogenic proteins. Oleate treatment induces UBXD8 trafficking to LDs and stabilizes ATGL by inhibiting its constitutive proteasomal degradation. On LDs, UBXD8 binds ATGL and, through its recruitment of p97/VCP, functions as a noncompetitive inhibitor that mediates dissociation of the activated ATGL–CGI-58 complex.
Finally, we used a bimolecular fluorescence complementation assay of split YFP-tagged ATGL and CGI-58 to directly interrogate the interaction between ATGL and CGI-58 (Fig. 6C). Whereas overexpression of UBXD8-S resulted in a small but significant decrease in fluorescence, UBAC2-S overexpression led to strongly enhanced fluorescence (Fig. 6D). Our data support the conclusion that UBXD8, together with p97/VCP, inhibits ATGL by promoting CGI-58 dissociation.
Discussion
Balancing lipogenesis and lipolysis is essential to the maintenance of cellular energy homeostasis (22). Although ATGL is expressed ubiquitously, emerging evidence indicates that different cell types have evolved different mechanisms to control this critical rate-limiting enzyme (22). In adipocytes, ATGL is inhibited directly by G0S2 and indirectly by hormonally regulated sequestration of CGI-58 by the adipose-specific perilipin, PLIN1 (22). Our results reveal that in nonadipocytic cells, ATGL is subject to two different, hitherto unrecognized mechanisms of metabolically controlled regulation: UBXD8- and p97/VCP-dependent dissociation of CGI-58 and regulated turnover by the proteasome.
p97/VCP is a hexameric ring-shaped enzyme that converts the chemical energy derived from ATP hydrolysis into mechanical force (13). Although this enzyme functions in a remarkably broad array of cellular processes, all appear to share a dependence on the segregase function of this enzyme to facilitate the dissociation of protein complexes (13). For example, p97/VCP segregase activity contributes to transcriptional activation by extraction of membrane-tethered transcription factors, contributes to quality control by selective removal of misfolded proteins from the surfaces of organelles, including the ER and mitochondria; and supports the remodeling of chromatin-associated proteins involved in the DNA damage response (13). Specificity is achieved by the ability of p97/VCP to interact with recruitment factors, including UBX domain-containing proteins like UBXD8. Together with a recent study documenting a role for Cdc48, the yeast ortholog of p97/VCP, in controlling the induced dissociation of a cullin-RING ligase E3 Ub ligase complex (24), our findings identify a noncanonical mode of enzyme regulation in which p97/VCP activity is coupled to the disassembly of an active enzyme complex (Fig. 6E). Thus, p97/VCP may play a more general role in the regulation of multimeric enzyme complexes than previously appreciated.
p97/VCP function is coordinated through interactions with ubiquitinated client proteins (13), and it is likely that Ub is involved in the actions of UBXD8 and p97/VCP in LDs. Such a role is suggested by the presence of a UBA domain at the C terminus of UBXD8, the identification of Ub conjugation machinery in LDs (2), and the demonstration of in vitro Ub conjugation activity in LD-enriched fractions (25). Nonetheless, the presence or absence of UBXD8 in LD does not appear to influence the half-life of ATGL (Fig. 5) or, in preliminary analyses, CGI-58. Additional studies are needed to decipher precisely how Ub contributes to the regulation of ATGL by UBXD8 and p97/VCP.
Our analysis reveals that metabolically controlled degradation of ATGL by the proteasome represents yet another unexpected mechanism by which this enzyme is regulated. Stabilization of ATGL by oleate initially seemed paradoxical, given that under these conditions, the balance must be tipped toward TAG storage, not hydrolysis. However, in the presence of oleate, we find that ATGL is nearly completely bound to UBXD8 and thus uncoupled from CGI-58. We propose that maintaining a stabilized pool of ATGL on LDs in an inhibited state may provide a mechanism for rapid activation of ATGL without the need for additional protein synthesis and trafficking.
In the ER, UBXD8 is poised to play an integrative role in lipid metabolism and has been proposed to function as a “sensor” that regulates TAG synthesis through its ability to specifically bind to unsaturated fatty acids (26). The mechanism of UBXD8 inhibition of TAG synthesis is not well understood, but our proteomic identification of ACSL3 and AGPAT1 in UBXD8-S affinity purified complexes (10) suggests that UBXD8 has the potential to regulate upstream TAG synthesis enzymes, perhaps by directing them to destruction by ERAD. UBXD8 also regulates de novo fatty acid synthesis through its recruitment of p97/VCP for the dislocation of ubiquitinated Insig-1, an ER-resident protein that controls the proteolytic activation of the master transcription factor SREBP-1 (11, 26). We propose that controlling the partitioning of UBXD8 between ER and LDs represents a potentially important mechanism for regulating cellular energy balance, and in this work we have identified the ER-resident rhomboid pseudoprotease UBAC2 as a selective UBXD8 ER receptor that restricts UBXD8 trafficking from the ER to LDs. Further studies are needed to determine the extent to which the interaction between UBAC2 and UBXD8 is responsive to metabolic conditions and contributes to net energy balance.
Methods
LD Fractionation.
As described previously (27), after 16–24 h of treatment with 200 µM oleate, cells were incubated in hypotonic lysis buffer [20 mM Tris⋅HCl (pH 7.4), 1 mM EDTA] for 10 min, dounce-homogenized, and centrifuged at 1,000 × g for 10 min. The postnuclear supernatant was transferred to a new tube, adjusted to a final concentration of 20% (wt/vol) sucrose, and overlaid with 4 mL of hypotonic lysis buffer containing 5% (wt/vol) sucrose and then with 4 mL of hypotonic lysis buffer. After centrifugation at 28,000 × g for 30 min, the buoyant LD-enriched fraction was collected using a Beckman tube slicer, and the remaining fractions were collected by pipetting. Equivalent percentages of each fraction were measured by immunoblot analysis. Under these conditions, quantification of immunoblots indicate that ∼75% of the ER proteins employed as ER markers, UBAC2 and Derlin-2, sedimented and were present in the pellet fraction (Fig. S1B). Analysis of the pellet fraction underestimated the amount of ER-localized proteins by ∼25%.
Fluorescence Microscopy.
Cells grown on poly-L-lysine–coated coverslips were fixed in 4% (wt/vol) paraformaldehyde, immunostained, and analyzed using a Zeiss Axiovert 200M fluorescence microscope. LDs were stained with BODIPY 493/503 (Invitrogen). ImageJ 1.42q was used to quantify LD number, size, and area. These experiments and analyses are described in more detail in SI Methods.
Endogenous Immunoprecipitation and Immunoblot Analysis.
LD-enriched fractions were solubilized in a final buffer containing 20 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, and 1% (vol/vol) Triton X-100 supplemented with Complete Protease Inhibitor Mixture (Roche). Endogenous immunoprecipitations were performed by incubation with antibody immobilized on agarose beads using the Direct Immunoprecipitation Kit (Thermo Fisher Scientific). Samples were separated by SDS/PAGE and evaluated by immunoblot analysis.
Flow Cytometry.
Bimolecular fluorescence complementation in HEK293 cells transiently transfected with split YFP constructs fused to the N terminus of YFP (Yn) or the C terminus of YFP (Yc), including ATGL-Yc, Yn-UBXD8, or Yn-CGI-58, was analyzed using a BD Biosciences LSRII flow cytometer, as described in SI Methods.
Supplementary Material
Acknowledgments
We thank the members of the Kopito laboratory, especially Ethan Greenblatt and Margaret Pearce, for discussions and critical reading of the manuscript. We also thank Robert Farese, Jr. (Gladstone Institute); Andy Nguyen (Gladstone Institute); J. Wade Harper (Harvard University); and Eric Bennett (University of California at San Diego) for helpful discussions. This work was supported by National Institutes of Health Grants DK095921 (to J.A.O.) and GM074874 (to R.R.K.). J.A.O. was also supported by National Research Service Award fellowship GM086026 from the National Institute of General Medical Sciences and C.M.R. was supported by a fellowship from the Cystic Fibrosis Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.A.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213738110/-/DCSupplemental.
References
- 1.Fujimoto T, Parton RG. Not just fat: The structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011;3:a004838. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zehmer JK, Bartz R, Liu P, Anderson RG. Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J Cell Sci. 2008;121(Pt 11):1852–1860. doi: 10.1242/jcs.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zehmer JK, et al. Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets. J Cell Sci. 2009;122(Pt 20):3694–3702. doi: 10.1242/jcs.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki M, et al. Derlin-1 and UBXD8 are engaged in dislocation and degradation of lipidated ApoB-100 at lipid droplets. Mol Biol Cell. 2012;23(5):800–810. doi: 10.1091/mbc.E11-11-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller B, Klemm EJ, Spooner E, Claessen JH, Ploegh HL. SEL1L nucleates a protein complex required for dislocation of misfolded glycoproteins. Proc Natl Acad Sci USA. 2008;105(34):12325–12330. doi: 10.1073/pnas.0805371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartz R, et al. Dynamic activity of lipid droplets: Protein phosphorylation and GTP-mediated protein translocation. J Proteome Res. 2007;6(8):3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto Y, et al. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 2004;1644(1):47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279(5):3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 10.Christianson JC, et al. Defining human ERAD networks through an integrative mapping strategy. Nat Cell Biol. 2012;14(1):93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JN, Zhang X, Feramisco JD, Gong Y, Ye J. Unsaturated fatty acids inhibit proteasomal degradation of Insig-1 at a postubiquitination step. J Biol Chem. 2008;283(48):33772–33783. doi: 10.1074/jbc.M806108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan VT, et al. The RasGAP proteins Ira2 and neurofibromin are negatively regulated by Gpb1 in yeast and ETEA in humans. Mol Cell Biol. 2010;30(9):2264–2279. doi: 10.1128/MCB.01450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14(2):117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 14.Schuberth C, Buchberger A. UBX domain proteins: Major regulators of the AAA ATPase Cdc48/p97. Cell Mol Life Sci. 2008;65(15):2360–2371. doi: 10.1007/s00018-008-8072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandru G, et al. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134(5):804–816. doi: 10.1016/j.cell.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenblatt EJ, Olzmann JA, Kopito RR. Derlin-1 is a rhomboid pseudoprotease required for the dislocation of mutant α-1 antitrypsin from the endoplasmic reticulum. Nat Struct Mol Biol. 2011;18(10):1147–1152. doi: 10.1038/nsmb.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279(45):46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 18.Ju JS, Miller SE, Hanson PI, Weihl CC. Impaired protein aggregate handling and clearance underlie the pathogenesis of p97/VCP-associated disease. J Biol Chem. 2008;283(44):30289–30299. doi: 10.1074/jbc.M805517200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomoda H, Igarashi K, Omura S. Inhibition of acyl-CoA synthetase by triacsins. Biochim Biophys Acta. 1987;921(3):595–598. [PubMed] [Google Scholar]
- 20.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 21.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 22.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis: A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50(1):14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lass A, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 2006;3(5):309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Yen JL, et al. Signal-induced disassembly of the SCF ubiquitin ligase complex by Cdc48/p97. Mol Cell. 2012;48(2):288–297. doi: 10.1016/j.molcel.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klemm EJ, Spooner E, Ploegh HL. Dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and endoplasmic reticulum (ER) protein quality control. J Biol Chem. 2011;286(43):37602–37614. doi: 10.1074/jbc.M111.284794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JN, et al. Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc Natl Acad Sci USA. 2010;107(50):21424–21429. doi: 10.1073/pnas.1011859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brasaemle DL, Wolins NE. Isolation of lipid droplets from cells by density gradient centrifugation. Curr Protoc Cell Biol. 2006:3.15.1–3.15.12. doi: 10.1002/cpcb.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.