Abstract
Appropriate control of immune responses is a critical determinant of health. Here, we show that choline acetyltransferase (ChAT) is expressed and ACh is produced by B cells and other immune cells that have an impact on innate immunity. ChAT expression occurs in mucosal-associated lymph tissue, subsequent to microbial colonization, and is reduced by antibiotic treatment. MyD88-dependent Toll-like receptor up-regulates ChAT in a transient manner. Unlike the previously described CD4+ T-cell population that is stimulated by norepinephrine to release ACh, ChAT+ B cells release ACh after stimulation with sulfated cholecystokinin but not norepinephrine. ACh-producing B-cells reduce peritoneal neutrophil recruitment during sterile endotoxemia independent of the vagus nerve, without affecting innate immune cell activation. Endothelial cells treated with ACh in vitro reduced endothelial cell adhesion molecule expression in a muscarinic receptor-dependent manner. Despite this ability, ChAT+ B cells were unable to suppress effector T-cell function in vivo. Therefore, ACh produced by lymphocytes has specific functions, with ChAT+ B cells controlling the local recruitment of neutrophils.
Keywords: colitis, inflammatory reflex, regulatory T cells, sepsis
Immunity requires successfully deciphering between noxious, pathogenic insults and innocuous, self-derived antigens. Reactions to self, or exaggerated immune responses, are detrimental, resulting in autoimmunity or immunopathology. To mitigate this, numerous regulatory mechanisms control innate and adaptive immunity, permitting robust responses while limiting damage. A striking example of immunopathology resulting in mortality following an exaggerated immune response is septic shock and multiorgan system dysfunction. This overwhelming immune response is typically preceded by bacteremia, although death can occur in the absence of viable bacteria. Stimulation by pathogen-associated molecular patterns through Toll-like receptors (TLRs) after infection or exposure to bacterially derived products causes immune effector molecule production that can have an adverse impact on normal physiology. For example, nitric oxide produced by endothelial cells and macrophages induces vasodilatation, as well as the acute circulatory failure common in severe sepsis. In addition, enzymes released from neutrophils recruited to the site of infection can damage the endothelium (1, 2). This endothelial injury and the resulting increased vascular permeability in the lung can have devastating clinical consequences resulting in pulmonary edema and acute lung injury or acute respiratory distress syndrome. Options for therapeutic intervention are limited, with varying degrees of success, highlighting the need for alternative approaches.
Regulation of immunity is normally accomplished by diverse mechanisms, including glucocorticoids, anti-inflammatory cytokines, soluble cytokine receptor antagonists, and the nervous system. Until recently, modulation of immune cell function by neurological inputs has been ignored despite the innervation of lymphoid organs, the close physical approximation of immune cells with neurons, and the effect of neurotransmitters on immune cells. Examples of neural immune communication can be found throughout the body, from the innervation of the thymus, spleen, and Peyer’s patches (PP) (3, 4) to the neural signaling induced by TLR ligands in the intestinal mucosa (5). The effect of this neural input on immunity is dictated by the chemical coding of the neurons, the receptors on the target cells, and the target cell type. For example, ACh induces signaling through muscarinic or nicotinic receptors, although only activation of nicotinic α7-receptors on macrophages specifically reduces TNF-α production during septic shock (6). Protection afforded by this “inflammatory reflex” occurs following electrical (6) or cholecystokinin (CCK) (7) stimulation of the vagus nerve. Stimulation and activation of vagal efferents terminating in the celiac ganglion induce norepinephrine (NE) release from neurons innervating the spleen, resulting in ACh release. It is important to note that these studies have dissected the efferent reflex arc using external stimulation (e.g., electrical stimulation) and that the origin of the intrinsic signals remains undetermined.
ACh is produced by the acetylation of choline, with acetyl-CoA as the donor in the presence of choline acetyltransferase (ChAT). As the sole enzyme that produces ACh, ChAT expression indicates cholinergic neurons that release ACh. Historically, this has led to a paradox in the spleen. Although electrical stimulation of the splenic ganglion induced ACh release, neuronal ChAT had not been observed by immunohistochemistry. This splenic ACh production was recently demonstrated to originate from CD4+ T cells using a reporter mouse expressing GFP under the control of the ChAT promoter (ChAT-GFP) (8). The ChAT-expressing T cells were both necessary and sufficient to restore the inflammatory reflex in nude mice, indicating that these cells provide the terminal neurotransmitter in a vagus nerve-controlled circuit. These data were in keeping with the ability of specific B- and T-cell lines to produce ACh (9). Despite these prior studies, the factors regulating ChAT expression, the site of induction, and whether other primary immune cells are similarly capable of ACh production were not determined.
Here, we have identified ChAT expression by B cells, dendritic cells (DCs), and macrophages. ChAT expression begins after microbial colonization, following birth, which requires MyD88-dependent signaling. These signals are derived from the intestinal microbiota, with ChAT+ cells appearing to originate in the mucosal-associated lymphoid tissue (MALT). Highlighting that ChAT+ B cells are unique compared with the previously described CD4+ ChAT+ T cells, they release ACh in response to different signals. Functionally, compared with T cells, ChAT+ B cells fulfill different roles that limit local neutrophil recruitment independent of signals from the vagus nerve.
Results
ChAT-GFP Is Expressed in Specific Immune Cell Populations.
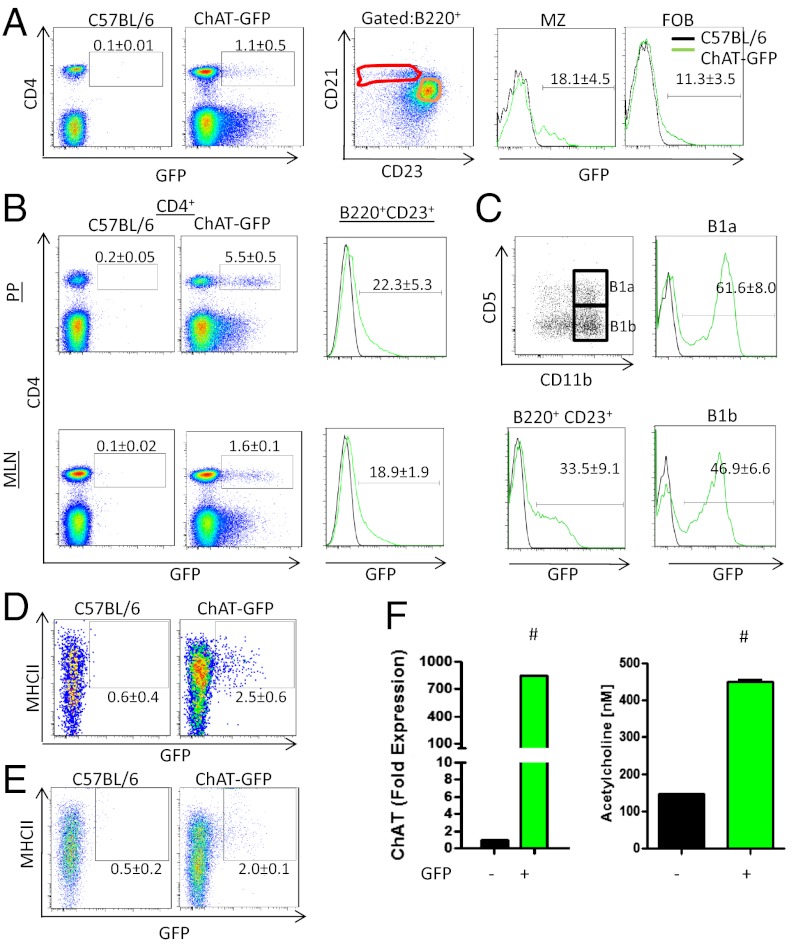
Recently documented in splenic CD4+ T cells, ChAT-GFP expression was assessed in a variety of immune cells. As indicated in Fig. 1, ChAT-GFP was confirmed in CD4+CD8− T cells and detected in follicular B cells (B220+CD21+CD23+) and marginal zone (B220+CD21+CD23int) B cells (Fig. 1A). This was not unique to the spleen, because GFP+ cells were also observed in secondary lymphoid organs, including mesenteric lymph nodes (MLNs), peripheral lymph nodes (pLNs; cervical, inguinal, brachial, and axial), PP, and peripheral blood (Fig. 1B and Fig. S1 A and B). In the thymus, no GFP expression was observed in CD4+CD8+, CD4+CD8−, or CD4−CD8+ T cells (Fig. S1C). Similarly, in the bone marrow, immature B cells (B220int) were GFP−, with expression restricted to mature recirculating B cells (B220hi+IgM−IgD+) (Fig. S1D). ChAT-GFP was also detected in peritoneal B2, B1a (B220+CD11b+CD5+), and B1b (B220+CD11b+CD5−) cells, with B1 populations comprising the largest percentage of ChAT-GFP+ B cells (Fig. 1C). Together, these data suggest that ChAT-GFP expression is induced in response to specific signals received after commitment to the CD4+ T-cell lineage in the thymus and B-cell development in the bone marrow.
Fig. 1.
ChAT-GFP is expressed in multiple immune cells. (A) Flow cytometry for GFP expression was conducted on splenocytes for CD4+ and CD8+ T cells (Left) and marginal zone (MZ) and follicular B (FOB) cells (Right). ChAT+ cells were also enumerated in the PP and MLN (B) and in peritoneal B1a, B1b, and B2 B cells (C). Splenic DCs (D) and macrophages (E) were assessed for ChAT-GFP. (F) ChAT and ACh production was assessed by qRT-PCR (Left) and by MS from splenic B220+CD23+ B cells (Right) (n = 3 mice repeated three times). #P <0.001 by ANOVA.
ChAT-GFP expression was also detected in splenic DCs (FSChiCD11b−CD11c+MHCII+F4/80− and CD11b+CD11c+MHCII+F4/80−) and macrophages (CD11b+CD11c−F4/80+) (Fig. 1 D and E). In assessing other tissues, we noted no GFP+ natural killer (NK) cells, NK T cells, or T cells in the liver (Fig. S1E), as well as no small intestinal intraepithelial lymphocytes (TCR-αβ CD8-αα, TCR-γδ CD8-αα; Fig. S1F).
We validated that GFP reporter expression correlated with ChAT expression and ACh production. As indicated in Fig. 1F, significantly increased endogenous ChAT mRNA was detected by quantitative RT-PCR (qRT-PCR) and ACh was detected by MS in FACS-sorted GFP+ B cells. These data expand on the immune cell populations that can produce ACh and suggest that these cells reside in unique niches.
Induction of ChAT Occurs by MyD88/Antigen Receptor-Dependent Signaling.
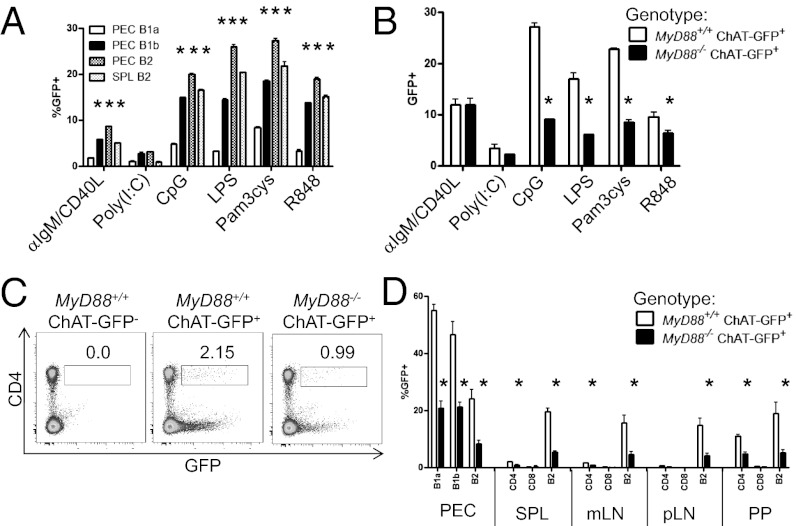
To determine which signals were capable of inducing ChAT expression, FACS-sorted GFP− splenic B2 cells (B220+CD23+) and peritoneal B2, B1a, and B1b cells were stimulated with anti-IgM/CD40L or TLR ligands. With the exception of B1a cells, stimulation with CpG, LPS, Pam3Cys, R848, or anti-IgM + CD40L but not the MyD88-independent stimuli polyinosinic:polycytidylic acid (Fig. 2A) induced ChAT-GFP expression.
Fig. 2.
MyD88-dependent signaling regulates ChAT expression in immune cells. (A) Sorted GFP− peritoneal (PEC) B1a, B1b, or B2 cells or splenic (SPL) B2 cells were stimulated (16 h) with anti-IgM/CD40 (10 μg/mL and 5 μg/mL, respectively), polyinosinic:polycytidylic acid [Poly(I:C); 100 μg/mL], CpG (10 μM), LPS (10 μg/mL), Pam3cys (1 μM), or R848 (1 μg/mL) and assessed for GFP. (B) FACS-sorted GFP− splenic B2 cells from MyD88+/+ Chat-GFP−, MyD88+/+ ChAT-GFP+, and MyD88−/− ChAT-GFP+ mice were stimulated with TLR ligands. ChAT expression in MyD88−/− mice was assessed by flow cytometry on SPL follicular B cells (C, Left) and CD4+ T cells (C, Right), summarized from PEC, SPL, MLN (mLN), pLN, and PP cells (D) (n = 3 mice repeated three times). *P < 0.05 by ANOVA.
To test the role of MyD88 in ChAT induction directly, sorted splenic GFP− B2 cells from ChAT-GFP+ MyD88−/− or MyD88+/+ mice were stimulated with TLR ligands. As indicated in Fig. 2B, ChAT-GFP induction was significantly reduced in MyD88−/− compared with MyD88+/+ mice. The ability of these TLR ligands to induce ChAT expression in a cell-intrinsic manner was tested using FACS-sorted splenic B220+CD23+GFP− cells from ChAT-GFP+ MyD88−/− and MyD88+/+ mice. Cells were differentially labeled with the tracking dyes cell trace violet (MyD88−/−) and efluor670 (MyD88+/+), mixed in a 1:1 ratio, and stimulated with TLR ligands. As before, TLR stimulation induced GFP expression in MyD88+/+ but not MyD88−/− cells, either stimulated alone or cocultured with WT cells (Fig. S2). MyD88 signaling was also required for in vivo induction of ChAT, with significant reductions in GFP+ B cells and CD4+ T cells observed in the bone marrow, spleen, peritoneum, PP, and MLN of the MyD88−/− mice (Fig. 2 C and D). Together, these results demonstrate that specific signaling events through the antigen receptor or transduced through MyD88 are required for the induction of ChAT expression in immune cells.
Production of ChAT-GFP+ Cells Occurs After Birth and Depends on Microbial Colonization.
Because TLR ligands induced ChAT-GFP expression in immune cells, we considered that postnatal microbial colonization might induce lymphocyte ChAT expression in vivo. Consistent with this hypothesis, B220+GFP+ splenocytes were absent in embryonic day (E) 18.5 fetuses confirmed to be ChAT-GFP+ by genotyping (Fig. S3A). Fetal B cells became activated by LPS or R848 as indicated by increased CD69 expression, and induced ChAT-GFP in a comparable manner to adult B cells (Fig. S3B). Analysis of neonatal mice on postnatal day 7 revealed the presence of B220+GFP+ B cells; however, this population was significantly smaller compared with adult mice (Fig. S3C). These data demonstrated that B cells in utero have not received the appropriate stimuli to induce ChAT expression and that this occurs after birth.
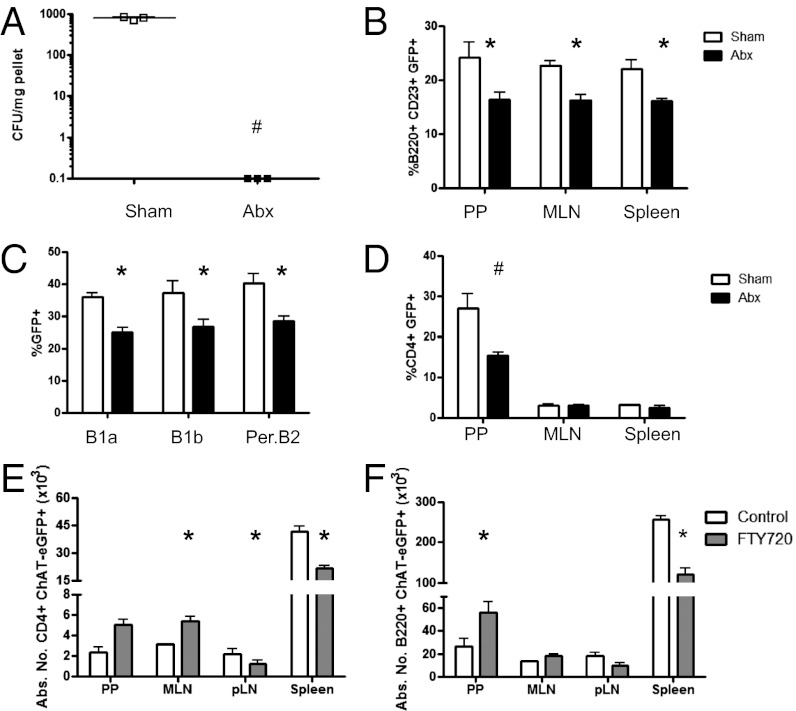
To test the role of the microbiota in the induction of ChAT directly, mice were treated at weaning with an antibiotic mixture in the drinking water for 4 wk (10). Antibiotic treatment significantly reduced the colonic microbiota (Fig. 3A) and the frequency of ChAT-GFP+ cells. With respect to B220+CD23+ B cells, this reduction occurred in the PP, MLN, and spleen, as well as in peritoneal B1a, B1b, and B2 B cells (Fig. 3 B and C). Depletion of the microbiota also reduced CD4+ ChAT-GFP+ cells in the PP but not in the MLN or spleen (Fig. 3D).
Fig. 3.
Intestinal microbiota contribute to ChAT+ lymphocyte development. (A) Antibiotics (Abx) were administered in the drinking water to deplete the microbiota. (B) Follicular B cells were assessed in control (white bars) and antibiotic-treated (black bars) mice in the PP, MLN, and spleen. (C) Effect of microbiota depletion was also assessed in peritoneal B1a, B1b, and B2 B cells. (D) Analysis of the CD4+ T-cell population was also conducted in the PP, MLN, and spleen. In separate experiments, mice were gavaged with vehicle (control, open bars) or FTY720 (solid bars). GFP expression was assessed on cells from the PP, MLN, pLN, and spleen in CD4+ T cells (E) and B220+ B cells (F) (n = 3 to 4 mice per group, two experiments). Abs. No., absolute number. *P < 0.05 and #P < 0.001 by ANOVA.
To assess which of these tissues contributed to ChAT induction in vivo, mice were treated with the synthetic sphingosine 1 phosphate analog FTY720 (1 mg⋅kg⋅d, administered for 7 d). Treatment with this compound has been demonstrated to prevent lymphocyte egress from secondary lymphoid organs (11). Highlighting the importance of the mucosal immune system in generating ChAT+ T cells and B cells, significantly increased numbers of CD4+GFP+ and B220+GFP+ cells were observed in the MLN, with corresponding decreases in the pLN and spleen (Fig. 3 E and F). These data indicate that signals to induce ChAT are received in MALT as opposed to other peripheral sites, such as the spleen.
ChAT-GFP+ Expression Is Transient, and ACh Is Produced on Demand.
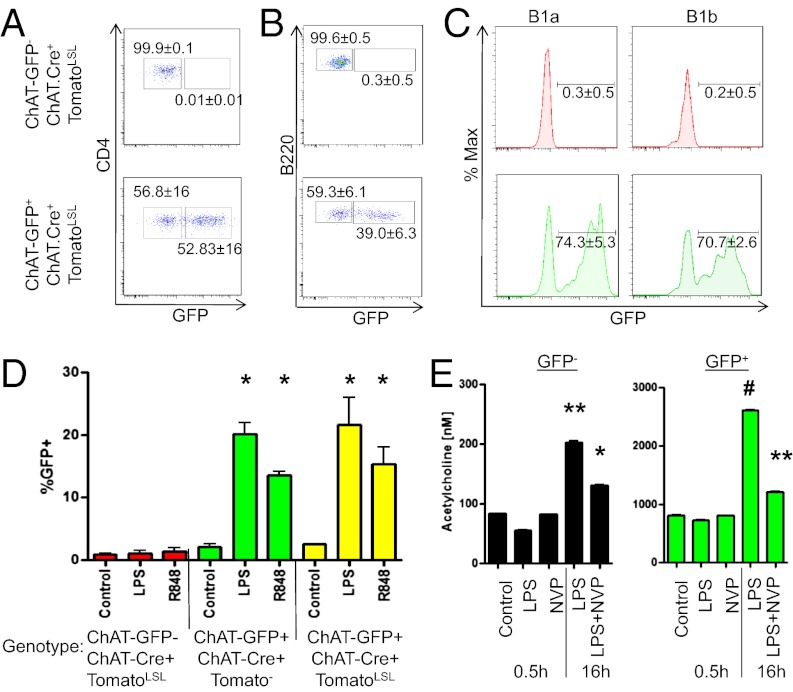
To determine if ChAT expression was stable in lymphocytes, a genetic fate-map approach was used. Mice expressing Cre recombinase under the endogenous ChAT promoter and dsTomatoLSL were bred to ChAT-GFP mice. Cells from these progeny currently expressing ChAT are GFP+Tomato+, whereas previous expression is indicated by GFP−Tomato+. Under steady-state conditions, Tomato+ B cells and T cells were GFP− and GFP+ (Fig. 4 A–C), indicating that ChAT expression can be induced and terminated in vivo. Highlighting this transient nature, LPS stimulation of sorted GFP−Tomato+ B cells resulted in GFP reexpression (Fig. 4D). Together, these data demonstrate that ChAT expression in lymphocytes is transient and can be reinduced by the appropriate stimuli.
Fig. 4.
Lymphocyte ChAT expression is transient and reinducible. ChAT-GFP mice were crossed to ChAT.Cre+TomatoLSL mice to allow for fate mapping. Cells from ChAT-GFP− ChAT.Cre+TomatoLSL (Upper) and ChAT-GFP+ ChAT.Cre+TomatoLSL (Lower) were assessed by flow cytometry. Tomato+ lymphocytes were assessed for GFP in splenic CD4+ T cells (A), B220+ B cells (B), and peritoneal B1a and B1b B cells (C). (D) Splenic follicular B GFP−Tomato+ cells were FACS-sorted from ChAT-GFP− ChAT.Cre+TomatoLSL+ and ChAT-GFP+ ChAT.Cre+Tomato+ and stimulated (16 h) with media, LPS (10 μg/mL), or R848 (1 μg/mL). As a control, B220+CD23+ GFP− cells from ChAT-GFP+ ChAT.Cre+Tomato− mice were sorted and stimulated. (E) FACS-sorted B2 GFP− or GFP+ cells were treated with LPS ± NVP (1 μM) for either 0.5 h or 16 h and were assessed by MS [n = 3 mice per group, representative of three experiments (A–C) and two experiments (D)]. *P < 0.05 by ANOVA; **P < 0.01; #P < 0.001.
In considering if ACh was stored or produced on demand by ChAT+ cells, FACS-sorted GFP− and GFP+ splenic B2 B cells were stimulated with LPS ± 4-(1-naphthyl)vinyl pyridine (NVP), a selective and irreversible inhibitor of ChAT. Short-term (0.5 h) treatment with LPS or NVP failed to alter the amount of ACh detected in cell lysates by MS (Fig. 4E). Long-term (overnight) exposure to LPS significantly enhanced ACh production from cells originally GFP− and GFP+ but was prevented by cotreatment with NVP. These data indicate that ACh detected in freshly isolated cells ex vivo is preformed, with inhibition of ChAT affecting de novo synthesis.
Specific Receptors Expressed by ChAT-GFP+ Immune Cells Facilitate ACh Release.
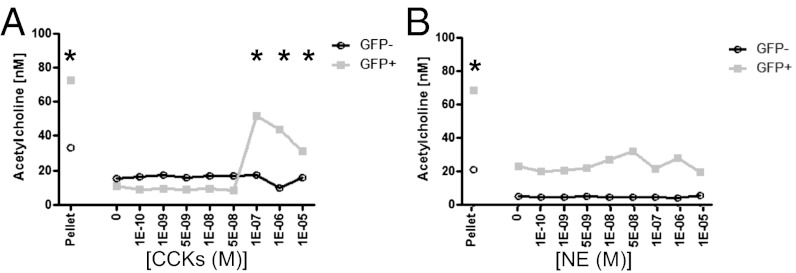
In the nervous system, ACh is preformed in vesicles and released following coordinated input of specific neurotransmitter signals. Using an 84-gene qRT-PCR array on mRNA from FACS-purified splenic ChAT-GFP+ or GFP− B2 cells, we identified ∼20 genes, including several neurotransmitters and hormone receptors, that are differentially expressed in GFP+ and GFP− cells (Tables S1 and S2). Validating this array approach, endogenous ChAT and ACh esterase (AChE) were expressed in B220+GFP+ cells but not in B220+GFP− cells. Demonstrating the expression and functionality of these receptors, stimulation of GFP+ but not GFP− B cells with CCKs caused ACh release as assessed by MS (Fig. 5A). Critically, these B cells respond to unique stimuli compared with ChAT-expressing CD4+ cells, because NE failed to induce ACh release (Fig. 5B). Together, these data demonstrate that B cells possess selective receptors for hormones and neurotransmitters, and that stimulation through specific receptors will drive ACh release.
Fig. 5.
Specific stimuli induce release of ACh. Release of ACh was determined from FACS-sorted B220+CD23+ ChAT-GFP− or ChAT-GFP+ splenic B cells, following stimulation with CCK (A) or NE (B) with 10 μM physostigmine bromide (20 min). Supernatants were mixed 1:1 with methanol (MeOH) and assessed by MS. As a control, sorted ChAT-GFP+ and ChAT-GFP− cells were lysed with MeOH and mixed 1:1 with a PBS “pellet” (n = 3, measured in duplicate). *P < 0.05 by ANOVA.
ChAT+ Lymphocytes Do Not Restrain Effector CD4 T-Cell Function.
The previously described ChAT+ CD4+ T cells were found to possess a phenotype characteristic of regulatory cells (e.g., CD45RBlo) (8). Here, we sought to determine if these GFP+ B-cell and T-cell populations would suppress effector T cells in the adoptive transfer model of colitis (12). Colonic inflammation occurs following the transfer of FACS-sorted effector CD4+CD45RBhi T cells into Rag1−/− mice. Six to eight weeks after transfer, mice begin to display characteristic hallmarks of disease, including weight loss, diarrhea, and extensive T-cell infiltration, as evidenced by histological examination following necropsy. Disease pathogenesis can be prevented by cotransfer of FACS-sorted CD45RBlo regulatory cells.
As expected, transfer of CD4+CD45RBhi T cells induced intestinal inflammation that was suppressed by cotransfer of CD45RBlo regulatory cells. Cotransfer of CD4+ ChAT-GFP+ cells but not CD4+ ChAT-GFP− cells also reduced hallmarks of disease, including weight loss (Fig. S4A), reduced colon length (Fig. S4B), macroscopic disease (Fig. S4C), histological damage (Fig. S4D), and IFN-γ–producing CD4+ effector T cells in the MLN (Fig. S4E).
Although these data suggested that ChAT+ T cells could suppress effector function, subsequent analysis indicated that this population was composed of known regulatory cell types. Intracellular staining following FACS sorting revealed enrichment for Foxp3+Helios− and Foxp3+Helios+ in CD4+ ChAT-GFP+ T cells (Fig. S4F), although in vitro regulatory T-cell (Treg) differentiation failed to induce ChAT-GFP expression (Fig. S4G). Using a cytokine secretion assay, we found that few ChAT-GFP+ cells produced IL-10 after in vitro stimulation (Fig. S4H). Thus, a significant proportion of CD4+ ChAT-GFP+ T cells are nTregs/iTregs but not type 1 regulatory (TR1) cells. As a whole, these data suggest that reduced colitis burden was not due to ACh production but was the result of transfer of ChAT+ cells that were classic Tregs. In support of this, suppression by CD4+ ChAT-GFP+ cells was not observed in recipients of CD4+CD45RBhi cells from mice with a dominant negative TGF-β1 receptor (CD4-dnTGFβRII; Fig. S5A).
With respect to the B cells, although a fraction of B220+CD23+ ChAT-GFP+ B cells produced IL-10, cotransfer with effector CD4 T cells failed to prevent colitis (Figs. S4I and S5B). Thus, these cells are discrete from the previously described regulatory B-cell population and do not act as suppressors of adaptive immune function.
ChAT+ B Cells Regulate Innate Immunity.
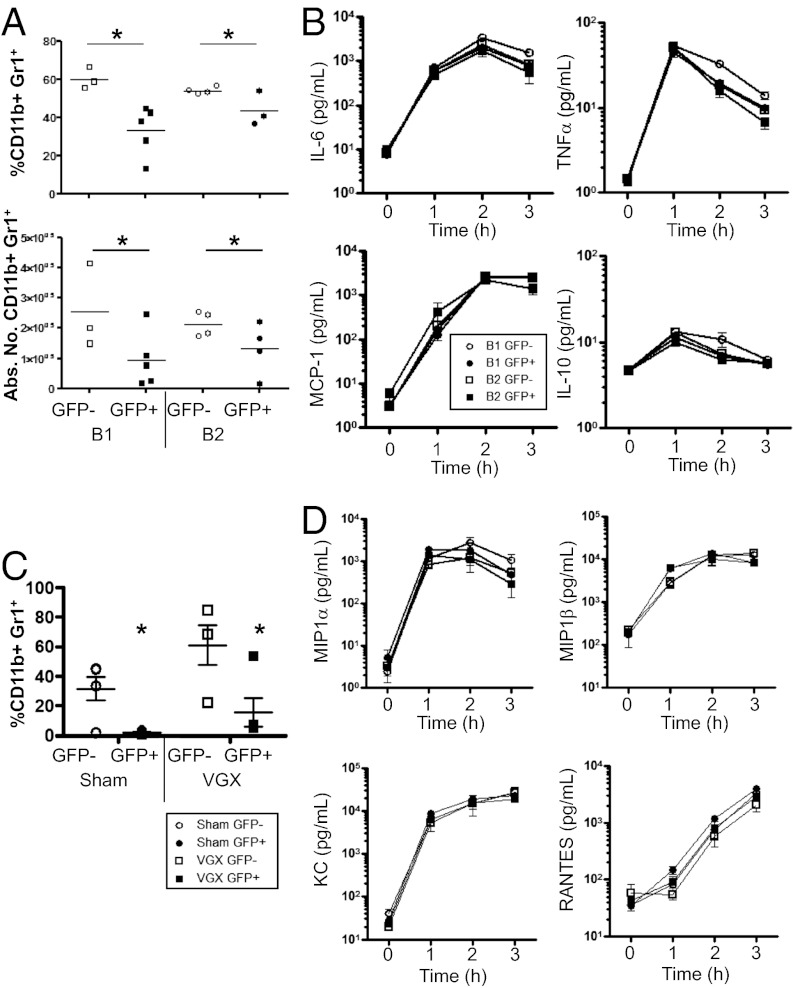
With the previous identification of CD4+ ChAT+ T cells being required for the inflammatory reflex arc, we assessed if ChAT+ B cells influence innate immunity. To this end, FACS-sorted GFP+ or GFP− B1 or B2 cells were transferred into Rag1−/− mice, with neutrophil recruitment and cytokine production assessed after LPS injection (0.2 mg/kg). Neutrophil (SSChiCD11b+GR1hi) recruitment into the peritoneum 3 h after LPS injection was significantly reduced in recipients of GFP+ but not GFP− B1 or B2 cells (Fig. 6A). Highlighting the difference between ChAT+ B cells and the previously described CD4+ ChAT+ T-cell population, we noted no reduction in TNF-α or other cytokines and chemokines [monocyte chemotactic protein-1 (MCP-1), IL-6, or IL-10] over the duration of the experiment (Fig. 6B). These data indicated that ACh-producing B cells limit neutrophil influx during sterile sepsis by a mechanism independent of innate immune activation.
Fig. 6.
B cell-derived ACh is required for neutrophil recruitment. Peritoneal B1 or splenic B2 cells were enriched and FACS-sorted based on ChAT-GFP expression. Cells were transferred (2 × 105, i.p.) to Rag1−/− recipients, and LPS (0.2 mg/kg) was injected 16 h after transfer. (A) Neutrophils were assessed 3 h after challenge with LPS. Abs. No., absolute number. (B) Serum was assessed for cytokine production by cytometric bead assay. (C) Rag1−/− sham or vagotomized (VGX) mice received FACS-sorted GFP− or GFP+ peritoneal B1 cells. Mice were challenged with LPS (0.2 mg/kg) and assessed for peritoneal neutrophil recruitment (C) and serum chemokines (D) (n = 3–7 mice per group in two separate experiments). *P < 0.05, two-tailed t test (A) and two-way ANOVA (C).
To ascertain if the reduced neutrophil recruitment required an intact vagal nerve, a subdiaphragmatic vagotomy (VGX) or sham operation was performed on Rag1−/− mice. After 2 wk, sorted GFP− or GFP+ B1 cells were adoptively transferred and challenged with LPS as before. Reduced polymorphonuclear cell recruitment was observed in Rag1−/− recipients of GFP+ B1 cells that had a VGX performed, excluding a role of the vagus nerve (Fig. 6C). In these experiments, we further sought to determine if reduced neutrophil recruitment was due to decreased production of typical chemokines. Analysis of the serum from these mice revealed no reduction in an expanded panel of chemokines, including eotaxin, MCP-1, macrophage inflammatory protein (MIP)-1α, MIP-β, keratinocyte-derived chemokine (KC), and regulated on activation, normal T-cell expressed and secreted (RANTES) or cytokines (Fig. 6D).
Attenuated recruitment without reduced innate immune activation suggested decreased interactions and transmigration across the endothelium. Previously, decreased expression of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM), and E-selectin has been observed following treatment with ACh and mimetics of ACh (13). Here, we assessed if ACh treatment of mouse endothelial cells would prevent TNF-α–induced increases in cell surface ICAM-1 and VCAM. As indicated in Fig. 7, ACh pretreatment reduced basal and induced adhesion molecule expression in a dose-dependent manner. Mechanistically, ACh-induced signaling through muscarinic receptors was required, with atropine pretreatment restoring TNF-α–induced ICAM and VCAM expression (Fig. 7 A and B). Excluding a role for nicotinic α7-receptors, the selective agonist PHA543613 failed to inhibit adhesion molecule expression induced by TNF-α (Fig. S6).
Fig. 7.
Endothelial adhesion molecules are down-regulated by ACh. Bend3 endothelial cells were pretreated (16 h) with physostigmine bromide (10 μM) and ACh (0–1,000 nM) ± atropine (10 μM) before LPS stimulation (1 ng/mL, 4 h). Cells were trypsinized and assessed for surface ICAM-1 (A) and VCAM (B) by flow cytometry (n = 3 separate experiments). *P < 0.05 (vs. “0 ACh”).
These data demonstrate that localized ACh release by peritoneal B cells can reduce neutrophil recruitment without reducing the production of proinflammatory factors. These cells influence recruitment through inhibition of adhesion molecule expression on endothelial cells.
Discussion
Communication between the nervous and immune systems occurs throughout the body and modulates numerous physiological processes. Examples of this neural–immune interaction are abundant, with neurotransmitters having an impact on adaptive and innate immunity, such as inhibition of TNF-α production by ACh (14). Activation of this inflammatory reflex arc significantly reduces mortality in models of septic shock through the ligation of nicotinic α7-receptor (6).
Immune cells can also function as integral components in neuronal circuits as opposed to target cells. Reduced mortality during sepsis by the inflammatory reflex arc was shown to be dependent on ACh producing T cells (8). These CD4+ ChAT+ T cells were intermediaries relaying neuronal signals to macrophages, reducing TNF-α production during sepsis. Whether other immune cells expressed this enzyme and the origin, development, and other immunological functions were not known.
The data presented in this study demonstrated that ChAT is expressed by B cells, macrophages, and DCs. This expression occurs in response to TLR agonists that elicit MyD88-dependent signal transduction in a cell-intrinsic manner. In accordance with these data, ablation of MyD88 significantly reduces ChAT expression following in vitro stimulation and reduces ChAT+ lymphocytes in vivo. This impact of MyD88 deficiency on ChAT expression appears to be unique to immune cells. Although a deficiency in MyD88 increases neural progenitor cell proliferation, ChAT expression is unaltered (15). This is in keeping with neuronal ChAT being induced at the transcription level by neurotrophins (16). Further highlighting the uniqueness of ChAT in immune cells, signals that induce expression occur after birth and originate from the host microbiota. Corroborating this, expression in lymphocytes is absent in utero (E18.5) but develops by postnatal day 7, a time when the microbiota is being established (17). Supporting this role, depletion of the microbiota by antibiotic treatment subsequently reduced ChAT expression in B cells and T cells. Although sites other than the colon represent unique niches for different commensal microorganisms, it appears that cells acquire the ability to produce ACh in intestinal MALT. Increased retention of ChAT+ lymphocytes was observed in MALT but not in other peripheral secondary lymphoid organs (e.g., spleen) following FTY720 treatment. These data would suggest that signals in the MALT induce ChAT expression, followed by emigration of these cells to other peripheral sites.
Our data show that the capability of immune cells to produce ACh is transient rather than being a defined lineage. Using a fate-map approach, cells that had previously expressed this enzyme were capable of reexpression, suggesting dynamic regulation as opposed to an enforced lineage. In a fashion similar to neurons, ACh is preformed by immune cells and is released following appropriate stimulation. These signals that induce ACh release are divergent from the previously described ChAT+ T-cell population. Although stimulation through β2-adrenergic receptors by NE induced ACh release by CD4+ ChAT+ T cells (8), NE was without effect on B cells. Several receptors for neurotransmitters and hormones were expressed by ChAT+ B cells, suggesting that specific inputs control the B-cell ACh pathway. It is tempting to speculate that additional neural inputs could modulate ACh release and fine-tune the response.
With complex regulation of ACh release from immune cells, it seemed plausible that ChAT-expressing lymphocytes could exert control over numerous immunophysiological processes. Having previously established the CD4+ ChAT+ cells in sepsis, the ability of these cells to modulate adaptive immunity was investigated. Using the T-cell adoptive transfer model of colitis, suppression of disease was observed with cotransfer of CD4+ ChAT-GFP+ T cells. Suppression of effector T-cell function, and prevention of colitis, appears to be due to the high degree of natural and inducible Tregs that comprise the ChAT-GFP+ T-cell population. In keeping with this, cotransfer of ChAT-GFP+ and effector T cells from mice with CD4 cells expressing CD4-dnTGFβRII resulted in disease. Therefore, suppression is likely due to TGF-β1 and not to ACh produced by the CD4+ ChAT+ population. Similarly, cotransfer of ChAT+ B cells failed to provide protection in the adoptive transfer model of colitis. These data indicate that ChAT expression is a property of known Treg populations, although ACh does not prevent effector T-cell function.
Modulation of innate immune responses by ACh is site-specific and dependent on the immune cells targeted. As evidence of this, although the release of ACh from T cells following stimulation of the vagal nerve reduces proinflammatory cytokines, this was not observed with B cells. Here, we identified that ChAT+ B cells do not have an impact on proinflammatory cytokine or chemokine production during sterile endotoxemia but reduce local neutrophil recruitment. Reduced recruitment should not be equated to reduced host defense, because ACh has been shown to increase bactericidal activity by neutrophils or macrophages in a muscarinic or nicotinic α4β2-receptor–dependent but not α7-receptor–dependent manner (18).
Systemic reduction of neutrophil recruitment following vagal nerve stimulation (VNS) has been described previously (19). This systemic effect is fundamentally different from the reduced localized recruitment into the peritoneum in recipients of ChAT-GFP+ B cells. In contrast to VNS, control afforded by B cells did not require an intact vagus nerve, because reduced recruitment was observed in mice that underwent a VGX. Although direct effects on neutrophils following systemic administration of selective nicotinic α7-agonists have been suggested previously (20), it is unlikely that endogenous ACh could act in this fashion. This effect on circulating cells is inconsistent with reduced localized recruitment, as well as with the short t1/2 of ACh in serum due to the presence of AChE.
Decreased peritoneal recruitment without impinging on cytokine or chemokine production suggested that endothelial adhesion molecule expression was reduced. Stimulation of mouse endothelial cells with TNF-α increased ICAM and VCAM-1 expression that was inhibited by pretreatment with ACh but not a highly selective α7-agonist. In agreement with our data, reduced TNF-α–induced expression of ICAM-1, E-selectin, and P-selectin has been observed with treatment of human microvascular endothelial cells (13) with ACh or mimetics. Suggesting a difference between mouse and human endothelial cells, these studies demonstrated reduced adhesion molecule expression with a nicotinic α7-agonist. Nicotine alone has, however, been reported to increase E-selectin, ICAM, and VCAM on human umbilical vein endothelial cells in vitro (21). Whether these differences are due to activation of non–α7-receptors or represent differences in endothelial cells isolated from different sources is uncertain. At this point, it is uncertain what muscarinic receptors are required for the observed reduction in ICAM-1 expression. Signaling through a G protein-coupled receptor that increases cAMP and c-Fos phosphorylation has been shown to block NF-κB–dependent gene expression (22). Whether this mechanism is responsible for our observations is not known and will require subsequent study.
This ability of ChAT+ B cells to control neutrophil recruitment and activity rapidly is in keeping with the protective effects of CD4+ T cells during sepsis. Although the recruited cells are bactericidal, release of effector molecules and enzymes, such as neutrophil elastase, causes significant tissue damage, contributing to pathology. Endothelial cell injury and the resulting increased vascular permeability in the lung can have devastating clinical consequences. Pulmonary edema, due to the leakage of protein-rich fluid across the microvascular endothelium, compromises lung function, resulting in oxygen therapy refractive cyanosis. Although a number of clinical presentations can precipitate the development of acute respiratory distress syndrome, sepsis is by far the most prevalent cause (2). Severe pathology from overabundant neutrophil activation or dysregulation of neutrophil proteolytic enzymes has also been observed in a model of intestinal inflammation (12). Therefore, immunopathology in a diverse number of tissues may be limited by ChAT+ B cells through reducing endothelial adhesion molecule expression, limiting damage due to neutrophil influx and effector release.
Materials and Methods
Detailed information on materials and methods used in this study is provided in SI Materials and Methods.
Mice.
All mice, including those that underwent a VGX (or sham operation) were purchased from Jackson Laboratories. Mice were treated with FTY720 by oral gavage (1 mg⋅kg⋅d). Microbiota depletion was accomplished using a mixture of oral antibiotics. Endotoxemia was induced by i.p. injection of LPS (0.2 mg/kg). T cell-induced colitis was performed by transfer of FACS-sorted cells as published previously (12).
Chemicals.
ACh, NE, physostigmine bromide, and LPS were from Sigma–Aldrich, and PHA543613 and CCKs were purchased from Tocris Bioscience.
Cells.
The mouse endothelial cell line Bend.3 was purchased from the American Type Culture Collection.
Flow Cytometry.
Isolated immune cells were assessed according to standard protocols.
qRT-PCR and qRT-PCRarray.
cDNA was prepared from sorted immune cells, and qRT-PCR was conducted using primers from the PrimerBank (23) or PCRarray (SABiosciences).
MS.
FACS-sorted immune cells were lysed or stimulated to release ACh. Samples were spiked with deuterated-ACh internal standard (Sigma–Aldrich) and analyzed by LC-tandem MS.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221655110/-/DCSupplemental.
References
- 1.Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995;332(1):27–37. doi: 10.1056/NEJM199501053320106. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Ottaway CA. In vitro alteration of receptors for vasoactive intestinal peptide changes the in vivo localization of mouse T cells. J Exp Med. 1984;160(4):1054–1069. doi: 10.1084/jem.160.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma B, von Wasielewski R, Lindenmaier W, Dittmar KEJ. Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol. 2007;36(1):62–74. doi: 10.1111/j.1439-0264.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 5.Bogunovic M, et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1770–G1783. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 7.Luyer MD, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202(8):1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80(24-25):2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 12.Reardon C, et al. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35(2):223–235. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeed RW, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201(7):1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 15.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, et al. Regulation of TrkA and ChAT expression in developing rat basal forebrain: Evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15(4):2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friswell MK, et al. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS ONE. 2010;5(1):e8584. doi: 10.1371/journal.pone.0008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Zanden EP, et al. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology. 2009;137(3):1029–1039. doi: 10.1053/j.gastro.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 19.Huston JM, et al. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009;183(1):552–559. doi: 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Stimulation of alpha 7 cholinergic receptors inhibits lipopolysaccharide-induced neutrophil recruitment by a tumor necrosis factor alpha-independent mechanism. Shock. 2007;27(4):443–447. doi: 10.1097/01.shk.0000245016.78493.bb. [DOI] [PubMed] [Google Scholar]
- 21.Albaugh G, et al. Nicotine induces mononuclear leukocyte adhesion and expression of adhesion molecules, VCAM and ICAM, in endothelial cells in vitro. Ann Vasc Surg. 2004;18(3):302–307. doi: 10.1007/s10016-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 22.Koga K, et al. Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity. 2009;30(3):372–383. doi: 10.1016/j.immuni.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Spandidos A, et al. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.