Abstract
Cancer of the cervix has the potential to be eradicated since the initiating cause is known. There was not much known about this cancer until the time of the Renaissance. In Malaysia, it is the second most common cancer among females after breast cancer. The strategies on prevention in this country are still not optimal. This article highlights the problems and also discusses the pathogenesis of this disease. The key to prevention is screening and the future is the era of molecular pap smear.
Keywords: Cancer of the cervix, cancer cervix in Malaysia, screening for cancer cervix, molecular pap smear
Introduction
The population of females in Malaysia in the year 2000 was approximately 10.5 million. Approximately thirty percent (30%) of these females are in the reproductive period or older and are at risk of developing cervical cancer. Cancer of the cervix is the second most common cancer among females in Malaysia after breast cancer. The incidence of this cancer is 11.6 per 100,000 populations, with the age standardized rate of 16.2 per 100,000. Cancer of the cervix is preventable. The precursor cancer cells can be easily detected provided the women do regular screening tests called pap smear. There is 30% reduction in the risk of developing cervical cancer if pap smear is done every 10 years, 80% every 5years and 90% every 3 years (1).
The Bleak Past
Little was known about cervical cancer until the time of the Renaissance. Before this era, Hippocrates, in 450 BC and Aetius of Amida, in 600 AD referred it as cancer of the uterus. In the early 15th century, ‘The Medieval Women’s guide to Health’ recorded a certain “cancer and festering of the wombs resulting from old injuries that have not healed well”. Mathew Bailie, in 1793 stated that this cancer did not cause enlargement of the uterus rather continuous ulceration of the uterus until the whole organ is destroyed. John Clark, in 1812 noted the tumour grew like peculiar cauliflower excrescence. The insight into the epidemiology came in the early 19th century when in 1842, Rigoni-Stern, an Italian physician examined the death records of the city of Verona for the years of 1760 to 1839. He noted that cancer of the cervix was high among married ladies and widows and low among the Jewish women, rare in the unmarried ladies and absent in Italian nuns. This was the first recorded reference, which incriminated ‘sexual’ events in its genesis. Samuel Ashley, in 1844 noted that the majority of the patients with cervical cancer were often of dark complexion, and F von Scazoni, in 1861 noticed the patients were among those with frequent sexual excitation. In 1872, deaths due to cervical cancer in South Carolina (USA) were much higher in black women, linking socio-economic status as one of the risk factors (2).
The earliest specific reference on the treatment of uterine cancer is recorded in the works of Hippocrates. ‘The disease was so destructive they were better left uncured than treated’. Hippocrates, in 450 BC tried local fumigation and irrigation using herbal concoctions. Aetius, in 600 AD said the disease could be ‘mitigated and alleviated’ by baths, poultices and irrigations using various herbs. In 1575, Ambrose Parre and in 1652 Tulpius, performed cervical amputation as a form of treatment. In 1837 Duparcque advocated blood letting and purging by releasing leeches into the cervix. Some physicians at that time favoured the application of red-hot irons or other powerful caustics.
The sensible form of treatment of this cancer began when JM Langenbeck in 1813 performed first vaginal hysterectomy and WA Freund in 1878 performed total abdominal hysterectomy. Freund performed the hysterectomy, using anesthesia and antiseptic measures. By 1885, 95 patients were treated with such surgical procedure. The world applauded Wilhelm Roentgen who discovered X-ray but it was when Marie Curie who discovered radium in 1898, that improved the treatment of cervical cancer. Ernest Wertheim introduced radical hysterectomy and published his results of 500 cases from 1899–1912. His operative mortality rate was high (up to 31.5%) and many of his patients had surgical complications. Radium therapy improved the mortality rate.
To the pathologists, it was Rudolf Virchow who made a difference. Virchow in 1855 stated that ‘every cell is derived from a cell’ (cellula a cellula) and that human disease processes was essentially disease of the cells. Virchow is considered the protagonist of the concept of Zellular pathologie or pathology based on the study of cells. However, It was George Nicholas Papanicolou, who made an impact on cytopathologists. Papanicolou, an American anatomist of Greek descent, together with a gynaecologist Herbert Traut, published the first major paper on the use of vaginal smear for the diagnosis of cancer of the uterus in 1941. Soon after, the pap smear (named after Papanicolou), a screening test for cervical cancer was born.
The Present Situation
Cancers in general will remain the number one concern of the human population worldwide. An estimate of 10 millions new cancers with 6. 2 million deaths and 22.4 million people were living with cancers in the year 2000. The most common cancers are cancers of the lungs (1.24 million), breasts (1.05 million), colo-rectum (945,000) and stomach (876,000). The top 9 cancers affecting women is illustrated in Figure 1 (3). There is no difference in the incidence, between developed and developing countries among cancers that have genetic basis in the carcinogenesis, such as cancers of the breasts and cancers of the colorectum. But cancers in which infection plays a significant role in the carcinogenesis such as cervical cancer (due to HPV infection), liver cancer (mainly due to Hepatitis B and C virus infection) and cancer of the esophagus (HPV infection plays some role), and cancer of the cervix, the incidence is much higher in developing countries than in developed countries.
Figure 1:
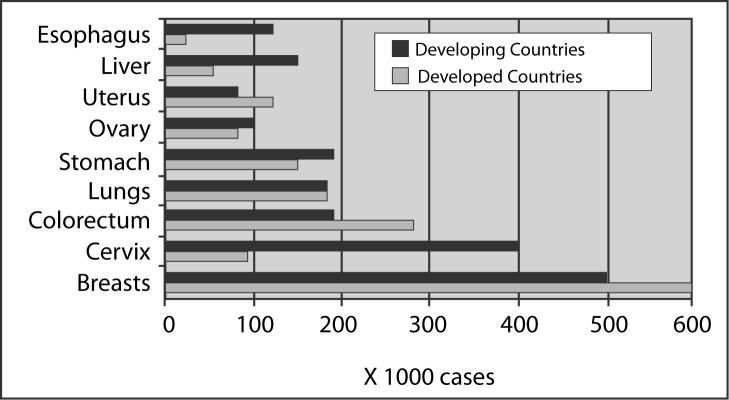
The 9 common cancers affecting women in the world in year 2000 (From Parkin D.M, Bray FI, Devesa SS,. Cancer burden in the year 2000; The global picture. European J of Cancer 2001;37s4–s66)
It is estimated that approximately 440,000 new cases of cervical cancers occur worldwide annually, and 80% of these cancers occur in developing and undeveloped countries (4). The bulk of cervical cancer is in Asia in which the countries are underdeveloped or developing, with nearly 330,000 cases. Centers for Disease Control and Prevention (5), reported that the incidence of invasive cervical cancer for Hispanic women was approximately twice that for non-Hispanic women indicating that cervical cancer is a disease of the economically disadvantaged population. In a developed country that has diverse population, a community that is in disadvantage economic situation may not receive optimal health services. Socioeconomic factors play an important role in the pathogenesis of the cancer the cervix.
Cancer of The Cervix In Malaysia
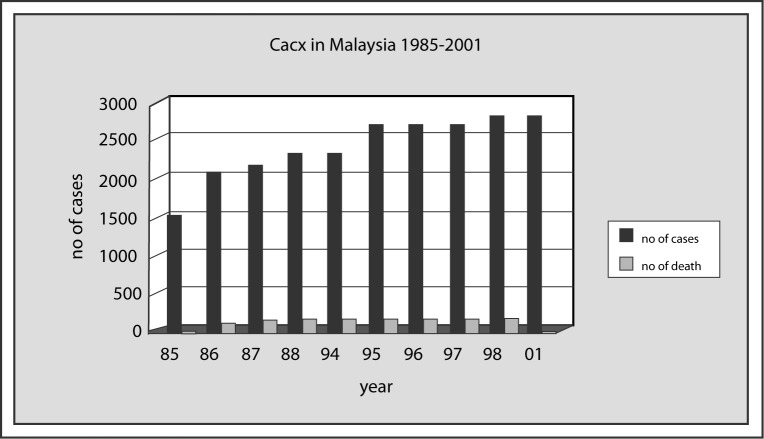
In 1999, Ministry of Health, Malaysia recorded that cancer was the third leading cause of death among medically certified deaths in the country (6). While cancer of the cervix is ranked 3rd, it is the second most common cancer among females in the country. Penang, a state in Peninsular Malaysia, which has an organized cancer registry, records the age-standardized rate of 14 per 100,000 females and Sarawak, 12.8 per 100,000 (7). In general there is an average of 2000–3000 hospital admissions of cancer of the cervix per year (8). Malaysia has introduced Pap smear since the late sixties and early seventies, however, we see no change in the pattern of the prevalence of this cancer indicating that the pap smear coverage have not targeted the population at risk (Figure 2). Pap smear coverage in the country was a dismal figure of less than 2% in 1992, 3.5% in 1995 and 6.2% in 1996 (8). The reasons for this poor screening performance are several. The nation-wide campaign for women to come forward for screening is not aggressively put forth. The general public is largely unaware of the benefits of screening. The screening, when done is not targeting the population at risk (9). Many pap smears are done on women who come for post natal check up (Table 1) (10) . This is a ‘wrong’ time to do pap smear. First, the epithelium of the cervix is denuded at delivery of the baby and secondly, there is significant amount of inflammation of the cervix as a results of the delivery, masking the cells of interest. Nor Hayati Othman et al (10–14) did several studies in Kelantan, a state in the north eastern part of Peninsular Malaysia and their studies showed that, 42.8% to 70.4% of the pap smears done in Hospital Universiti Sains Malaysia and Kota Bharu hospital did not contain endocervical cells nor presence of metaplastic cells to indicate the samples were not taken from the area of the cervix where the cancer arises from, the transformation zone. More than 7000 women in their studies were mostly those in the late twenties and the early 30s and they were mainly women attending post natal check-ups (Table 1) (10). They suggested that if Malaysia was to have a serious look at reducing the rate of cervical cancer, the country must have a proper screening system and the age of the women targeted are those above 35 years and no samples should be done for women who just had deliveries for reasons mentioned above.
Figure 2:
The Prevalence of Cancer of the Cervix in Malaysia 1985–2001 [From Sistem matlumat dan Dokumentasi Kementerian Kesihatan Malaysia]
Table 1:
The age of the women when the pap smears were taken in HUSM and HKB in 1996 (from Nor Hayati O,., Ayub, M. C., Aziz, W. A. A., Muda, M., Wahid, R. & Selvarajan, S. Pap Smears – Is It An Effective Screening Methods for Cervical Cancer Neoplasia? – An Experience with 2289 Cases.The Malaysian J. of Medical Sciences 1997; 4(1): 45–50)
| Age (years) | No of smears | Percentage |
|---|---|---|
| <20 | 90 | 3.9 |
| 21–30 | 755 | 32.9 |
| 31–40 | 944 | 41.2 |
| 41–50 | 377 | 16.5 |
| 51–60 | 88 | 3.8 |
| 61–70 | 20 | 0.8 |
| >71 | 15 | 0.6 |
Key : HUSM – Hospital Universiti sains Malaysia HKB – Hospital Kota Bharu
Before 1998, the gynaecologists and the pathologists were not using the same language when referring to the same cervical lesion. In November 1998, Ministry of Health organized a meeting to discuss the consensus on pap smear screening in the country. The results of the meeting; a National Pap Smear Screening Program was born (15). This is in the form of a guide booklet, issued to all hospitals in the country, including private hospitals.
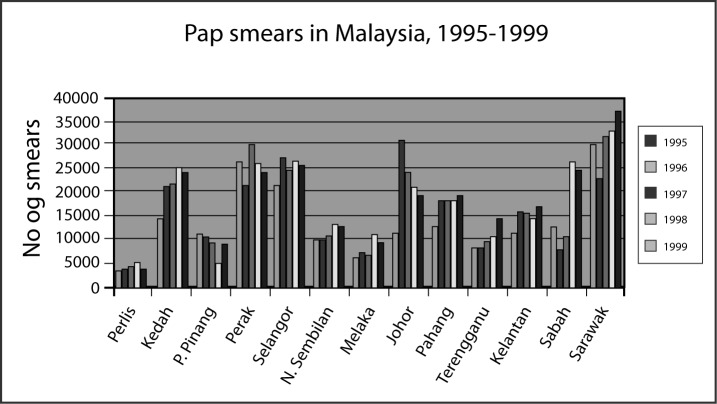
Figure 2 also shows the prevalence of death due to cancer of the cervix. This falsely low number of deaths per year is due to poor documentation of deaths in Malaysia. Many of the terminally ill patients chose to die at home, thus contributing to the low figure of hospital deaths. Many states in Malaysia with the exception of Johore show an increasing number of pap smears (Figure 3) taken from 1995 to 1999 (8). Sarawak for example, has the highest coverage yet the disease in Sarawak remains high with an incidence of 16.2 per 100,000 females.
Figure 3:
The number of pap smears done in Malaysia according to states, 1995–1999
Cancer Of The Cervix In Kelantan
Kelantan is a state in Malaysia in which the population is predominantly Malays and presumably Muslims. In the past, circumcision was thought to be protective against cancer of the cervix when early epidermiological studies noted that this cancer was lower among Jewish women who presumably had sexual relationship with Jewish men who have circumcision soon after birth. Muslim men too get circumcision before puberty, i.e. before the age of committing sexual relationship. As we now know, circumcision does not prevent transmission of HPV from the men to their sexual partners.
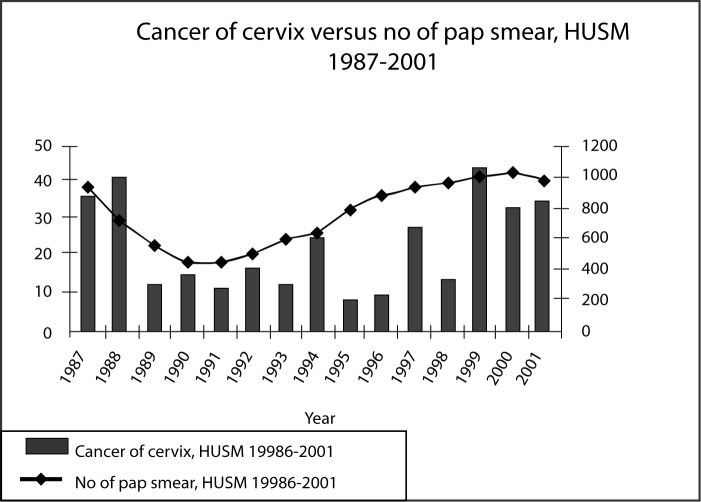
Hospital Universiti Sains Malaysia (HUSM) is regarded as the referral center for the East Coast region. From 1987 to 2001, the Hospital have seen and treated 328 cases of cancer of the cervix, with an average of 21–22 cases per year. In a population census of the year 2000, Kelantan has a population of 1.3 millions. 41.5% of these were children below the age of 14. The male to female ratio is 1:1. This calculation implies that there are about 379,600 women above the age of 14 years. Projecting the annual incidence of 21–22 cases of cancer of the cervix every year for this state, the incidence of cancer of the cervix is approximately 5.5 per 100,000 females. This figure is much lower than the incidence for the country. However, if we speculate only 50% of the women in the state of Kelantan come to get treatment in HUSM, while the rest goes to Hospital Kota Bharu, the incidence is almost equivalent to that recorded for Malaysia. The number of pap smears done in HUSM is displayed in Figure 4. It is interesting to note that the pattern of the incidence of cervical cancer is almost parallel to the number of smears taken rather than reciprocal to it. This chart shows that pap smears are being used for diagnosis rather than for screening of the disease.
Figure 4:
The number of cases of cancer of the cervix and the number of pap smears taken from 1987–2001 in HUSM
Cancer Of The Cervix In Malaysia As Compared To Other Countries
Malaysia is a rapidly developing country. One of the early indicators of economic growth in any country is her health care service. While we are almost at par with Singapore and Hong Kong in terms of the economic growth we are in a lesser situation in terms of health services particularly pertaining to cancer of the cervix. We are in the same rank as Guam, a country much lesser developed in the Western Pacific. Australia, a developed country follows a comprehensive screening protocol with 63.9% pap smear coverage. The age-standardized rate of the cancer is 1.8 per 100,000 while in Malaysia 16.2 per 100,000. The downward trend in the incidence of cervical cancer in Australia (16) confirms the effectiveness of their pap smear campaign.
The Disease
Nearly all cancer of the cervix arises from a region called the transformation zone. At this region, mucus secreting columnar cells abruptly change to squamous epithelium. This zone differs in location slightly according to the age of the women, lower in teens and higher in elderly. Frequent sexual intercourse is perceived as a form of trauma to the fragile columnar epithelium thus an adaptive phenomenon called squamous metaplasia takes place whereby the columnar epithelium changes to another kind of epithelium which is stronger; – the squamous epithelium. Pap smear sampling is said to be adequate if the transformation zone cells or the metaplastic (squamous) cells are sampled. Slight modification is needed in the technique of pap smear sampling in women of different ages (12,14).
The Culprit
The organism that causes cancer of the cervix is Human Papilloma Virus (HPV), a small double stranded virus with circular genome. It is a group of viruses that infect skin and mucous membranes of human. There are more than 130 subtypes of HPV and about 70 subtypes infect human. Out of these, 35 subtypes infect genital tracts. Of those that infect the genital tracts they are classified to low risk (LR HPV) and high risk HPV groups (HR HPV) according to the risk of development of cervical cancer. The LR HPV subtypes are 6,11,42, 43, 44 and the HR HPV are 16,18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68. The prevalence of HPV DNA in cancer of the cervix is 99.7% (17). The high risk HPV, are those most often found in invasive cancers (18). The presence of HPV in virtually all cervical cancers implies the highest worldwide attributable fraction so far reported for a specific cause of any human cancer (17).
Persistence infection by HPV gives women more than hundred times relative risk to develop cervical cancer. The association of HPV with cervical cancer is very high, much higher than the association of tobacco smoking and lung cancer. The Odds Ratio (OR) of the association of HPV DNA (pooled) to cervical cancer is 70 (Confidence Interval=57–88). The association is equally strong for both squamous cell carcinoma (OR=74) and for adenocarcinoma (OR=50). The OR for HPV 16 is 150, HPV18=182; HPV31=60; HPV33=78; HPV59=347 (19). HPV 16 has a number of variants, the AA (Asian-American) variant is associated with a 21 times higher risk of developing cervical cancer (OR=27.0; 95% confidence interval) compared to the E (European) variant (OR=3.4; 95% Confidence interval) (20).
The Pathogenesis
Pleasurable event called sexual intercourse, potentially introduces a few physiological and pathological phenomena to a woman. Firstly, she may get pregnant, which may not be a problem if that is what she wanted out of the relationship. Secondly, repeated sexual intercourse leads to squamous metaplasia of the transformation zone of the cervix, an adaptive defence mechanism of the body, in which the fragile columnar epithelium is converted to a stronger epithelium, the squamous epithelium. The third event is the possible introduction of an incriminating virus called Human Papillomavirus (HPV).
Infection by the virus causes no immediate or direct untoward symptoms to the woman. The open reading frames of the virus which are of interest in pathogenesis of cervical cancer is E2, E6 and E7. The E2 region of the virus attaches and integrates its genome to the genome of the woman’s cells at the basal layer of the metaplastic epithelium. Upon integration, the ring molecule unrolls, disrupting the continuity of the viral gene. Basal cells are stem cells, which have the potential to differentiate along squamous, glandular and neuro-endocrine cells. The basal cells are constantly dividing. In normal condition, the basal cells (squamous) will mature to form suprabasal cells and intermediate cells, which in turn form the mature keratinocytes.
There are many genes that regulate cell cycle and the ones of interest in understanding the pathogenesis of cancer of the cervix are two genes; the p53 and the Rb (Retinoblastoma) genes. p53 is the guardian of genome and is involved in responding to DNA damage either by repairing the damage or by inducing cell death called apoptosis and the Rb gene regulates the cells entering the cell cycle. p53 and Rb are tumour suppressor genes located on chromosome 17 (p53 gene) and 13 (Rb gene). The actual mechanism how the Rb and the p53 genes get degraded is complex. The E6 protein inactivates p53 gene and E7 protein binds with the Rb gene products causing inactivation of these tumour suppressor genes. When these genes are inactivated, cells with damaged DNA content are allowed to enter the cell cycle thus generating new clones of cells which are abnormal (dysplastic).
There is a preferential integration site within both the host and viral genomes (21). The site of HPV 18 DNA integration in a cell line derived from an HPV 18 DNA positive cervical carcinoma has been localized to chromosomes 8q24 and 2p24. The chromosomes positions 8q24-qter and 2p23–24 coincide with the location of c-myc and N-myc respectively, the genes which are responsible for cellular proliferation.
The virus remains in the cells for a long time. Since the virus evokes no cell death, nor does the virus attack immune presenting cells located in the epithelium, the body does not recognize these infected cells as foreign. Furthermore, there is no blood-borne phase of infection, the immune system outside the epithelium has little opportunity to detect the virus (22). The virus can reassemble its viral genome and multiply inside the host cells. In the immediate supra-basal zone, there is expression of the early regions of the virus, and as the cells differentiate, there is induction of all viral genes, as well as viral DNA synthesis, leading to assembly and production of virions in the cells just beneath the surface. These cells are recognized as koilocytic atypia (KA) (23).
The Risk Factors
The younger the age of the woman in indulging in sexual activity the higher is her risk to get HPV infection. The same goes; if she has multiple sex partners or the male partners has multiple sex partners. The risk is higher if the male partners have wives or spouses or have had sexual relations with women who later died of cancer of the cervix. Infection by HPV is very common however, not every woman who gets the virus develops dysplasia or invasive cancer, indicating that most of the infection is cleared spontaneously. Why do certain women progress to cancer? The answer is the presence of co-factors or co-carcinogens which are needed to switch on the infected cells to neoplastic transformation.
HPV acts as initiator of oncogenesis, it needs interaction with somatic environmental factors to cause cancer. The role of smoking either active or passive smoking in cervical cancer has not been studied until of recent years. Coker et al, 2002, showed that passive cigarette smoke exposure was significantly (p<0.05) associated with High Grade Surface Intra-epithelial Lesion (HSIL) with adjusted odds ratio (aOR) of 2.2. They noted that passive smoke was also associated with low grade Surface Intra-epithelial Lesion (LSIL) with aOR of 1.8 (24).
Low immune status plays a role in many cancers. It is not clear if the presence of HIV infection gives the woman a higher risk in getting invasive cervical cancers if she also has HIV infection or AIDS. However, it is observed that cervical cancers among women with and HIV/AIDS have poor clinical behaviour. The presence of other genital infections besides HPV has also been studied as the possible co factor in carcinogenesis. The organisms include herpes viruses, chlamydia, candida and actinomycetes. Using blood and cervical specimens from 1,263 women with cervical cancer and 1,117 women without cervical cancer, Smith JS, (25) detected HSV-2 antibodies in the blood of 44.4% of women with squamous-cell carcinoma and 43.8% of women with adenocarcinoma or adenosquamous-cell carcinoma. The infections by these organisms however are very common and multiple re-infections can occur in one woman making it difficult to study if they play any role in carcinogenesis.
The Morphological Changes
The earliest change noted by light microscope is virus induced cytopathic change called koilocytes. Under light microscope koilocytes are cells with immature nuclei and have peri-nuclear halo (Figure 5). They are often thought to be pathognomonic for HPV infection. Cytological or histological absence of KA however, does not imply absence of HPV infection.
Figure 5:
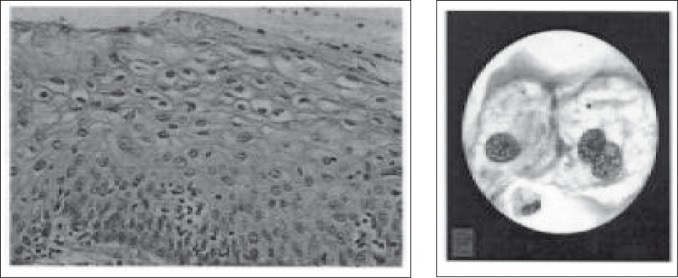
Koilocytes – The histology (left), and the cytology (right). The cytoplasm have perinuclear halo and the nuclei are large and immature.
The subsequent morphological changes are more obvious. This is called dysplasia commonly called cervical intra-epithelial neoplasia (CIN). Dysplasia is divided into 3 grades according to the thickness of the epithelial cells involved. Mild dysplasia (CIN1) is termed when the dysplastic cells are involving one third of the whole thickness of the cervical epithelium, measured from the basal layer. Moderate dysplasia (CIN2) is termed when the involvement is two third of the epithelium. Severe dysplasia is when the whole thickness of the epithelium is involved. Severe dysplasia is also called carcinoma in-situ. Micro-invasion is when the cancer cells have breached the basement membrane but less than 3 mm in thickness.
The Current Technology And The Problems Faced
Pap smear screening has been effective and since its introduction more than 50 years ago, it has reduced the incidence of cervical cancer by 43% and decreases its mortality by 46% (26). In more than 50% the cancers are seen in women who never had pap smear done. In about 10%, these women did not have pap smears done in the last 10 years. In a small percentage they are detected in women who had negative pap smear. The sensitivity of pap smear has a wide range from as low as 30% to as high as 87% indicating that many cases could be missed by this screening test .The specificity is 86–100% indicating that there are false positive diagnoses made (27)
Some of the reasons cited for poor sensitivity include sampling error, detection error and technical error in sampling or in fixation of the cervical cells. Sampling error is when the abnormal cells are not collected from the women or if collected are not transferred to the slides. The tools used for sampling also determine the adequacy of samples obtained. Martin-Hirsch et al, 2002 (28) reviewed thirty-four trials and six observational comparative studies of cervical specimen adequacy. The Ayre spatula was shown to be less effective compared with extended tip spatulas for collecting endocervical cells in eight trials (odds ratio 2.25, 95% confidence interval 2.06 to 2.44). Use of a spatula with the cytobrush was more effective than spatula alone at collecting endocervical cells (odds ratio 3.33, 95% 3.05 to 3.63) and the same effect was present for adequate smear rates (odds ratio 1.51 95% 1.19–1.92) (28). Detection error occurs when the abnormal cells are missed or misinterpreted by the cytotechnologists and pathologists. To reduce detection error, many laboratory introduces re examination of 10% of the slides which initially reported negative as quality assurance measure.
In some big laboratories, computerized screening devices using neural network or algorithmic classifier such as PAPNET and AutoPap are used. So far no studies have indicated that these automated screeners are superior than human in detecting abnormal cells. Many of the studies using these computerized system did not apply the use of the new technology to the same samples that use conventional pap smear therefore, the relative sensitivity and specificity cannot be calculated (27).
Recently, our group (29–38) investigated the applicability of two conventional and two hybrid artificial neural networks as an early diagnostic system for cervical cancer. We worked on radial basis function (RBF), multilayered perceptron (MLP), hybrid radial basis function (HRBF) and hybrid multilayered perceptron (HMLP) networks to classify the type of cervical cancer in its early stage. We called this system ‘Neuralpap’ [Phd dissertation of Mat-Isa, 2002]. NeuralPap is an automatic pap smear diagnosis system. It produces 94.3%, 89.4% and 100.0% of accuracy, sensitivity and specificity respectively. These figures are much more impressive than the currently available commercial pap smear screener Papnet, however it is still in the early phase and further field testing is required before it can be used for diagnostic purposes. Papnet is capable to classify normal and abnormal cervical cells with the accuracy between 73% and 85% (39, 40).
Liquid based cytology was introduced to reduce detection error by overcoming technical difficulty in reading pap smears due to thick layer of cells and cells masked by thick mucus or blood. In general there is an increase in the rate of detection of cervical dysplasia and reduction of smears diagnosed in the ‘uncertain’ category, the ASCUS (atypical squamous cells of unknown significance) and The AGUS (atypical glandular cells of unknown significance) category under the Bethesda classification. There was an 11.7% increase in the rate of normal smears, a 27% increase in the rate of atypical cells and a 52% increase in detection of cervical dysplasia (41). However not all studies indicated favourable findings. New Zealand’s National Cervical Screening Programme (NCSP), based on the report made by New Zealand Health Technology Assessment decided not to purchase or endorse liquid-based cytology for its population-based national screening program (42) as it is not cost effective and is not shown to be overtly superior compared to conventional cytology.
Pap smear coverage of the target population for any particular nation has always been a problem, lesser in developed and problematic in developing countries. The problems are multi-factorial. In developing countries, access to health care is limited by funds and the national expenditure is more focused on treatment of diseases rather than prevention. The women are not well informed on the benefits of regular screening. The database of the population at risk, is not available. While all these problems may not exist in developed countries, it is interesting to look at the results of one survey done by Dr O’Malley and colleagues (2001) who sought why non indigent patients do not seek cervical cancer screening among the women who live in the suburb of Detroit city in 2001. Using a phone survey, 90% of whom covered by health insurance, 4% of them have never had pap smear and 13% had not had one within 3 years (43). Is this an early sign that the public is losing faith with the conventional screening test?
Conventional pap smear is now challenged (44). If HPV participates in the genesis of nearly all cases of cervical cancers, it is only sensible, for screening purposes, we examine the presence of this organism in the cervical samples. Identification of HPV remains on research basis except in a very few major centers. The sensitivity of HPV testing for detecting a histologically proven HGSIL (High grade surface epithelial lesions) using Hybrid Capture ll assay is 100%, higher than that of conventional (68.1%), and liquid based cytology (87.8%) (45). Hybrid capture (HC) testing system utilizes the ‘capture’ of the hybrids formed by RNA probes recognizing oncogenic HPV single stranded DNA in the cervical cells. The single stranded DNA is obtained by denaturing the pap smear samples taken in a usual manner, and inserted into Specimen Transport MediumTM (Dygene Corporation). These hybrids are ‘captured’ on the side of the wells or tubes (depending on the generation of the HC system used) which are coated with antibody that recognizes DNA-RNA hybrids. Adding a second antibody tagged with alkaline phosphatase permits detection of the bound hybrids using chemiluminescent read out. The amount of light emitted is expressed as relative to the light emitted by the positive control (PC) sample which contains 10pg/ml HPV DNA. The unit used is Relative light unit (RLU/PC). Test specimens in whom the RLU/PC equal or exceed 1.0 is said to be positive. The second generation Hybrid Capture (Hybrid Capture ll®) is said to be more effective and sensitive in HPV detection compared to the earlier generation with the detection threshold of 1.0 pg/ml. HC ll also permits semi-quantitative measurements, in which 1.0 pg/ml is calculated to contain 100,000 HPV genomes calculated based on HPV plasmid controls. The severity of the disease can be roughly estimated by the viral load in women who are HPV positive above the cut-off point of 1.0pg/mL. The median HPV DNA in cancer is 100.7pg/ml with corresponding medians of various grades; high grade SIL 84.6pg/ml, low grade 76.8pg/ml and normal 13.0pg/ml (46).
Serum tumour markers for different kinds of cancers are now widely available in many laboratories. Squamous cell carcinoma antigen (SCC-Ag) is the most common tumour marker for cancer of the cervix. SCC-Ag is often well correlated with stage and size of the tumour, the presence of metastasis and the survival of the patients. Other tumour markers are CA 125, and CA19-9. The upper limit of SCC-Ag is 1.5ng/ml, CA 125 35U/ml and CA19-9 37U/ml. These tumour markers are mainly used to monitor treatment response (47,48). Caution is advised on interpreting tumour marker levels. Persistent false positive serum SCC-Ag could be related to benign skin disorders such as psoriasis, or eczema and chronic pulmonary disease (48).
The Dilemma
HPV infection is extremely common. The prevalence of genital HPV infection below the age of 30 years is about 50%. The prevalence decreases with age, with approximate decrease by 10% as the age increases by 10 years. Who gets treatment and who does not? If the women are infected, is it worth treating her when 80% of the infection clears spontaneously? Why do some women clear the infection while some do not? If the HPV organism does not incite inflammatory response, how does the body defense mechanism clear the infection? Since HPV infection in women is sexually acquired, shall the males be screened for HPV as well? No country has adopted a policy including males in the cervical cancer screening strategy.
The Bright Future - Molecular Pap Smear
Conventional pap smear will remain the main screening form for this cancer as it has been proven to reduce the incidence by 43%. However, the sensitivity of pap smear is variable from 30–87%. it is time to move forward and embrace molecular pap smear (49). Identification of HPV should no longer be at research basis since the evidence that this virus cause cancer is monumental. HPV could be identified from the cervical samples by the conventional PCR (Polymerase Chain Reaction) and Hybrid Capture (HC) assay. While PCR is very sensitive it is also more time consuming compared to HC. In countries limited by financial resources, it is not cost effective to screen for HPV for women younger than 30 years. All the high risk and low risk HPV could also be identified by DNA chips where by an array of HPV DNAs of these strains are printed on glass slides and reacted with the patients RNA. The technique of micro-array is getting popular in many laboratories.
Since the viral genome integrates into the genome of the host cells, the change in cell kinetics of these infected cells could be examined by flowcytometry. There are no studies on the feasibility of identifying the infected cervical cells by flowcytometry yet. Flowcytometry have been used routinely in diagnosis of leukemia and other cancers. If it can be applied to pap smears flowcytometry has the potential to be used for mass screening thus overcoming the shortage of cytopathologists Malaysia is facing (50). Flowcytometry of cervical cancer cells while undergoing radiation therapy has been studied (51).
Other molecular markers, which are still under experimental stage but appear promising include looking for micro-satellite instability in exfoliated cervical cells DNA (52) and detecting HPV in cervical samples by SSCP (Single-Strand DNA Conformation Polymorphism) (53). The effect of HPV on cells and the cell cycle are also investigated. This include measurement of telomerase activity and identification of Mib −1 and PCNA in the cervical samples. Telomerase activity is significantly related to the level of dysplasia (54). Mib-1 could be used to improve the diagnostic accuracy of cervical low grade surface epithelial lesions (55).
Recently, an innovative technique to collect cervical smears for HPV testing was introduced in India. Kailash U eta al, 2002 (56) used simple paper smear method to collect cervical smears/scrape for HPV analysis by PCR. The cervical scrapes, obtained by Ayer’s spatula were smeared on 3MM Whatman paper, cut to the size of a small glass slide. The remainder of cells in the spatula was transferred to collection vials containing 5ml of PBS and stored at −70ºC. The paper smears were placed in individual auto-seal (ziplock) polythene bags. Half of these were stored at room temperature while the rest at 4ºC until it was to do the PCR amplification. Imprint biopsies, blood and fine-needle aspirates were also collected by this method. The quality and the quantity of DNA of the sample in dry paper smears was comparable with the same sample following collection with PBS.
Vaccination
Vaccination against HPV is the most appropriate preventive measure since this virus is the root cause of cervical cancer. Vaccination is more relevant in developing countries as comprehensive screening programs are not feasible due to limited resources. However development of the vaccine has been slowed by a number of problems related to the biology of HPV. Immunologists are still intrigued at what cause spontaneous remission in most individuals. Local inflammatory response was thought to play a role in those cases that regress (57). HPV is difficult to be cultured in vitro nor there are animal models for HPV. There is no ready source of live virus available for attenuated live vaccine like the polio vaccine. HPV infection has a very minimum blood phase therefore natural antibody against it does not developed. HPV infection remains in the epithelium, thus antibodies must traverse the basement membrane to reach the layers of the skin or mucosa to be effective in preventing infection (58). The infected patients do not produce significant IgA mucosal antibody. The immunity against this virus is mainly through cellular immune response; by CD8, CD4 cytotoxic T cells.
Having said the problems in vaccine development, it is enlightening to note that vaccine against HPV is on its way to the market probably in the next couple of years. Many types of vaccines are in phase 1 and phase 11 clinical trials. The major breakthrough in prophylactic vaccine development was the discovery that the capsid protein L1 can self-assemble into virus-like particles (VLPs), when independently expressed at high levels in cultured cells. These VLPs resemble the HPV virions morphologically and immunologically (59). The VLPs do not contain any viral DNA and thus non infectious and non oncogenic. They are the most attractive candidate for developing a prophylactic vaccine against genital HPV infection (60). Preliminary data on phase 1 and phase 2 clinical trials with HPV L1-VLP, given intra muscularly on healthy volunteers showed a strong humoral response (61). The L1 protein can also be expressed into attenuated bacterial vectors such as Mycobacterium bovis (62). The incidence of prevalence of neutralizing antibody in non pathological cervix (85.7%) is statistically significant (p<0.0001) compared to the presence of neutralizing antibodies in CIN1 (21.5%), CIN 2 and 3 (15.7%) and cervical cancer (0%) indicating that vaccines that can promote neutralizing antibodies may be effective in eliminating CIN lesions (63).
Recently, the break-through on vaccination came from a group of researchers in United States, where a double blind study involving 2392 women was done (64). In this study they showed a 100 percent efficacy; at 95% confidence interval, when HPV-16-like particle vaccine (40 ug per dose) was administered at interval of 0, 2 and 6 months.
The IEC Policy
The countries that are serious in combating cervical cancer should have better information, education and communication (IEC) policy. There should be a complete data base of the target population, ie all females in the country. The high risk women should be identified. Education on the prevention of the disease should be taught early in schools. Every member of the society has a role to play. The country should use effective mass media to disseminate information on the pathogenesis of cancer of the cervix. The women should be invited to come forward for screening when she reaches 30 years of age. The screening program must be comprehensive and friendly to the public. The cost of screening should be affordable by most women. There should be a national policy on screening protocol, which the entire nation subscribes so that the cost per each test could be made cheap. At the first visit, the pap smears are taken and the HPV tests done on the same cervical samples. The results are notified by mail. The women should come for re screening every 3 years if the pap smears are normal and the HPV test is negative. If the HPV test is positive, she should come every 2 years until the HPV clears up. All hospitals and health centers should use the same treatment policy for the women who have abnormal pap smears or HPV tests.
Conclusion
Cervical cancer is preventable. It has a known cause. Careful and effective screening can prevent it. New technologies of screening are becoming available to general population. Many countries are improving the education, communication and the information to the target population. Unlike Dr Giard who was pessimistic in his editorial article entitled “False-Negative Rate of Cervical Cytology: Sense and Sensitivity” (64), I remain an optimist. He said “the disappointing results of screening for cervical cancer have defied the logic of screening for this cancer based on scientific speculation. Cervical cancer is not always detectable, it is not always preventable, and it is not always curable”. On the contrary, I beg to differ from him. Cancer of the cervix HAS THE POTENTIAL to be eradicated and time and science have proved it.
References
- 1.Kuie TS. Cervical Cancer: Its Causes and Prevention. Singapore: Times Book Int; 1996. [Google Scholar]
- 2.Shingleton HM, Orr JW. Cancer Of The Cervix, Diagnosis And Treatment. Churchill Livingstone Publication; 1987. [Google Scholar]
- 3.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000; The global picture. European J of Cancer. 2001;37:s4–s66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 4.Masood SA. Plea for a Worldwide Volunteer Cervical Cancer Education and Awareness Program. Acta Cytologica. 1999;43(4):539–542. doi: 10.1159/000331144. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and prevention Invasive Cervical Cancer Among Hispanic and Non-Hispanic Women - United States, 1992–1999. MMWR. 2002;51(47):1067–1070. [PubMed] [Google Scholar]
- 6.Ministry of Health Malaysia. Malaysia’s Health Technical Report of The Director-General of Health, Malaysia 1999
- 7.Sarawak Cancer Statistics 1996; http://sarawak.health.gov.my/cancerre/cancerre.htm
- 8.Annual reports of Ministry of Health (1980–1998)
- 9.Nor Hayati O, Ayub MC, Aziz WAA, Muda M, Wahid R, Selvarajan S. Pap Smears – Is It An Effective Screening Methods for Cervical Cancer? – An Experience with 2289 Cases. The Malaysian J of Medical Sciences. 1997;4(1):45–50. [Google Scholar]
- 10.Nor Hayati Othman, Ayob Mukarramah Che, Pap Smear Study: The Squeal Is there a need to change the sampling tool. Malaysian Journal of Pathology. 1997;19(1):77. (abstract) [Google Scholar]
- 11.Nor Hayati O, Ayob MC, Wahid RA. Is Pap Smear Screening Program Effective? A Kelantan Experience with 5000 cases. Malaysian J of Pathology. 1995;17(1):53. [Google Scholar]
- 12.Nor Hayati Othman. The fancies and the fallacies of specimen collections - the need to break bad habits. Malaysian Journal of Pathology. 1995;17(1):55. [Google Scholar]
- 13.Nor Hayati Othman. Cancer of the cervix- Is it a preventable disease? Medical on Line. Nov, 2000.
- 14.Nor Hayati Othman. The facts and fallacies of specimen sampling. What you should and should not do. Universiti Sains Malaysia Buletin CME. 1998;1(2):7–8. [Google Scholar]
- 15.National Pap smear Screening Program. 1998. Family Health Development Division, Ministry of Health, Malaysia.
- 16.Cancer of the cervix in New South Wales 1972–1992. http://www.cancercouncil.com.au/cncrinfo/research/reports/sumCervix.htm
- 17.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human Papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathology. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Stoler MH. The Biology of Human Papillomaviruses. Pathology Case Reviews. 1997;2(1):8–20. [Google Scholar]
- 19.Nubia Munoz. Human Papillomavirus and cancer: the epidemiological evidence. J of Clin Virol. 2000;19:1–5. doi: 10.1016/s1386-6532(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 20.Berumen J, Ordonez RM, Lazcano E, Salmeron J, Galvan SC, Estrada RA, Yunes E, Garcia-Carranca A, Gonzales-Lira G, Madrigal-de la Campa A. Asian-American variants of Human papillomavirus 16 and risk for cervical cancer: a case control study. J of national Cancer institute. 2001;93(7):1325–1330. doi: 10.1093/jnci/93.17.1325. [DOI] [PubMed] [Google Scholar]
- 21.Corden SA, Sant-Cassia LJ, Easton AJ, Morris AG. The integration of HPV-18 DNA in cervical carcinoma. J of Clinical Pathology: Mol Pathology. 1999;52:275–282. doi: 10.1136/mp.52.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nature Reviews. 2002 Jan;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 23.Stoler MH. Human papillomavirus and Cervical Neoplasia: A Model for Carcinogenesis. Int J Gynaecol Pathol. 2000;19(1):16–28. doi: 10.1097/00004347-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Coker AL, Bond SM, Williams A, Gerasinomova T, Parisi L. Active and passive smoking, high risk human Papilloma virus and cervical neoplasia. Cancer Detect Prevent. 2002;26(2):121–8. doi: 10.1016/s0361-090x(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 25.Smith JS, Herrero R, Bosetti C, Munoz N, Bosch FX, Eluf-Neto J, Castellsague X, Meijer CJ, Van Den Brule AJ, Franceschi S, Ashley R. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;6,94(21):1604–13. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- 26.Cohn DE, Herzog TJ. New Innovations In Cervical Cancer Screening. Clin Obstet and Gynecol. 2001;44(3):538–549. doi: 10.1097/00003081-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB. The Accuracy of The papanicolaou Test In Screening For And Follow-up Of Cervical Cytologic Abnormalities: A systematic Review. Ann Intern Med. 2000;132:810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Hirsch P, Jarvis G, Kitchener H, Lilford R. Collection devices for obtaining cervical cytology samples. Cochrane Database Syst Rev. 2000;2:CD001036. doi: 10.1002/14651858.CD001036. [DOI] [PubMed] [Google Scholar]
- 29.Mat-Isa NA, Mashor MY, Nor Hayati O. Diagnosis of Cervical Cancer Using Hybrid Radial Basis Function Network. Student Conference on Research and Development. 2001:37. [Google Scholar]
- 30.Mat-Isa NA, Mashor MY, Nor Hayati O. Pap Smear Image Segmentation Using Modified Moving K-Means Clustering. International Conference on Biomedical Engineering. 2002:44–46. [Google Scholar]
- 31.Mat-Isa NA, Mashor MY, Nor Hayati O. Automatic Seed Based Region Growing for Pap Smear Image Segmentation. International Conference on Biomedical Engineering. 2002:167–169. [Google Scholar]
- 32.Mat-Isa NA, Mashor MY, Nor Hayati O. Diagnosis of Cervical Cancer Using Hierarchical Radial Basis Function (HiRBF) Network. International Conference on Artificial Intelligence in Engineering and Technology. 2002:458–463. [Google Scholar]
- 33.Mashor MY, Mat-Isa NA, Nor Hayati O. Automatic Pap Smear Screening Using HMLP Network. Artificial Intelligence in Engineering and Technology. 2002:453–457. [Google Scholar]
- 34.Mat-Isa NA, Mashor MY, Nor Hayati O. Application of Neural networks for early stage of cervical cancer diagnosis - International Journal of Computer. 2002 Dec; [Google Scholar]
- 35.Mat-Isa NA, Mashor MY, Nor Hayati O. Region growing based features extraction. International Conference on Image Processing (ICIP); Sep 14–17, 2003. (paper submitted for publication, December 2002) [Google Scholar]
- 36.Mat-Isa NA, Mat-Sakim HA, Mashor MY, Nor Hayati O. A Review on Application of Neural Network in Cancer Disease: Case of Cervical and Breast Cancer. International Conference on Robotics, Vision, Information and Signal Processing.; Jan 22–24, 2003. (Paper accepted) [Google Scholar]
- 37.Mat-Isa NA, Mashor MY, Nor Hayati O. Comparison of Segmentation Performance of Clustering Algorithms for Pap Smear Images. Accepted for International Conference on Robotics, Vision, Information and Signal Processing.; 22–24 January, 2003; 2003. (paper accepted) [Google Scholar]
- 38.Mat-Isa NA, Mashor MY, Nor Hayati O. Application of Moving K-Means Clustering for Pap Smear Image Processing. International Conference on Robotics, Vision, Information and Signal Processing; Penang. 22–24 January, 2003; 2003. (Paper accepted) [Google Scholar]
- 39.Cabaniss D, Cason Z, Lemos L, Benghuzzi H. The Assessment of An Endocervical Component in Cervicovaginal Smears with The Papnet System. Proc. of 16th Southern Conf on Biomedical Engineering; 1997. pp. 357–361. [Google Scholar]
- 40.Ashfaq R, Solares B, Saboorian M. Detection of Endocervical Component by Papnet System on Negative Cervical Smears. Diagnosis Cytopathology. 1996;15(2):121–123. doi: 10.1002/(SICI)1097-0339(199608)15:2<121::AID-DC7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Papillo JL, Zarka MA, St John TL. Evaluation of thinprep Pap test in clinical practice. A seven- month, 16,314-case experience in Northern Vermont. Acta Cytol. 1998;42:2203–208. doi: 10.1159/000331547. [DOI] [PubMed] [Google Scholar]
- 42.Broadstock M. Liquid-based Cytology –An Alternative International View. Cytopathology. 2001;12:141–143. doi: 10.1046/j.1365-2303.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- 43.O’Malley DM, Munkarah AR, Morris RT, Deppe G, Malone JM, Gray N. Reasons for failure to seek cervical cancer screening in non-indigent population. Abstrat 835, 37th Annual Meeting of The American Society of Clinical Oncology; May 12–15th 2001; San Fransico, California. [Google Scholar]
- 44.Herzog TJ. Update On Cervical Cancer: Where Do We Go From Here?. 37th Annual meeting of The American Society Of Clinical Oncology; 2002; conference coverage. [Google Scholar]
- 45.Clavel C, Masure M, Bory J-P, Mangeonjean C, Nazeyrollas P, Gabriel R, Quereux C, Birembaut P. Human Papillomavirus testing in primary screening For The detection of High Grade Cervical Lesions: a Study Of 7932 women. British J of Cancer. 2001;89(12):1616–1623. doi: 10.1054/bjoc.2001.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffman M, Herero R, Hildesheim A, herman ME, Bratti M, Wacholder S, Alfaro M, Hutchinson M, Morales J, Greenberg MD, Lorincz AT. HPV DNA Testing in Cervical Cancer Screening. Results From Women I a High-Risk Province Of Costa Rica. JAMA. 2000;283(1):87–93. doi: 10.1001/jama.283.1.87. [DOI] [PubMed] [Google Scholar]
- 47.Takeda M, Sakuragi N, Okamoto K, Todo Y, minobe S-I, Nomura E, Negeshi H, Oikawa M, Yammoto R. Fujimoto S Preoperative Serum SCC, Ca 125, and CA19-9 Levels And Lymph Node Status In Squamous Cell Carcinoma Of The Uterine Cervix. Acta Obstet Gynecol Scan. 2002;81:451–457. doi: 10.1034/j.1600-0412.2002.810513.x. [DOI] [PubMed] [Google Scholar]
- 48.Esajas MD, Duk JM, de Brujin HWA, Aalders JG, Willemse PHB, Sluiter W, Pras B, Hoor KT, Hollema H, van der Zee A. Clinical Value Of Routine Serum Squamous Cell Carcinoma Antigen Follow-Up of patients With Early-Stage Cervical Cancer. J Clin Oncology. 2001;19:3960–3966. doi: 10.1200/JCO.2001.19.19.3960. [DOI] [PubMed] [Google Scholar]
- 49.Stoler MH. HPV for Cervical Cancer Screening: Is the Era of The Molecular Pap Smear Upon Us? Journal of Histochemistry and Cytochemsitry. 2001;49(9):1197–1198. doi: 10.1177/002215540104900918. [DOI] [PubMed] [Google Scholar]
- 50.Nor Hayati Othman. 2003. proposal for IRPA PR research. Cancer of the cervix — Understanding its pathogenesis, developing its screening, preventive and treatment strategy.
- 51.Higuchi KH, Nakano T, Tsuboi A, Suzuki Y, Ohno T, Oka K. Flowcytometric and Ki-67 immunohistochemical analysis of cell cycle distribution of cervical cancer during radiation therapy. Anticancer Res. 2001;21(4A):2511–8. [PubMed] [Google Scholar]
- 52.Chang CL, Wong SY, Wu CC, Su TH, Wang KL, Chen HS, Yang YC. Microsatellite alterations in exfoliated cervical epithelia deoxyribonucleic acid as a marker for high grade dysplasia. Am J Obstet Gynecol. 2001;185:108–115. doi: 10.1067/mob.2001.114919. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa H, Sugano K, Fujii T, Kubushiro K, Tsukazaki K, Nozawa S. (Frequent detection of Human Papilloma viruses in cervical dysplasia by PCR single-strand DNA-conformational polymorphism analysis. Anticancer Res. 2002;3:1655–60. [PubMed] [Google Scholar]
- 53.Herbsleb M, Knudsen UB, Orntoft TF, Bichel P, Norild B, Knudsen A, Mogensen O. Telomerase Activity, Mib-1, PCNA, HPV 16 and p53 as Diagnostic Markers for Cervical Intraepithelial Neoplasia. APIMS. 2001;109(9):607–17. doi: 10.1034/j.1600-0463.2001.d01-182.x. [DOI] [PubMed] [Google Scholar]
- 54.Pirog EC, Baergen RN, Soslow RA, Tam D, DeMattia AE, Chen YT, Isacson C. Diagnostic accuracy of Cervical Low Grade squamous Intra epithelial Lesions Is Improved with Mib –1 Immunostaining. Am J Surg. 2002;26(1):70–5. doi: 10.1097/00000478-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Kailash U, Hedau S, Gopalkrishna V, Katiyar S, Das BC. A simple ‘paper smear’ method for dry collection, transport and storage of cervical cytological specimens for rapid screening of HPV infection by PCR. J Med Microbiol. 2002;51(7):606–10. doi: 10.1099/0022-1317-51-7-606. [DOI] [PubMed] [Google Scholar]
- 56.Nasiell K, Roger V, Nasiell M. Behaviour of mild cervical dysplasia during long term follow-up. Obstet Gynaecol. 1986;67:665–669. doi: 10.1097/00006250-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Carrasco D, Straten MV, Tyring SK. Prophylactic And Therapeutic Vacines For Genital Papillomavirus Infection. J Investig Dermatol Symp Proc. 2001;6(3):238–43. doi: 10.1046/j.0022-202x.2001.00036.x. [DOI] [PubMed] [Google Scholar]
- 58.Schiller JT, Hidesheim A. Developing HPV virus-like particle vaccines to prevent cervical cancer: a progress report. J Clin Virol. 2000 Oct;19(1–2):67–74. doi: 10.1016/s1386-6532(00)00091-3. [DOI] [PubMed] [Google Scholar]
- 59.Da Silva DM, Eiben GL, Ausch SC, Wakabayashi MT, Rudolf MP, Velders MP, Kast WM. Cervical Cancer Vacine: Emerging Concepts and Developments. J Of Cellular Physiology. 2001;186:169–182. doi: 10.1002/1097-4652(200102)186:2<169::AID-JCP1023>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 60.Harro CD, Pang S, Roden RRBS, Hildesheim A, Wang Z, Dilner J, Robinson R, Schiller JT, Lowy DR. A safety and immunogenecity trial of a Human Papilloma virus type 16 L1 virus-like particle vaccine healthy young adults human volunteers. 18th International Papilloma Virus Conference, Barcelona, Spain program and abstract book; 2000; abstract 362. [Google Scholar]
- 61.Maclean J, Passmore J, Burgers W, Marais DJ, Steyn L, Rose RC, Williamson A. Human Papillomavirus specific immunity induced following immunoziation with recombinant Mycobacterium bovis baciile calmette-guerin (BCG) expressing HPV L1 protein. 18th International Papillomavirus Conference, Barcelona Spain. Program and Abstract Book; 2000; Abstract 457. [Google Scholar]
- 62.Kawana K, Yasugi T, Kanda T, Kawana Y, Hirai Y, Yoshikawa H, Taketani Y. Neutralizing antibodies against omcogenic hman papillomavirus as a possible determinant of the fate of low grade cervical intra epithelial neoplasia. Biochem Biophys Res Commun. 2002;296(1):102–5. doi: 10.1016/s0006-291x(02)00843-4. [DOI] [PubMed] [Google Scholar]
- 63.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. Controlled Trial of a Human Papillomavirus Type 16 Vaccine. New Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 64.Giard RWM. False-Negative Rate of Cervical Cytology: Sense and Sensitivity. Diagnostic Cytopathology. 2001;25(5):75–277. doi: 10.1002/dc.2054. [DOI] [PubMed] [Google Scholar]