Abstract
Paleoanthropologists have long argued—often contentiously—about the climbing abilities of early hominins and whether a foot adapted to terrestrial bipedalism constrained regular access to trees. However, some modern humans climb tall trees routinely in pursuit of honey, fruit, and game, often without the aid of tools or support systems. Mortality and morbidity associated with facultative arboreality is expected to favor behaviors and anatomies that facilitate safe and efficient climbing. Here we show that Twa hunter–gatherers use extraordinary ankle dorsiflexion (>45°) during climbing, similar to the degree observed in wild chimpanzees. Although we did not detect a skeletal signature of dorsiflexion in museum specimens of climbing hunter–gatherers from the Ituri forest, we did find that climbing by the Twa is associated with longer fibers in the gastrocnemius muscle relative to those of neighboring, nonclimbing agriculturalists. This result suggests that a more excursive calf muscle facilitates climbing with a bipedally adapted ankle and foot by positioning the climber closer to the tree, and it might be among the mechanisms that allow hunter–gatherers to access the canopy safely. Given that we did not find a skeletal correlate for this observed behavior, our results imply that derived aspects of the hominin ankle associated with bipedalism remain compatible with vertical climbing and arboreal resource acquisition. Our findings challenge the persistent arboreal–terrestrial dichotomy that has informed behavioral reconstructions of fossil hominins and highlight the value of using modern humans as models for inferring the limits of hominin arboreality.
Keywords: Australopithecus, human pygmy phenotype, phenotypic plasticity
Paleoanthropologists have long argued—often contentiously—over the climbing abilities of early hominins and whether a foot adapted to terrestrial bipedalism constrained regular access to trees (1). Central to this debate is Australopithecus afarensis, which possessed a hindlimb adapted to terrestrial bipedalism, including a rigid ankle (2, 3) and an arched, nongrasping midfoot (refs. 4 and 5; but see refs. 6–8). Such traits represent a major shift from an ape-like foot, but there is disagreement over the behavioral implications of this shift. Some researchers interpret the ankle and foot of Au. afarensis as being functionally incompatible with climbing and thus definitive markers of terrestriality (2, 9), whereas others have argued that the hindlimb is compatible with significant arboreality (6, 7, 10). There is also disagreement over the forelimb of Au. afarensis. A suite of traits—including long and curved fingers (6, 11), a cranially oriented glenoid fossa (6), and greater muscularity relative to modern humans (12)—is considered by some to indicate significant arboreality (6, 7, 10), whereas others regard these traits as primitive retentions with marginal adaptive significance (13) or call attention to derived (modern human-like) features of the forelimb (14, 15). Nevertheless, the long-term retention of plesiomorphic forelimb traits associated with arboreality suggests a functional role for such traits and could imply stabilizing selection on climbing abilities (1, 16).
A common assumption in the debate over the locomotor repertoire of Au. afarensis is that a bipedally adapted ankle and foot would fully compromise performance variables in an arboreal milieu, rendering individuals incompetent in trees (2, 3, 9, 13). This assumption has received critical attention (7, 10, 17, 18), but it has not been subject to empirical tests. Most relevant kinematic studies have focused on captive and wild apes (e.g., refs. 3 and 19), yet some consider the locomotion of modern apes to be of marginal relevance to the reconstruction of early hominin locomotor behavior (20, 21). Nevertheless, form–function inferences for hominins demand consideration of the locomotor diversity of both extant apes and modern humans. In comparison with chimpanzees, the diversity of modern human locomotion has received little attention (22, 23). For example, modern humans who climb trees routinely remain unstudied, despite their relevance for inferring potential anatomical constraints on hominin arboreality.
Many hunting and gathering populations climb trees, primarily to collect honey. African pygmy populations are particularly reliant on honey (24, 25); for instance, the Mbuti of the central Ituri Forest (Democratic Republic of Congo) consume 0.83 kg of honeycomb per person per day during the 3-mo honey season (26) or “honey holiday” (27). To meet this demand for honey, men climb trees regularly (Fig. 1). In the northern Ituri Forest, Efe men devote 33.8% of their foraging time to honey acquisition and climb as high as 51.8 m (mean = 19.1 m; SD = 9.7 m, n = 34) (28). Such foraging behaviors are a testament to the high caloric and nutritive value of honey and the accompanying brood (29, 30), as well as the social prestige associated with provisioning a favored resource (26). However, the energetic cost of vertical climbing is high (31), and foraging at great heights is inherently dangerous (Fig. 1). The chance of death for modern humans from falling is 100%, 77.8%, 56.2%, and 44.4% from heights of >19.2, 19.2, 15.6, and 12 m, respectively (32), suggesting that tree climbing could be a substantial cause of mortality for rainforest hunter–gatherers. Indeed, accidental falls from trees account for 6.6% of deaths among Aka men in the Central African Republic (33).
Fig. 1.
Hunter–gatherers climb trees and acquire arboreal resources using a variety of techniques. (A) An Mbuti man climbs in the pursuit of honey and uses smoldering leaves to subdue stinging bees. The plantar surface of the foot is applied to the tree trunk. Dorsiflexion of his right ankle occurs as his left foot pushes off the tree (photograph by Rebecca Blackwell; reproduced with permission). (B) Vertical climbing is risky and often requires extraordinary bridging abilities in the understory and canopy (photograph by Bruno Zanzottera; reproduced with permission). (C) In Malaysia, the Batek use small-diameter lianas to access resources in the canopy (photograph by Kirk Endicott; reproduced with permission).
Hunting and gathering populations in Southeast Asia also climb trees and exploit honey extensively. For example, in Taman Negara (Malaysia), a Batek camp acquired 260.3 kg of honey in 93 d (34). Batek men are reported to climb 50-m heights daily, often at night, but fatalities appear to be rare (35). Data from the Agta (northeast Luzon, Philippines) show that falls from trees accounted for 4 of 238 deaths (1.7%) among adult men (> 17 y of age) between 1962 and 2010 (36).
Safe and efficient climbing is therefore expected to carry substantial fitness advantages for hunter–gatherers. To enhance safety during climbing, hunter–gatherers sometimes use material culture, such as harnesses and pegs, particularly when resource-bearing trees are too thick to climb directly (34) (Fig. S1 A–C). However, unassisted climbing involving (i) ankle and metatarsophalangeal dorsiflexion, (ii) extreme hip abduction coupled with ankle inversion, and (iii) hallucal grasping (Fig. 1 and Fig. S1 D–F) also occurs during honey and fruit collection (e.g., refs. 34 and 37), as well as during the active pursuit (38, 39) and ambush of prey in trees (28, 40).
The adaptive significance of unassisted vertical climbing is illustrated by the fitness benefits of extracting high-value resources from dangerous heights without reliance on material culture. Because climbing appears to be associated with foods that are central to the diets of tropical rainforest hunter–gatherers (e.g., refs. 26, 28, and 35), and even some savanna woodland populations (41), it is possible that natural selection has favored postcranial anatomies that facilitate safe climbing. The interpretation of chimpanzee postcranial traits as safety adaptations (42) was premised in part on the observations that tree falls accounted for 4% of mortality over a 2-y span at Gombe, Tanzania (43), and that 30.8% of Gombe chimpanzees suffered postcranial fractures consistent with falls from trees (44). Some modern human foragers have climbing-related mortality rates (up to 6.6%) exceeding those of chimpanzees (33).
A mobile tibiotalar (ankle) joint is advantageous for vertical climbing because it enables the climber to reduce the distance between his center of mass and the tree. Accordingly, chimpanzees use high degrees of dorsiflexion and inversion at the tibiotalar joint during climbing (3). The extent to which modern human hunter–gatherers use similar techniques during climbing is unexplored. Anecdotal reports of hunter–gatherers (34, 35, 45, 46) indicate that modern humans can climb small-diameter trees by applying the plantar surface of the foot directly to the trunk and “walking” upward with the arms and legs advancing alternately (Fig. 1A). This climbing technique, termed changwod in Malaysia (45), resembles that of chimpanzees (3) and has been proposed as a candidate climbing style for Au. afarensis (10). Theoretical considerations predict that a high degree of dorsiflexion and inversion of the ankle will bring the climber’s center of mass closer to the tree (47), thus mitigating energetic expenditure and the safety risks associated with vertical ascent.
Here, we report a comparative analysis of vertical climbing behavior and its anatomical correlates between hunter–gatherers (Twa) and nonclimbing agriculturalists (Bakiga). The Twa* are a population of former hunter–gatherers living near Bwindi Impenetrable National Park (BINP) in Uganda (49). Twa males have an average height of 153 cm (50, 51). This adult stature exemplifies the pygmy phenotype, which is strongly associated with rainforest habitats (24). The Bakiga are an agricultural population (52) that has coexisted with the Twa for at least five centuries (53).
To test the assumption that modern humans cannot achieve dorsiflexion at the ankle joint during tree climbing similar to that observed in chimpanzees (3), we recorded the tree-climbing behavior of experienced Twa honey-gatherers and used movie stills to measure maximum dorsiflexion at the ankle joint.
Results and Discussion
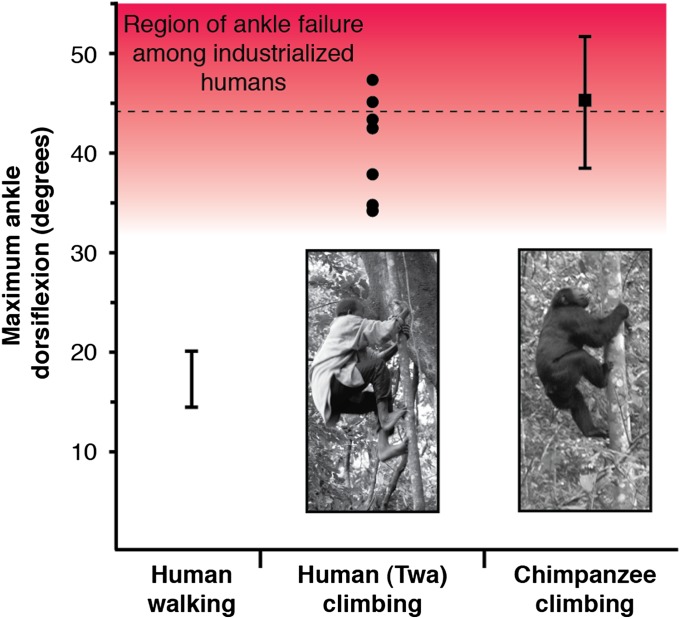
Twa hunter–gatherers exhibited extreme dorsiflexion during climbing [40.73 ± 5.14° (mean ± SD)]. These values are comparable to those reported for wild chimpanzees (Fig. 2), although the mean difference was marginally lower (Welch two-sample t test, t8.78 = 2.25, P = 0.052) and fell within the range of expected ankle failure under loading, as measured experimentally in cadavers of industrialized humans (54) (Fig. 2).
Fig. 2.
Maximum ankle dorsiflexion during walking and climbing. For industrialized human populations, maximum dorsiflexion during walking ranges from 15.0° to 20.0° (55–57), and gross injury or microfilament damage occurs at a mean angle of 44.0° (hashed line) ± 10.9° (SD; red shaded region) (54). For Twa men (n = 7 individuals; filled circles), maximum dorsiflexion ranged from 34.4 to 47.0° during vertical climbing (photograph by George H. Perry, reproduced with permission). The average maximum dorsiflexion of wild chimpanzees (photograph by Jeremy DeSilva; reproduced from ref. 3) occurred at a mean angle of 45.5° (filled square) ± 7.1° (SD; whiskers) (3).
The morphologies of the distal tibia of tree-climbing hunter–gatherers may permit or reflect extreme ankle dorsiflexion, as in chimpanzees (3). However, the size-standardized mediolateral widths of the anterior distal tibiae of six pygmy males from habitually climbing populations in the Ituri Forest (28) did not differ from those of other human populations (Fig. S2). It is possible that these particular individuals were not habitual climbers, but this result suggests that anatomical mechanisms other than bony morphology can reflect and/or permit extreme ankle dorsiflexion. Indeed, the ankle joint is a complex of bone, ligaments, and muscles, all of which collectively constrain dorsiflexion. For example, the inverse relationship between muscle fiber length and stiffness in the gastrocnemius muscle-tendon unit (58) raises the possibility that increased fiber length decreases joint stiffness and could facilitate hindlimb force production during extreme dorsiflexion.
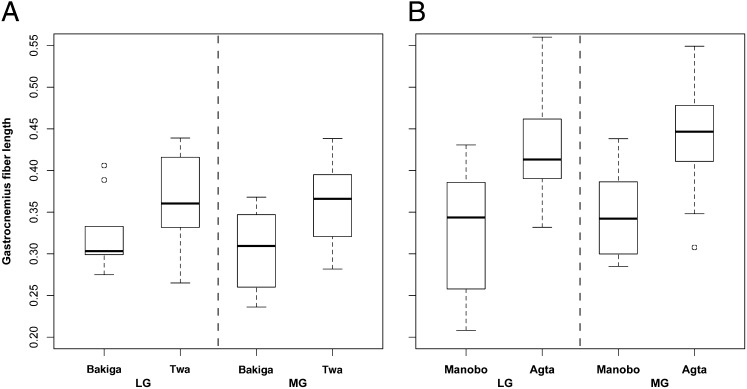
To explore this possibility, we used ultrasonography (Fig. 3) to compare the gastrocnemius muscles of the Twa and the Bakiga, who seldom climb trees. The normalized muscle fibers of Twa men were significantly longer than those of Bakiga men (Welch two-sample t test, lateral head: t14.6 = −2.44, P = 0.03; medial head: t13.0 = −3.30, P < 0.01; Fig. 4A), whereas the fiber lengths of nonclimbing women were comparable (lateral head: nTwa = 26, nBakiga = 17; t40.5 = −0.79, P = 0.43; medial head: nTwa = 25, nBakiga = 17; t36.0 = 0.06, P = 0.95).
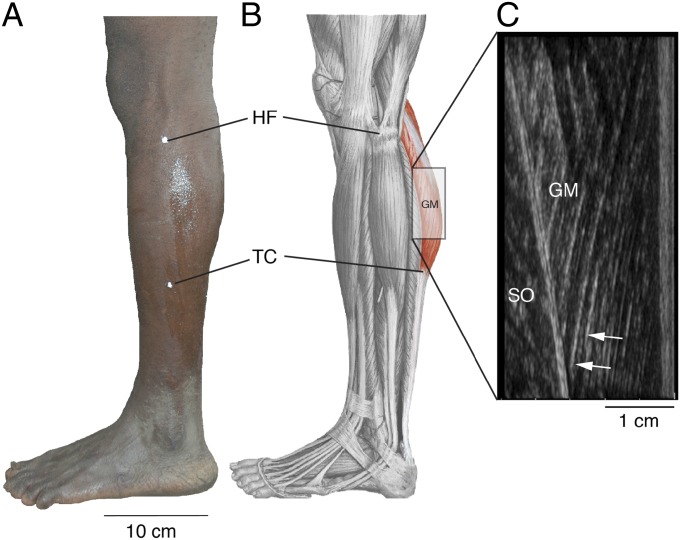
Fig. 3.
Depiction of anatomical measurements in this study. (A) Anatomical landmarks indicated on left lower leg of Twa male, in lateral view (HF, head of fibula; TC, tendocalcaneal complex). (B) Depiction of musculature in lateral view. The lateral head of the gastrocnemius muscle (GM; red shade) originates from the lateral condyle of the femur and inserts at the TC. (C) Ultrasound image of the GM and soleus muscle (SO), separated by the deep aponeurosis. Arrows indicate individual fibers of GM.
Fig. 4.
Muscle architecture measurements in African (Twa and Bakiga) and Philippine populations (Agta and Manobo). (A) Boxplot of fiber length, normalized for muscle length, of the lateral (LG) and medial (MG) gastrocnemius muscles of Bakiga men (n = 9) and Twa men (n = 29), shown on left. (B) Boxplot of fiber length comparing Manobo men (nManobo-LG = 18, nManobo-MG = 16) and Agta (nAgta-LG = 23, nAgta-MG = 24) men.
We also compared the gastrocnemius fiber lengths of men in two Philippine populations, Agta hunter–gatherers (40) and Manobo agriculturalists (59). The Agta express the pygmy (negrito) phenotype (24), and men climb trees regularly to collect honey, especially in June, July, and August (60). The Manobo are an indigenous population of agriculturalists (59). The Agta had significantly longer normalized fiber lengths (Welch two-sample t test, lateral head: t29.5 = −4.71, P < 0.0001; medial head: t32.1 = −5.63, P < 0.0001; Fig. 4B).
These results suggest that habitual climbing by Twa and Agta men is related to the muscle architecture associated with ankle dorsiflexion. The plasticity of soft tissues appears to play a role in increasing ankle mobility, thereby permitting hindlimb force production, whether passive or active, with the ankle highly flexed. Thus, ankle dorsiflexion during vertical climbing is not limited to the extant great apes and could be among the primary mechanisms allowing hunter–gatherers to access arboreal resources. The extraordinary climbing abilities of hunter–gatherers demonstrate that significant amounts of arboreal behaviors, particularly vertical climbing, are possible without an abducted hallux and flexible midfoot. These considerations do not necessarily imply Au. afarensis was a habitual climber, but they do suggest that arboreal capabilities in this species would not have been compromised to the extent assumed by many paleoanthropologists.
This study has implications for inferring both behavior (10) and adaptive change (13) from the hominin fossil record. Although it is possible that early hominins climbed in a fashion kinematically distinct from any modern primate, the unassisted vertical climbing styles of modern humans (Fig. 1 and Fig. S1) could be realized with the total morphological pattern evinced by Au. afarensis (e.g., styles described in refs. 7, 10, 17, 18, and 61). The humerofemoral indices of African pygmies are high among modern humans but are well below that of Au. afarensis (62). Such body proportions, in addition to small body size (31), are regarded as theoretically favorable for safety and efficiency during vertical climbing (61, 63). It is important to stress that pygmy stature and body proportions—in addition to the behavioral ecology of rainforest hunter–gatherers more generally—are likely highly derived (24, 64) and should not be assumed to be homologous with those of early hominins. Indeed, the universal importance of vertical climbing to the foraging ecology of rainforest hunter–gatherers lends support to the hypothesis that locomotion (65), including climbing (24, 66), within dense habitats has driven the convergent evolution of the pygmy phenotype.
Stabilizing selection for arboreal adaptations in Au. afarensis is supported by the long-term conservatism of the postcranial skeleton (1, 6, 16) across a range of wooded and open habitats (67). This pattern suggests the existence of strong ecological incentives for climbing by Au. afarensis within the context of a broad-spectrum foraging ecology. Possibilities include foraging, resting and sleeping, or escape, all of which are linked with climbing and use of trees by savanna-living primates (10, 11, 68). As observed among hunter–gatherers (Fig. S1), Au. afarensis might be expected to climb on tree trunks and near the central core of trees, rather than within a fine-branch niche (18).
Such ecological considerations based on the study of nonhuman primate and modern human behavior can lend crucial insights into the locomotor ecology of hominins. In particular, stable isotopes could be instructive for assessing the importance of arboreal resources, such as fruit, leaves, and honey, in the diet of hominins. These resources are predicted to be 13C-depleted (low δ13C values), and both Ardipithecus ramidus and Australopithecus sediba evince low δ13C values, indicating the exploitation of C3 plants in a woodland or closed-forest habitat (69, 70). These findings are consistent with clear anatomical signatures for arboreality in these species (21, 71), yet a nonnegligible C4 signature in the enamel of Ar. ramidus and grass/sedge phytoliths in the dental calculus of Au. sediba indicate some terrestrial foraging (69). Similar considerations might apply to Au. afarensis. Evidence of a diet based on C4 biomass would be a strong indicator of a terrestrial diet. Conversely, a mixed C3–C4 diet would be consistent with the consumption of either terrestrial or arboreal C3 resources and would thus be uninformative with respect to the locomotor ecology of Au. afarensis.
Divergent interpretations of the postcranial skeleton have long hindered consensus about the role of arboreality in Au. afarensis (1, 16). Skeletal signatures of vertical climbing in modern humans could provide a crucial comparative context for functional interpretations of Au. afarensis morphology, particularly with respect to traits that differentiate Au. afarensis from chimpanzees while uniting this species with nonclimbing modern humans. Such traits have provided compelling evidence for limited arboreality in Au. afarensis (3), yet they were not examined in habitually climbing modern humans, among whom genetically determined or plastic skeletal traits linked with climbing could be expressed. The existence of such traits would suggest that Au. afarensis climbed less than modern climbing humans, but we found no evidence of anteriorly expanded distal tibiae in a small sample of African rainforest hunter–gatherers (Fig. S2). Assuming these individuals were habitual climbers, this result could be due to the relatively slow speed and lower frequency at which humans climb compared with extant apes. Nevertheless, climbing-related changes in muscle, tendon, and skeletal architecture of the foot, knee, or hip could be particularly pronounced in climbing populations such as the Efe, who spend 8% of time away from camp climbing or perched in trees (64). Climbing begins at a young age in hunter–gatherers (refs. 34, 38, and 39; Fig. S1D) and may be reflected in ontogenetically plastic traits, such as phalangeal curvature, which respond to habitual climbing during early life (ref. 72; but see ref. 21).
Although debate will undoubtedly persist regarding the gait mechanics and functional role of plesiomorphic characters in Au. afarensis (1, 73), this study shows that the foot and ankle of Au. afarensis are not incompatible with climbing behavior—at least as it is performed by some modern hunter–gatherers. The diverse locomotor repertoire evinced by facultatively arboreal modern humans cautions against using even the most derived (modern human-like) traits in Au. afarensis as unequivocal evidence of negligible arboreality.
Materials and Methods
Study Sites and Subjects.
Southwest Uganda.
Research on the Twa and Bakiga was conducted in the BINP and surrounding settlements: Bikuto (0°54.45'S; 29°38.78'E), Buhoma (0°58.17'S; 29°36.99'E), Byumba (0°55.52'S; 29°41.61'E), Kebiremu (0°50.82'S; 29°38.51'E), Kitariro (0°52.97'S; 29°43.23'E), Mpungu (0°59.32'S; 29°41.60'E), and Rurangara (0°55.89'S; 29°39.86'E). Both the Twa and Bakiga are commonly unshod, although the use of footwear is variable. Permission to conduct research in BINP was approved by Uganda National Council for Science and Technology Permit HS617 and Uganda Wildlife Authority Permit UWA/FOD/RES/50.
Northeast Luzon, Philippines.
Research on the Agta was conducted in two settlements, Dibungko (17°03.968’N; 122°26.626’E) and Kanaipang (16°57.646’N; 122°27.910’E), in the province of Isabela. Permission to conduct research was approved by National Commission on Indigenous Peoples and the Department of Environment and Natural Resources Permit 03-2010.
Northern Mindanao, Philippines.
Research on the Manobo was conducted in Pangaylen (09°15.967’N; 125°34.750’E), a permanent settlement, or barangay, in the province of Surigao del Norte. Permission to conduct research was approved by the National Commission on Indigenous Peoples.
Climbing and Dorsiflexion.
Consenting Twa men (n = 46) climbed a 6.5-cm diameter liana to a height of 6.8 m twice in succession. A majority of men ascended the rigid liana by positioning their feet on a nearby tree. The present analysis is focused on seven men, all experienced honey-gatherers, who instead used the liana as their primary substrate and climbed in the changwod style (45). A similar style of climbing is depicted in Movie S1.
Standardized video of each climber was captured with a Sony HDR-SR12 digital camera mounted on a tripod. We isolated movie stills that depicted the right ankle in lateral view at the point of left-foot push-off (i.e., when the right foot supported the weight of the climber). Following DeSilva (3), ankle dorsiflexion was estimated with the angle tool in ImageJ (74) as the angle between two straight lines: one running from the knee to the heel (approximately bisecting the tibia) and another from the heel to the metatarsophalangeal joint of the fifth metatarsal (Fig. S3). By convention, dorsiflexion was calculated by subtracting the measured angle from 90°. To avoid angular errors associated with height, movie stills were only analyzed if the subject was <5 m above the ground. Maximum dorsiflexion refers to the greatest measured angle for an individual.
Distal Tibia Skeletal Measurements.
Measurements with digital calipers were taken on six male specimens housed at the University of Geneva. The specimens were labeled “Ituri pygmées” and are thus likely to have belonged to populations of Mbuti or Efe, among whom climbing is frequent (28, 64, 65). The age of individuals ranged from 17 to 60 y. It is unknown whether or how frequently these individuals climbed during life. Six measurements were taken on the anterior aspect of the left tibial articular surface to assess dorsiflexion capability. Methods closely followed DeSilva (3) to ensure direct comparability between studies. Repeated measurements were taken 4 d apart and were found to be within 5% of each other. The following measurements were taken: maximum mediolateral length of the anterior aspect of the articular surface (MLAA), the maximum mediolateral length of the posterior aspect of the articular surface, the maximum mediolateral length at the midpoint of the articular surface, the maximum anteroposterior width of the most medial aspect of the articular surface, the maximum anteroposterior width of the most lateral aspect of the articular surface, and the maximum anteroposterior width at the midpoint of the articular surface. The geometric mean was calculated by raising the product of the six measurements to the 1/6 power. The measure of interest (MLAA) was divided by the geometric mean, following the size adjustment protocol established by Darroch and Mosimann (75).
Gastrocnemius Muscle Architecture.
The head of the fibula and the proximal end of the tendocalcaneal complex were determined by manual palpation and ultrasonography, respectively (MicroMaxx ultrasound system outfitted with an L52e transducer; SonoSite). Corresponding surface marks (white body paint) were applied and photographed (Fig. 3A). Next, ImageJ was used to estimate the length of the gastrocnemius muscle (Fig. 3B). To measure fiber lengths, sonographic images of each head of the gastrocnemius muscle were recorded in the sagittal plane at muscle midlength as subjects stood upright in a neutral anatomical position. Fiber length was measured in ImageJ by tracing a line across a visible fiber bundle between the superficial and deep aponeuroses (Fig. 3C). This research was approved by the Committee on the Protection of Human Subjects of Dartmouth College (approval 22410) and the Research and Ethics Committee of Makerere University (approval 2009-137).
Supplementary Material
Acknowledgments
We thank our study participants, the Batwa Development Programme and National Commission on Indigenous Peoples, and E. E. Butler, A. J. Cunningham, R. L. Lieber, L. D. Dagsaan, J. M. DeSilva, K. M. Endicott, J. G. Fleagle, H. Glowacka, T. N. Headland, M. A. McPeek, G. H. Perry, J. W. Ridges, E. G. Snow, and R. H. Tuttle. Alicia Sanchez-Mazas and Mathias Currat (University of Geneva) facilitated the study of the Ituri pygmy skeletal material. Two anonymous reviewers provided comments that greatly improved the quality of the manuscript. This work was supported by National Science Foundation Graduate Research Fellowships (T.S.K. and V.V.V.); David and Lucile Packard Foundation Fellowship 2007-31754; and the Friedman Family Foundation at Dartmouth College.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.W. is a guest editor invited by the Editorial Board.
*The Batwa ethnonym has a complicated history (48), and widespread use of the term has confused cultural and genetic differences between populations. A recent trend is to distinguish the Batwa of Burundi, eastern Democratic Republic of Congo, Rwanda, and southwestern Uganda as lacustrine (48) or Great Lakes Batwa (49). Here we follow ethnographic convention by omitting the Bantu prefix Ba- when referring to the Twa of southwestern Uganda.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208717110/-/DCSupplemental.
References
- 1.Ward CV. Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand? Am J Phys Anthropol. 2002;45(Suppl 35):185–215. doi: 10.1002/ajpa.10185. [DOI] [PubMed] [Google Scholar]
- 2.Latimer B, Ohman JC, Lovejoy CO. Talocrural joint in African hominoids: Implications for Australopithecus afarensis. Am J Phys Anthropol. 1987;74(2):155–175. doi: 10.1002/ajpa.1330740204. [DOI] [PubMed] [Google Scholar]
- 3.DeSilva JM. Functional morphology of the ankle and the likelihood of climbing in early hominins. Proc Natl Acad Sci USA. 2009;106(16):6567–6572. doi: 10.1073/pnas.0900270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward CV, Kimbel WH, Johanson DC. Complete fourth metatarsal and arches in the foot of Australopithecus afarensis. Science. 2011;331(6018):750–753. doi: 10.1126/science.1201463. [DOI] [PubMed] [Google Scholar]
- 5.DeSilva JM, Throckmorton ZJ. Lucy’s flat feet: The relationship between the ankle and rearfoot arching in early hominins. PLoS ONE. 2010;5(12):e14432. doi: 10.1371/journal.pone.0014432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JT, Jr, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol. 1983;60(3):279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- 7.Stern JT, Jr, Susman RL. “Total morphological pattern” versus the “magic trait”: Conflicting approaches to the study of early hominid bipedalism. In: Coppens Y, Senut B, editors. Origin(s) of Bipedalism in Hominids. Paris: National Center for Scientific Research; 1991. pp. 99–111. [Google Scholar]
- 8.Harcourt-Smith WEH. 2002. Form and function in the hominoid tarsal skeleton. PhD dissertation (Univ College London, London)
- 9.Latimer B, Lovejoy CO. Hallucal tarsometatarsal joint in Australopithecus afarensis. Am J Phys Anthropol. 1990;82(2):125–133. doi: 10.1002/ajpa.1330820202. [DOI] [PubMed] [Google Scholar]
- 10.Susman RL, Stern JT, Jr, Jungers WL. Arboreality and bipedality in the Hadar hominids. Folia Primatol (Basel) 1984;43(2-3):113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle RH. Evolution of hominid bipedalism and prehensile capabilities. Philos Trans R Soc Lond B Biol Sci. 1981;292(1057):89–94. [Google Scholar]
- 12.Drapeau MSM. Articular morphology of the proximal ulna in extant and fossil hominoids and hominins. J Hum Evol. 2008;55(1):86–102. doi: 10.1016/j.jhevol.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Latimer B. Locomotor adaptations in Australopithecus afarensis: The issue of arboreality. In: Coppens Y, Senut B, editors. Origin(s) of Bipedalism in Hominids. Paris: National Center for Scientific Research; 1991. pp. 169–176. [Google Scholar]
- 14.Drapeau MSM, Ward CV, Kimbel WH, Johanson DC, Rak Y. Associated cranial and forelimb remains attributed to Australopithecus afarensis from Hadar, Ethiopia. J Hum Evol. 2005;48(6):593–642. doi: 10.1016/j.jhevol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Drapeau MSM, Ward CV. Forelimb segment length proportions in extant hominoids and Australopithecus afarensis. Am J Phys Anthropol. 2007;132(3):327–343. doi: 10.1002/ajpa.20533. [DOI] [PubMed] [Google Scholar]
- 16.Kimbel WH, Delezene LK. “Lucy” redux: A review of research on Australopithecus afarensis. Am J Phys Anthropol. 2009;140(Suppl 49):2–48. doi: 10.1002/ajpa.21183. [DOI] [PubMed] [Google Scholar]
- 17.Prost JH. Origin of bipedalism. Am J Phys Anthropol. 1980;52(2):175–189. doi: 10.1002/ajpa.1330520204. [DOI] [PubMed] [Google Scholar]
- 18.Rose MD. Food acquisition and the evolution of positional behavior: The case of bipedalism. In: Chivers DJ, Wood BA, Bilsborough A, editors. Food Acquisition and Processing in Primates. New York: Plenum; 1984. pp. 509–524. [Google Scholar]
- 19.Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126(1):66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- 20.Sayers K, Lovejoy CO. The chimpanzee has no clothes. A critical examination of Pan troglodytes in models of human evolution. Curr Anthropol. 2008;49:87–114. [Google Scholar]
- 21.Lovejoy CO, et al. Combining prehension and propulsion: The foot of Ardipithecus ramidus. Science. 2009;326(5949):72e1–72e8. [PubMed] [Google Scholar]
- 22.Watanabe H. Running, creeping, and climbing: A new ecological and evolutionary perspective on human locomotion. Mankind. 1971;8(1):1–13. [Google Scholar]
- 23.Devine J. The versatility of human locomotion. Am Anthropol. 1985;87(3):550–570. [Google Scholar]
- 24.Perry GH, Dominy NJ. Evolution of the human pygmy phenotype. Trends Ecol Evol. 2009;24(4):218–225. doi: 10.1016/j.tree.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Wrangham RW. Honey and fire in human evolution. In: Sept J, Pilbeam D, editors. Casting the Net Wide: Papers in Honor of Glynn Isaac and his Approach to Human Origins Research. Oakville, CT: Oxbow; 2011. pp. 149–167. [Google Scholar]
- 26.Ichikawa M. Ecological and sociological importance of honey to the Mbuti net hunters, eastern Zaire. Afr Study Monogr. 1981;1:55–68. [Google Scholar]
- 27.Terashima H. Honey and holidays: The interactions mediated by honey between Efe hunter-gatherers and Lese farmers in the Ituri Forest. Afr Study Monogr. 1998;25:123–134. [Google Scholar]
- 28.Bailey RC. The Behavioral Ecology of Efe Pygmy Men in the Ituri Forest, Zaire. Ann Arbor: Univ of Michigan; 1991. [Google Scholar]
- 29.Marlowe FW, Berbesque JC. Tubers as fallback foods and their impact on Hadza hunter-gatherers. Am J Phys Anthropol. 2009;140(4):751–758. doi: 10.1002/ajpa.21040. [DOI] [PubMed] [Google Scholar]
- 30.Crittenden A. The importance of honey consumption in human evolution. Food Foodways. 2011;19(4):257–273. [Google Scholar]
- 31.Hanna JB, Schmitt D, Griffin TM. The energetic cost of climbing in primates. Science. 2008;320(5878):898. doi: 10.1126/science.1155504. [DOI] [PubMed] [Google Scholar]
- 32.Risser D, Bönsch A, Schneider B, Bauer G. Risk of dying after a free fall from height. Forensic Sci Int. 1996;78(3):187–191. doi: 10.1016/0379-0738(95)01885-9. [DOI] [PubMed] [Google Scholar]
- 33.Hewlett BS, van de Koppel JMH, van de Koppel M. Causes of death among Aka pygmies of the Central African Republic. In: Cavalli-Sforza LL, editor. African Pygmies. New York: Academic; 1986. pp. 45–63. [Google Scholar]
- 34.Endicott KM, Endicott KL. The Headman Was a Woman. Long Grove, IL: Waveland; 2008. [Google Scholar]
- 35.Endicott KM. The hunting methods of the Batek negritos of Malaysia: A problem of alternatives. Canberra Anthropol. 1979;2(2):7–22. [Google Scholar]
- 36.Headland TN, Headland J, Uehara RT. 2011. Agta Demographic Database: Chronicle of a Hunter-Gatherer Community in Transition (SIL International, Dallas), Version 2.0.
- 37.Demps K, Zorondo-Rodríguez F, García C, Reyes-García V. Social learning across the life cycle: Cultural knowledge acquisition for honey collection among the Jenu Kuruba, India. Evol Hum Behav. 2012;33(5):460–470. [Google Scholar]
- 38.Hill K, Hawkes K. Neotropical hunting among the Aché of eastern Paraguay. In: Hames RB, Vickers WT, editors. Adaptive Responses of Native Amazonians. New York: Academic; 1983. pp. 139–188. [Google Scholar]
- 39.Lye T-P. Changing Pathways: Forest Degradation and the Batek of Pahang, Malaysia. Lanham, MD: Lexington; 2004. [Google Scholar]
- 40.Griffin PB, Estioko-Griffin A. The Agta of Northeastern Luzon: Recent Studies. Cebu City, Philippines: Univ of San Carlos; 1985. [Google Scholar]
- 41.Marlowe FW. The Hadza: Hunter–Gatherers of Tanzania. Berkeley: Univ of California Press; 2010. [Google Scholar]
- 42.Pontzer H, Wrangham RW. Climbing and the daily energy cost of locomotion in wild chimpanzees: Implications for hominoid locomotor evolution. J Hum Evol. 2004;46(3):317–335. doi: 10.1016/j.jhevol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Belknap; 1986. [Google Scholar]
- 44.Jurmain R. Skeletal evidence of trauma in African apes, with special reference to the Gombe chimpanzees. Primates. 1997;38(1):1–14. [Google Scholar]
- 45.Evans IHN. The Negritos of Malaya. London: Cambridge Univ Press; 1937. [Google Scholar]
- 46.Turnbull CM. Wayward Servants. New York: Natural History Press; 1965. [Google Scholar]
- 47.Cartmill M. Pads and claws in arboreal locomotion. In: Jenkins FA, editor. Primate Locomotion. New York: Academic; 1974. pp. 45–83. [Google Scholar]
- 48.Schadeberg T. Batwa: The Bantu name for the invisible people. In: Biesbrouck K, Elders S, Rossel G, editors. Central African Hunter-Gatherers in a Multidisciplinary Perspective: Challenging Elusiveness. Leiden: CNWS Publications; 1999. pp. 21–40. [Google Scholar]
- 49.Lewis J. The Batwa Pygmies of the Great Lakes Region. London: Minority Rights Group International; 2000. [Google Scholar]
- 50.Gusinde M. Pygmies and pygmoids: Twides of tropical Africa. Anthropol Q. 1955;28:3–61. [Google Scholar]
- 51.Ghesquiere JL, Karvonen MJ. Some anthropometric and functional dimensions of the pygmy (Kivu Twa) Ann Hum Biol. 1981;8(2):119–134. doi: 10.1080/03014468100004861. [DOI] [PubMed] [Google Scholar]
- 52.Edel MM. The Chiga of Uganda. New Brunswick, NJ: Transaction; 1996. [Google Scholar]
- 53.Ngologoza P. Kigezi and its People. Kampala, Uganda: Fountain; 1998. [Google Scholar]
- 54.Parenteau CS, Viano DC, Petit PY. Biomechanical properties of human cadaveric ankle-subtalar joints in quasi-static loading. J Biomech Eng. 1998;120(1):105–111. doi: 10.1115/1.2834289. [DOI] [PubMed] [Google Scholar]
- 55.Siegler S, Chen J, Schneck CD. The three-dimensional kinematics and flexibility characteristics of the human ankle and subtalar joints—Part I: Kinematics. J Biomech Eng. 1988;110(4):364–373. doi: 10.1115/1.3108455. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg A, Goldie I, Kalin B, Selvik G. Kinematics of the ankle/foot complex: Plantarflexion and dorsiflexion. Foot Ankle. 1989;9(4):194–200. doi: 10.1177/107110078900900409. [DOI] [PubMed] [Google Scholar]
- 57.Rome K. Ankle joint dorsiflexion measurement studies. A review of the literature. J Am Podiatr Med Assoc. 1996;86(5):205–211. doi: 10.7547/87507315-86-5-205. [DOI] [PubMed] [Google Scholar]
- 58.Csapo R, Maganaris CN, Seynnes OR, Narici MV. On muscle, tendon and high heels. J Exp Biol. 2010;213(15):2582–2588. doi: 10.1242/jeb.044271. [DOI] [PubMed] [Google Scholar]
- 59.Garvan JM. 1931. The Manóbos of Mindanáo, Memoirs of the National Academy of Sciences (Government Printing Office, Washington), Vol 23, 1st Memoir.
- 60.Estioko AA, Griffin PB. The Ebuked Agta of Northeastern Luzon. Philipp Q Cult Soc. 1975;3:237–244. [Google Scholar]
- 61.Stanley SM. An ecological theory for the origin of Homo. Paleobiology. 1992;18(3):237–257. [Google Scholar]
- 62.Jungers WL. Interlimb proportions in humans and fossil hominins: Variability and scaling. In: Grine FE, Fleagle JG, Leakey RE, editors. The First Humans: Origin and Early Evolution of the Genus Homo. New York: Springer; 2009. pp. 93–98. [Google Scholar]
- 63.Jungers WL. Body size and scaling of limb proportions in primates. In: Jungers WL, editor. Size and Scaling in Primate Biology. New York: Plenum; 1985. pp. 345–381. [Google Scholar]
- 64.Bailey RC, Headland TN. The tropical rain forest: Is it a productive environment for human foragers? Hum Ecol. 1991;19(2):261–285. [Google Scholar]
- 65.Turnbull CM. Survival factors among Mbuti and other hunters of the equatorial African rain forest. In: Cavalli-Sforza LL, editor. African Pygmies. New York: Academic; 1986. pp. 103–123. [Google Scholar]
- 66.Diamond JM. Why are pygmies small? Nature. 1991;354(6349):111–112. doi: 10.1038/354111a0. [DOI] [PubMed] [Google Scholar]
- 67.Reed KE. Paleoecological patterns at the Hadar hominin site, Afar Regional State, Ethiopia. J Hum Evol. 2008;54(6):743–768. doi: 10.1016/j.jhevol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 68.Washburn SL, Devore I. The social life of baboons. Sci Am. 1961;204:62–71. [Google Scholar]
- 69.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 70.Zipfel B, et al. The foot and ankle of Australopithecus sediba. Science. 2011;333(6048):1417–1420. doi: 10.1126/science.1202703. [DOI] [PubMed] [Google Scholar]
- 71.Henry AG, et al. The diet of Australopithecus sediba. Nature. 2012;487(7405):90–93. doi: 10.1038/nature11185. [DOI] [PubMed] [Google Scholar]
- 72.Richmond BG. 1998. Ontogeny and biomechanics of phalangeal form in primates. PhD dissertation (State Univ of New York, Stony Brook)
- 73.Stern JT. Climbing to the top: A personal memoir of Australopithecus afarensis. Evol Anthropol. 2000;9(3):113–133. [Google Scholar]
- 74.Rasband WS. 2011. ImageJ (National Institutes of Health, Bethesda). Available at http://imagej.nih.gov/ij/. Accessed June 4, 2012.
- 75.Darroch JN, Mosimann JE. Canonical and principal components of shape. Biometrika. 1985;72(2):241–252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.