Abstract
The retinoblastoma gene was the first tumour suppressor gene identified that was altered not only in retinoblastomas but has been described in a wide variety of human neoplasms. The retinoblastoma gene encodes a nuclear phosphoprotein that in its hypophosphorylated state plays an important role in regulating the cell cycle, thus preventing from tumour formation. Expression of retinoblastoma gene protein product (pRB) was investigated in 118 formalin-fixed, paraffin-embedded cervical tissues by immunohistochemistry using commercially available antibody directed against RB protein. Ten normal ectocervical epithelium, 16 cervical intraepithelial neoplasia (CIN) I, 13 CIN II, 14 CIN III, 53 invasive squamous cell carcinoma, 11 adenocarcinoma and 1 small cell carcinoma were selected for this study. The proportions of pRB-positive cells as well as the extent of pRB expression in ectocervical squamous epithelium were assessed and compared among the lesions. The pRB expression was observed in 100% of normal ectocervical epithelium (n=10), 100% of CIN lesions (n=43) and 98.5% of invasive carcinoma of the uterine cervix (n=65) and were statistically significant when CIN or CIN/invasive were compared to normal cases (P < 0.01, P < 0.05 respectively). While in invasive squamous cell carcinoma (SCC), 81.8% (9/11) pRB-positive cells were found in much higher percentages in well differentiated SCC compared to 64.3% (18/28) of moderately differentiated cases and only 7.1% (1/14) of poorly differentiated SCC (P < 0.01, respectively). The results of this study suggest that loss of RB protein expression is rare in carcinoma of the uterine cervix and this protein may be important in the pathogenesis of cervical carcinoma.
Keywords: Uterine cervix, pRB, immunohistochemistry
Introduction
Cervical cancer is one of the leading causes of malignancy related-death among women worldwide (1). Both the incidence and the mortality rate have been dramatically reduced in the United State and other developed countries during the last several decades. This phenomenon has been contributed by the introduction of organised cervical cytological screening programme (2). The majority of invasive cancer of cervix is squamous cell in type, however, invasive adenocarcinoma is increasingly being detected (3).
Many epidemiological studies over the last 20 years have established a strong association between the high risk of human papillomavirus (HPV type 16 and 18) and cervical cancer, up to 95% of cases (4). However viral infection alone is not sufficient to initiate malignant transformation. Additional genetic changes must occur in order for malignant transformation to take place (5).
The retinoblastoma (RB) gene was the first tumour suppressor gene discovered and was originally identified as gene involved in hereditary retinoblastoma (6). Alteration in the RB gene has also been described in a variety of common epithelial and mesenchymal malignancies (7,8). Transcription of RB gene gives rise to mRNA that encodes a protein (pRB) that plays an important role in regulating the ability of cells to enter S phase, the period when DNA is synthesised. Loss of normal RB function may allow cells to proliferate in uncontrolled manner, which is an important feature of neoplastic cells (9).
In human cervical cancers, a limited number of studies had been conducted concerning allelic loss and mutations of the RB gene as well as inactivation of RB protein expression (10). On the protein level, previous analysis of pRB expression in cervical tissues ranging from normal to invasive carcinoma revealed a statistical difference in staining pattern of pRB between normal / reactive cervical biopsies and CIN lesions (p< 0.05) (11), while immunohistochemical analysis by another author showed no loss of pRB expression in all cases of cervical carcinoma studied (12).
The study was designed to determine pRB expression in a group of cervical tissues comprised of normal, premalignant (cervical intraepithelial neoplasia) and malignant (invasive carcinoma) lesions and to compare the different patterns of pRB expression in these lesions.
Materials and Methods
Tissues
One hundred and eighteen cases were obtained from the files of the Department of Pathology, Universiti Kebangsaan Malaysia and Kuala Lumpur Hospital. Paraffin blocks of normal and CIN lesions diagnosed in the Department of Pathology, Kuala Lumpur Hospital in 1999 and of invasive carcinoma diagnosed between 1992 and 1995 in the Department of Pathology UKM were retrieved. The respective Haematoxylin and Eosin (H&E) slides were also retrieved and reviewed. Of the patients, 45 were Malays, 45 were Chinese and 28 were Indians. The median age was 49 years (range: 39 to 76 years old).
The normal cervical tissues were taken from hysterectomy specimen done for removal of uterine leiomyoma. Neoplastic lesions of the cervix encompasses of cervical intraepithelial lesions and invasive carcinoma of cervix. Histologically, there were 10 cases of normal cervix, 16 of CIN I, 13 of CIN II and 14 of CIN III. Among the invasive carcinoma groups, there were 11 cases of well-differentiated squamous cell carcinoma, 28 of moderately differentiated squamous cell carcinoma, 14 of poorly differentiated squamous cell carcinoma, 11 of invasive adenocarcinoma and 1 of small cell carcinoma. Classification of the carcinoma is based on Modified World Health Organisation criteria (13). The microscopic grading is derived from the modification of the Broder method, which was originally described the degree of differentiation in squamous cell carcinoma of the lip (14).
pRB immunohistochemistry
Immunohistochemistry was performed using the monoclonal mouse anti-human Retinoblastoma gene product RB1 (DAKO M 7131) as primary antibody followed by labelled streptavidin biotin method (LSAB + kit, DAKO K 0690) to detect the primary antibody.
Three μm sections were cut from the formalin-fixed, paraffin-embedded tissue blocks. The sections were picked up on poly-L-Lysin coated glass slides, air-dried overnight and placed in a 60°C oven for 20 minutes. The slides were then deparaffinised by two changes of xylene followed by rehydration in a series of decreasing concentration of alcohol. The slides were then washed under running tap water.
Subsequently, the slides were placed in 3% hydrogen peroxide for 5 minutes at room temperature to block endogenous peroxidase activity. They were then washed again with running tap water. Tissue sections were then subjected to pre-treatment process using microwave for antigen retrieval. The slides were immersed in target retrieval solution (DAKO S1699) in microwave for 8 minutes at 600 Watt followed by 30 minutes at 200 Watt. The cool-down period was 20 minutes.
The slides were then washed under running tap water followed by three changes of TBS. Subsequently, the tissue sections were incubated with primary antibody at room temperature for one and half hour. The antibody dilution used was 1: 40.
After several washes with TBS, sufficient amount of biotinylated linking antibody was applied and the sections were incubated at room temperature for 15 minutes followed by washing in three changes of TBS. Then the Labelled Streptavidin Biotin complex (LSAB) was applied and the slides were again incubated at room temperature for 15 minutes and subsequently washed with three changes of TBS.
Localisation of pRB was visualised by incubating the tissues sections for 10 minutes with 3,3 diaminobenzidine tetrahydrochloride (DAB). The slides were finally washed under running tab water, counterstained with haematoxylin, dehydrated, cleared and mounted.
Positive control was a case of a well-differentiated adenocarcinoma of colon known to have pRB positivity and negative control was of the same case as above but with omission of the primary antibody. Admixed nonneoplastic inflammatory cells served as internal control.
The immunostaining for RB protein was considered positive if there was brown nuclear staining of the epithelial cells. The sections were examined by light microscopy. The pRB expression was assessed by estimating the percentage of cell showing nuclear reactivity and each sample was then assigned to one of the following groups: 0 (0%); +1 (1–24%); +2 (25–49%); +3 (50–74%) and +4 (75–100%)(15).
The tissues were examined for the extent of pRB expression within the squamous epithelium for normal and CIN cases (11). The squamous epithelium was divided into three layers, the basal third, middle third and upper third. Basal two-third category comprised of basal third and middle third whereas full thickness was comprised of all the three layers.
On the other hand, normal and aberrant pRB expressions were determined for invasive carcinoma cases. Normal pRB expression was reflected by nuclear staining in all areas of tumour tissue with cell-to-cell heterogeneity and variable staining intensity. pRB expression was considered aberrant if either the whole section or major focal areas within the section showed no nuclear staining with preserved reactivity in immediately adjacent non neoplastic tissue. Cytoplasmic reactivity was disregarded (16).
Statistical analysis
The results were analysed statistically by using Chi-square test with Yate’s correction at confidence level of at least 95%.
Results
A total of 118 cervical tissues were selected for the study. Of the 118 cases, 10 were histologically diagnosed as normal and 43 cases were diagnosed as CIN lesions (16 CIN I, 13 CIN II and 14 CIN III). The remaining 65 cases were of invasive carcinomas consisting of 53 cases of squamous cell carcinoma, 11 cases of adenocarcinoma and 1 of small cell carcinoma.
The pattern of pRB immunostaining and the histological diagnosis is summarised in Table 1 and their representative immunostainings are shown in Figures 1 to 3.
Table 1.
Expression of retinoblastoma protein (pRB) in normal cervical squamous epithelium, cervical intraepithelial neoplasia and invasive carcinoma of the uterine cervix.
| Histological type | Total | % of pRB immunostaining | ||||
|---|---|---|---|---|---|---|
| 0 (0%) | +1 (1–24%) | +2 (25–49%) | +3 (50–74%) | +4 (75–100%) | ||
| Normal | 0 | 0 | 0 | 8/10(80%) | 2/10(20%) | 0 |
| Cervical intraepithelial neoplasia (CIN) # | ||||||
| CIN I ** | 16 | 0 | 0 | 7/16(43.7%) | 9/16(56.3%) | 0 |
| CIN II ** | 13 | 0 | 0 | 3/13(23.3%) | 9/13(69.3%) | 1/13(7.7%) |
| CIN III ** | 14 | 0 | 0 | 1/14(7.1%) | 9/14(64.3%) | 4/14(28.6%) |
| Invasive carcinoma # | ||||||
| Squamous cell carcinoma | ||||||
| Well differentiated * | 11 | 0 | 0 | 2/11(18.2%) | 3/11(27.3%) | 6/11(54.5%) |
| Moderately differentiated ## | 28 | 0 | 1/28(3.6%) | 9/28(32.1%)) | 18/28(64.3%) | 0 |
| Poorly differentiated *## | 14 | 0 | 9/14(64.3%) | 4/14(28.6%) | 1/14(7.1%) | 0 |
| Adenocarcinoma | 11 | 0 | 0 | 4/11(36.4%) | 4/11(36.4%) | 3/11(27.2%) |
| Small cell carcinoma | 1 | 1/1 (100.0%) | 0 | 0 | 0 | 0 |
P>0.05
P<0.01
P<0.01
P>0.05
Figure 1.
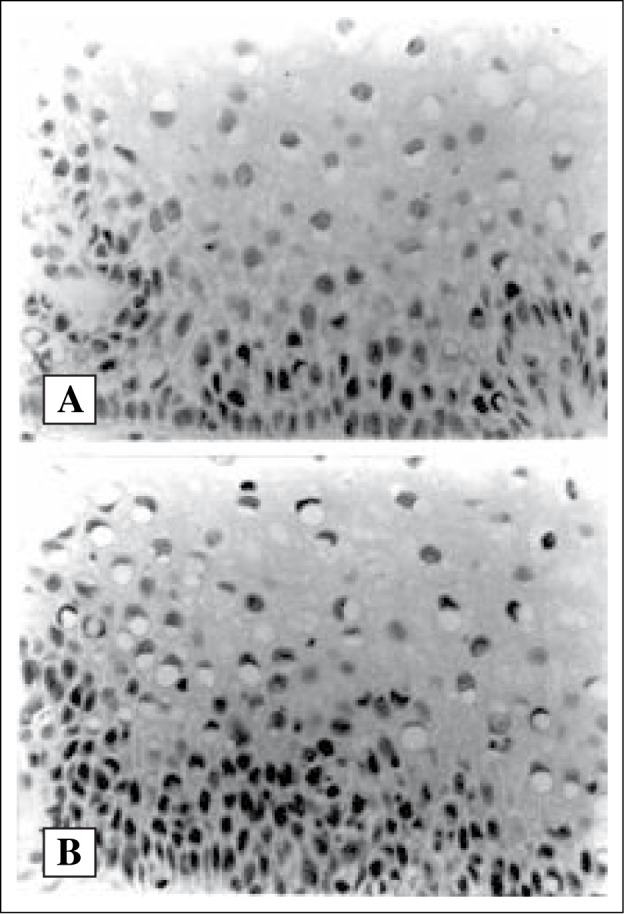
pRB immunoreactive cells are seen in the parabasal layer of normal squamous cells (A,X400) and confined to the basal third layer of in CIN I (B,X400)
Figure 3.
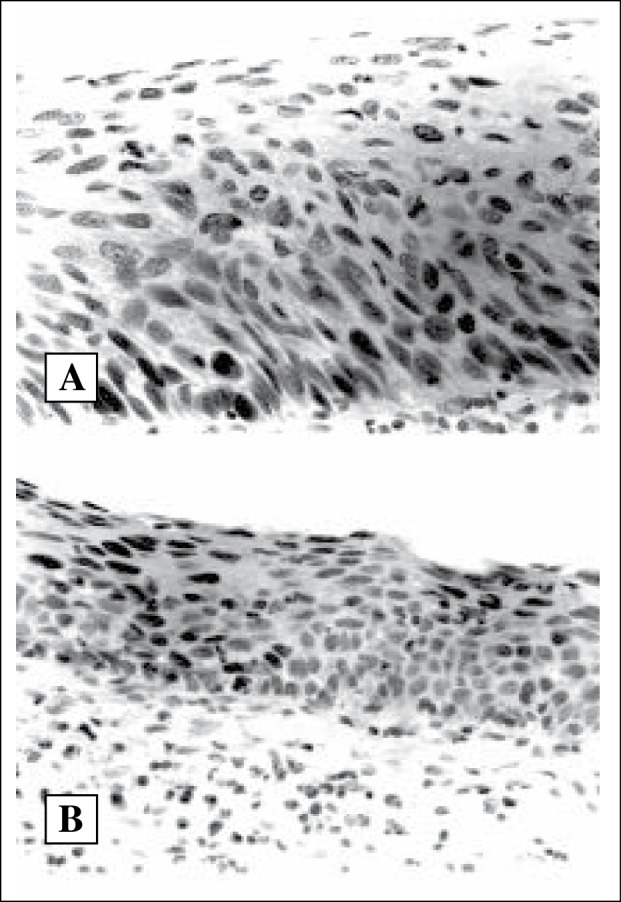
Invasive well differentiated squamous cell carcinoma of cervix showing 75–100% pRB immunoreactive cells (X400)
pRB expression in normal cervical epithelium
RB protein immunoreactivity was observed in all 10 cases. The majority (8/10) of the cases showed + 2 (25 – 49%) pRB immunoreactive cells. The pRB immunostaining was found in parabasal cell layers confined to the basal-third of the squamous cervical epithelium in 8 cases (80.0%, n=10) (Table 2). In these cases, the squamous epithelial cells in other cell layers were completely negative for pRB protein expression (Figure 1A). Two of 10 normal cases (20%) showed pRB-positive cells in basal two-third or full thickness of cervical epithelium.
Table 2.
Pattern of pRB immunostaining in normal cervical squamous epithelium and cervical intraepithelial neoplasia.
| Histological type | Total |
pRB epithelial staining
|
|
|---|---|---|---|
| Basal third | Basal two-third to full thickness | ||
| Normal* | 10 | 8/10(80.0%) | 2/10(20.0%) |
| Cervical intraepithelial Neoplasia (CIN)* | |||
| CIN I | 16 | 9/16(56.3%) | 7/16(43.7%) |
| CIN II | 13 | 1/13(7.7%) | 12/13(92.3%) |
| CIN III | 14 | 0 | 14/14(100%) |
Nuclear pRB immunoreactivity was also seen in normal endocervical glands and admixed with nonneoplastic inflammatory cells and endothelial cells that served as internal control.
pRB expression in cervical intraepithelial neoplasia (CIN)
The immunohistochemical results are summarised in Table 1 and representative pRB immunostaining are shown in Figures 1B, and 2. 56.3% of CIN I (9/16) cases showed +3 (50–74%) of pRB-positive cell while majority (69.3%) of CIN II (9/13) and 64.3% of CIN III (9/14) cases showed + 3 pRB immunoreactivity. One of 13 (7.7%) CIN II cases and 4 of 14 (28.6%) CIN III cases demonstrated +4 pRB cells.
Figure 2.
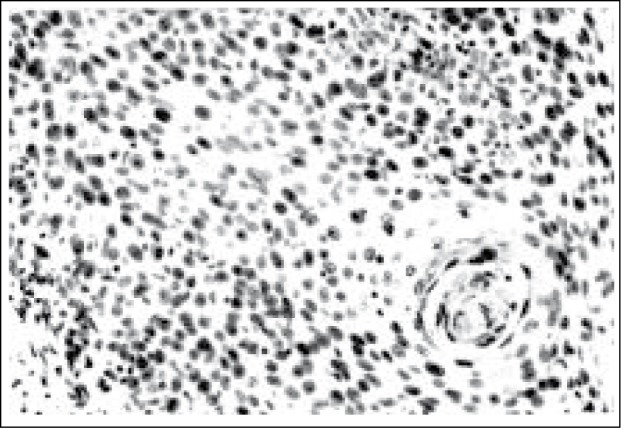
pRB immunoreactive cells involving the basal two-third of CIN II (A,X400) and entire of the thickness of CIN III lesions (B,X400)
The compartmental pRB immunostaining within cervical squamous epithelial of CIN lesions are summarised in Table 2. Basal third staining of pRB-positive cells were observed in 9/16 of CIN I (56.3%) cases. Only one (7.7%, n= 13) CIN II case showed basal third pRB staining. No CIN III cases showed pRB staining confined to the basal third layer. Thirty-three of 43 CIN cases (76.7%) showed pRB-positive cells in basal two-third or full thickness of cervical epithelium.
pRB expression in invasive carcinoma
64 cases (98.5%, n= 65) exhibited immunoreactivity for pRB (Figure 3). The percentage of pRB-positive cells is shown in Table 1.
Among the invasive squamous cell carcinoma, 9/11 well-differentiated cases (81.8%) showed higher percentage of pRB-positive cell (6 cases + 4 and 3 cases + 3). In contrast the poorly differentiated tumours revealed lower percentage of pRB positive cells (13/14) of the cases (4 cases + 2 and 9 cases + 1).
The adenocarcinoma group showed variable staining proportions ranging from 25 – 49% (+2) to 75 – 100% (+4).
Aberrant pRB expression was seen in the only one case of small cell carcinoma. In this case, total loss of pRB was observed.
Comparison studies between pRB expression in normal, cervical intraepithelial neoplasia and invasive cervical lesions.
Comparison of pRB expression in normal and CIN categories is illustrated in Table 1&2. 20.0% of normal cases (2/10) and majority (76.7%) of CIN cases (33/43) showed more than 50% pRB immunoreactivity (>= +3). Statistical significant difference was obtained when these two categories were compared for pRB expression (P < 0.01).
Comparison between pRB expressions in different grades of cervical epithelial lesions is shown in Table 1. There were no significant differences of pRB expression between CIN I and CIN II, CIN I and CIN III, CIN II and CIN III and between of low grade squamous intraepithelial lesions (comprised of CIN I) and high grade squamous epithelial lesions (comprised of CIN II and CIN III) for pRB expression (P > 0.05).
Comparison of pRB immunostaining between normal and neoplastic cervical lesions is shown in Table 3. Two of ten of normal (20%) and 67 of 108 neoplastic cases (62.0%) showed more than 50% pRB positive cell (>= +3). There was a statistically significant difference for pRB expression between these categories (P < 0.05).
Table 3.
Comparison of pRB immunostaining between normal and neoplastic cervical lesions
P<0.05
Comparison of pRB immunostaining between CIN cases and carcinoma of cervix is shown in Table 1. 32 of 43 CIN cases (74.4%) and 35 of 65 invasive carcinoma cases (53.8%) showed more than 50% pRB-positive cells (≥ + 3). There was no statistical significant difference between these two categories in relation to pRB expression (P > 0.05).
Comparison between pRB expressions in tumour differentiation of squamous cell carcinoma (SCC) is illustrated in Table 1. There was significant difference in pRB immunoreactivity between well-differentiated and poorly differentiated SCC and between moderately differentiated and poorly differentiated SCC (P < 0.01). However, comparison between well and moderately differentiated SCC groups did not show any statistical significant correlation (P > 0.05). Twenty-eight of 53 squamous cell carcinoma cases (52.8%) and 7 of 11 adenocarcinoma cases (63.6%) showed more than 50% pRB expression (Table 1).
Discussion
Alterations of tumour suppression genes and their role in the process of carcinogenesis have been extensively described (6,17, 18, 19, 20, 21,22). The genomic changes of 13 q and 17 p regions, where the retinoblastoma (RB) gene and p53 tumour suppressor gene reside, have been reported (10,19, 20,23, 24).
The protein product of the RB gene (pRB) is a nuclear phosphoprotein that plays an important role in regulating the cell cycle (9). Therefore loss of normal RB function may allow cells to proliferate in uncontrolled manner, not only to initiate event in tumourigenesis, but also as a step associated with malignant progression and progressive outcome (8, 25). Mutations and deletion of the RB gene are found in retinoblastoma (6, 22) and loss of RB function has been described in a variety of human malignancies (7, 8,19,26). In selected neoplasms, it has been reported that alteration of RB gene is associated with poor prognosis (26,27).
The RB gene is large and contains 27 exons, therefore genetic analysis of this gene at DNA level is technically challenging. The availability of commercially prepared monoclonal antibodies against pRB has made its immunohistochemical detection in cell lines, frozen sample and archival tissue, be feasible (16).
The majority of RB mutations lead to absence of RB mRNA and its protein product. A complete absence of nuclear reactivity in all areas of tumour associated with a positive internal control has shown a strong indication of underlying RB mutations (16). Production of truncated protein product or full-length mutant protein product has been reported in association with certain RB gene mutations. In this case, immunohistochemical analysis may not be able to distinguish mutant pRB from wild-type pRB (28). However, properly controlled pRB staining with presence of positive nonneoplastic cell as internal control, the immunoanalysis of RB protein generally provides an accurate reflection of RB status (29).
Expression of RB protein product (pRB) in normal ectocervical squamous epithelium and normal oesophageal mucosa (78% and 100% respectively) was reported in previous studies (11,15). These authors showed the expression was mainly observed in the basal third layer of the normal ectocervical epithelium (90%, 19/21) and normal oesophageal mucosa (100%, 10/10) with predilection at the parabasal layers. This study also showed similar findings with 100% pRB expression which was limited to the basal third layer in 80% (8/10) of neoplastic cases. The pRB immunoexpression was restricted at the parabasal region in these 8 cases (8/10) while the remaining 2 cases (2/10) showed extension up to the middle third of the epithelial thickness.
Our study, pRB immunoreactivity was found in all (100 %, 43/43) cervical intraepithelial lesions as compared to previous analysis in which 34 out of 36 (94.4%) CIN lesions (17 CIN I and 17 CIN II-III) showed positive pRB immunoreactivity (11). Higher proportions of pRB positive cells were observed in these premalignant lesions as compared to normal cervical epithelium (Table 1) and this comparison was statistically significant (p < 0.01) in contrast to the previous report (11). When the neoplastic group (CIN and invasive carcinoma) was compared with normal group for pRB expression there was significantly higher percentage of pRB expression in the former (P < 0.05) and the findings were consistent with report previously mentioned (11).
Increased pRB expression in premalignant and malignant lesions in general may be due to an increase proportions of proliferating cells (11,15). This is supported by fact that hyperphosphorylated pRB is increased during G2/M phases (9).
The pRB immunoreactive tumour cells were found in 64 out of 65 (98.5%) cases of invasive cervical carcinomas in this study. One study has reported pRB expression in all 74 (100%) primary carcinomas of cervix analyzed (12), while another study showed pRB expression in 43/50 (86%) (8).
The pRB expression in comparisons to grades of tumour differentiation is shown in Table 1. There was a significant difference of pRB expression between well-differentiated and poorly differentiated squamous cell carcinoma (P < 0.01). Similarly, the statistical difference was achieved when comparing moderately differentiated with poorly differentiated squamous cell carcinoma (P < 0.01). In general, the pRB over expression, i.e. higher percentage of pRB distribution was noted in well differentiated tumours as compared to the poorly differentiated ones.
Aggressive cancer is associated with loss or reduction in pRB expression. We found 6.5% of invasive cancers (1 small cell carcinoma and 9 poorly differentiated squamous cell carcinoma) in our series had low or loss (negative or +1) pRB expression. Loss or reduction of pRB expression and its association with aggressive behaviour of breast cancer was studied by Ceccarelli et al who found 8.5% of invasive breast carcinoma (7).
Loss of pRB function has been described as initiating factor in the development of retinoblastoma and several other common malignancies (6,7,19,22,23). The pRB expression was observed in both slow and fast growing tumours. In this study, the only one case of small cell carcinoma showed loss of pRB expression. There was no aberrant pRB expression in all cases of squamous cell carcinoma and adenocarcinoma. It has been suggested that in rapidly growing tumours, the rate of pRB mutation increases resulting in loss of pRB expression (7).
The infrequent aberrant pRB expression found in this study is in keeping with a few previous studies in other malignancies (15,29,30,31). It has been proposed that mutations in RB gene play a limited role in these tumours and may represent the late event in carcinogenesis. It has been stressed that the presence of distinct pRB nuclear staining of admixed non-neoplastic elements in the tumour is required to exclude non specific loss of pRB reactivity due to necrosis, overfixation, inappropriate processing of the tissue or incomplete staining of the section (16,30).
In this study, the majority of the pRB positive cases showed a heterogeneous staining pattern. The intensity of the nuclear staining varied from cells to cells with variable staining proportions of cells having an unstained nucleus. Similar findings have been observed and reported in earlier studies of pRB expressions (15,16,29). This variation in staining probably resulted from asynchronous progression of the cells through the cell cycle.
Conclusion
This study showed overexpression of retinoblastoma protein product (pRB) in majority of premalignant and malignant lesions of the uterine cervix as compared with normal cervical squamous epithelium. Statistical analysis showed significant differences in pRB expression between normal and CIN lesions. Proportions of pRB immunoreactivity are higher in better differentiated cancers, lower in carcinomas and complete loss in undifferentiated carcinomas.
Acknowledgments
This study was supported by Universiti Kebangsaan Malaysia (Grant No. F 5/99)
The authors thanked Encik Rosli Nasir of Fakulti Perubatan, Universiti Kebangsaan Malaysia for preparing the photographs.
References
- 1.Scheffner M, Romanezuk H, Munger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus protein in human pathogenic papilloma - viruses. In: zur Hausen H, editor. Current topics in microbiology and immunology. Vol. 186. Berlin: Springer-Verlag; 1994. p. 83. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Young JL, Jr, Brinton LA, Fraumeni JF., Jr Recent trends in cervix uteri cancer. Cancer. 1989;64:2184–2190. doi: 10.1002/1097-0142(19891115)64:10<2184::aid-cncr2820641034>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States - A 24 years population ñ base study. Gynecol Oncol. 2000;78(2):97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 4.Young LS, Bevan IS, Johnson MA, Blomfield PI, Bromidge T, Maitland NJ, Woodman CBJ. The polymerase chain reaction. A new epidemiologic tool for investigating cervical human papilloma virus infection. Br Med J. 1989;298:14–18. doi: 10.1136/bmj.298.6665.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- 6.Knudson AG. Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccarelli C, Santini D, Cheico P, Taffurelli M, Gamberini M, Pileri SA, Marrano D. Retinoblastoma (RB1) gene product expression in breast carcinoma. Correlation with Kiñ 67 growth fraction and biopathological profile. J Clin Pathol. 1998;51:818–824. doi: 10.1136/jcp.51.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetty R, Bramdev A, Aguirre-Arteta A, Pegoraro RJ, Sataar N. Relationship between retinoblastoma and p53 proteins in human papilloma viruses 16/18 positive and negative cancers of the uterine cervix. J Clin Pathol. 1997;50:413–416. doi: 10.1136/jcp.50.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodrich DW, Wang NP, Qian YW, Lee EYHP, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67:293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- 10.Kim JW, Lee CG, Han SM, Kim KS, Kim JO, Lee JM, Kim IK, Namkoong SE. Loss of heterozygosity of the retinoblastoma and p53 genes in primary cervical carcinoma with human papilloma virus infection. Gynecol Oncol. 1997;67:215–221. doi: 10.1006/gyno.1997.4847. [DOI] [PubMed] [Google Scholar]
- 11.Amortegui AJ, Meyer MP, Elborne VL, Amin RM. P53, retinoblastoma gene product and cyclin protein expression in human papillomavirus DNA-positive cervical intraepithelial neoplasia and invasive cancer. Mod Pathol. 1995;8(9):907–912. [PubMed] [Google Scholar]
- 12.Skomedal H, Kristensen GB, Lie AK, Holm R. Aberrant expression of the cell cycle associated protein TP53, MDM2, p21, p27, cdk 4, cyclin D1, RB and EGFR in cervical carcinomas. Gynecol Oncol. 1999;73(2):223–8. doi: 10.1006/gyno.1999.5346. [DOI] [PubMed] [Google Scholar]
- 13.Scully RE, Poulson H, Sobin LH, World Health Organisation . Histologic typing of female genital tract tumour. Berlin: Springer-Verlag; 1994. International histological classification of tumours; pp. 4–9. [Google Scholar]
- 14.Broders AC. Squamous cell epithelioma of the lip: A study of five hundred and thirty seven cases. JAMA. 1920;74:656–664. [Google Scholar]
- 15.zur Hausen A, Sarbia M, Heep H, Willers R, Gabbert HE. Retinoblastoma-protein (pRB) expression and prognosis in squamous cell carcinoma of the oesophagus. Int J Cancer (Pred. Oncol.) 1999;84:618–622. doi: 10.1002/(sici)1097-0215(19991222)84:6<618::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Geradts J, Hu SX, Lincoln CE, Benedict WF, Xu HJ. Aberrant RB gene expression in routinely processed, archival tumour tissues determined by three different anti RB antibodies. Int J. Cancer. 1994;58:161–167. doi: 10.1002/ijc.2910580203. [DOI] [PubMed] [Google Scholar]
- 17.Diamandis EP. Clinical applications of tumour suppressor genes and oncogenes in cancer. Clinical Chimica Acta. 1997;257:157–180. doi: 10.1016/s0009-8981(96)06442-x. [DOI] [PubMed] [Google Scholar]
- 18.Fearon ER. Human cancer syndrome: Clues to the origin and nature of cancer. Science. 1997;278:1043–1050. doi: 10.1126/science.278.5340.1043. [DOI] [PubMed] [Google Scholar]
- 19.Friend SH, Bernards R, Rogelj S, Weinberg R, Rappaport JM, Albert DM, Dryja TB. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 20.Knudson AG. Antioncogenes and human cancer. Proc Natl Acad Sci USA. 1993;90:10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arends MJ, Buckley CH, Wells M. Aetiology, pathogenesis and pathology of cervical neoplasia. J. Clin Pathol. 1998;51:96–103. doi: 10.1136/jcp.51.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphree AL, Benedict WF. Retinoblastoma: Clues to Human Oncogenesis. Science. 1984;223:1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- 23.Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venter DJ, Bevan KL, Ludwig RL. Retinoblastoma gene deletions in human glioblastoma. Oncogene. 1991;6:445–448. [PubMed] [Google Scholar]
- 25.Locker J. Tumour suppressor gene and the practice of surgical pathology. Hum Pathol. 1995;26(4):359–361. doi: 10.1016/0046-8177(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 26.Cordon-Cardo C, Wartinger D, Petrylak D, Dalbagni G, Fair WR, Fusk Z, Reuter VE. Altered expression of the retinoblastoma gene product : prognostic indicator in bladder cancer. J. Cancer Inst. 1992;84:1251–1256. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- 27.Xu HJ, Quilan DC, Davidson AG, Xu SX, Summers CK, Li J, Benedict WF. Altered retinoblastoma protein expression and prognosis in early-stage of non-small-cell lung carcinoma. J. Natl Cancer Inst. 1994;6:695–699. doi: 10.1093/jnci/86.9.695. [DOI] [PubMed] [Google Scholar]
- 28.Geradts J, Kratzke RA, Crush-Stanton S, Wen SF, Lincoln CE. Wild-type and mutant retinoblastoma protein in paraffin sections. Mod Pathol. 1996;9(3):339–347. [PubMed] [Google Scholar]
- 29.Niemann TH, Yilmaz AG, McGaughy VR, Vaccarello L. Retinoblastoma protein expression in endometrial hyperplasia and carcinoma. Gynecol Oncol. 1997;65:232–236. doi: 10.1006/gyno.1997.4664. [DOI] [PubMed] [Google Scholar]
- 30.Geradts J, Andriko JW, Abbondanzo SL. Loss of tumour suppressor gene expression in high-grade but not low-grade Non-Hogkinís Lymphomas. Am J. Clin. Pathol. 1998;109:669–674. doi: 10.1093/ajcp/109.6.669. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RR, Linnoila RI, Geradts J, Teneriello MG, Nash JD, Park RC, Birrer MJ. Abnormal expression of the retinoblastoma gene in ovarian neoplasms and correlation to p53 and k-ras mutations. Gynecol Oncol. 1995;58:307–311. doi: 10.1006/gyno.1995.1235. [DOI] [PubMed] [Google Scholar]


