Abstract
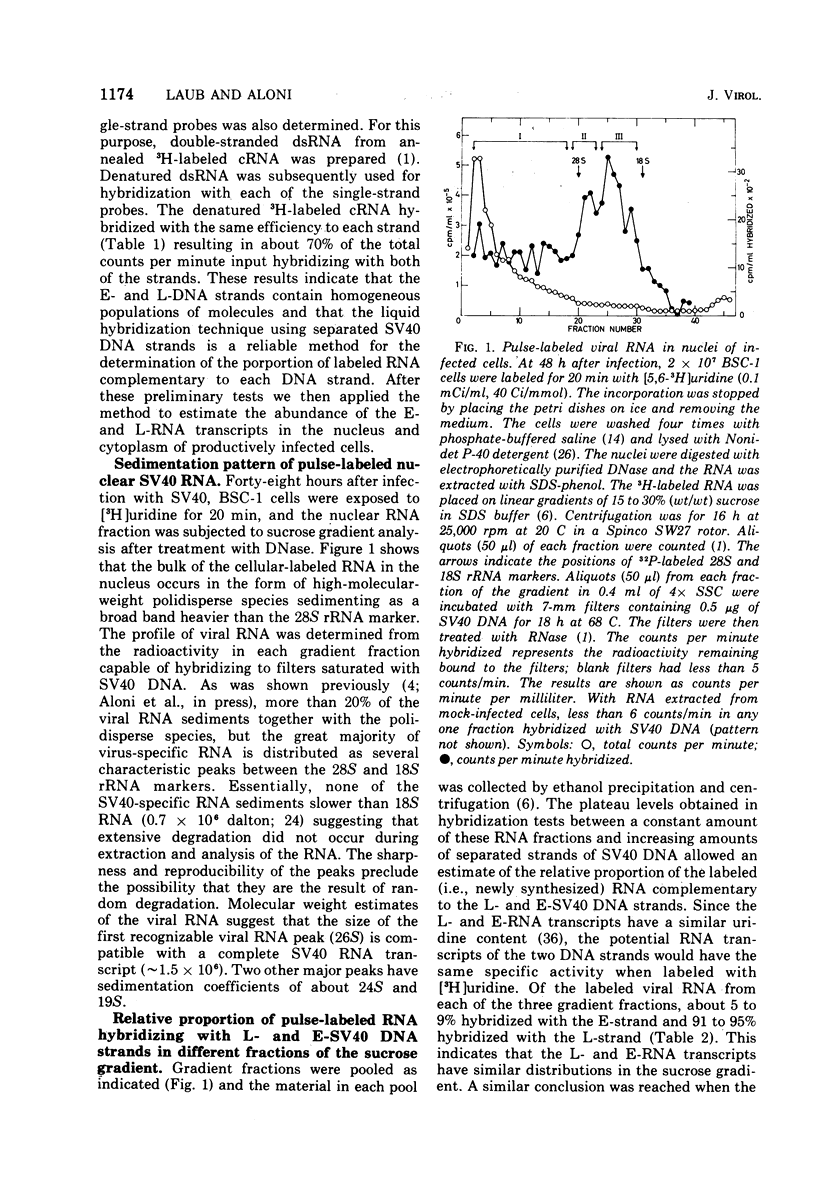
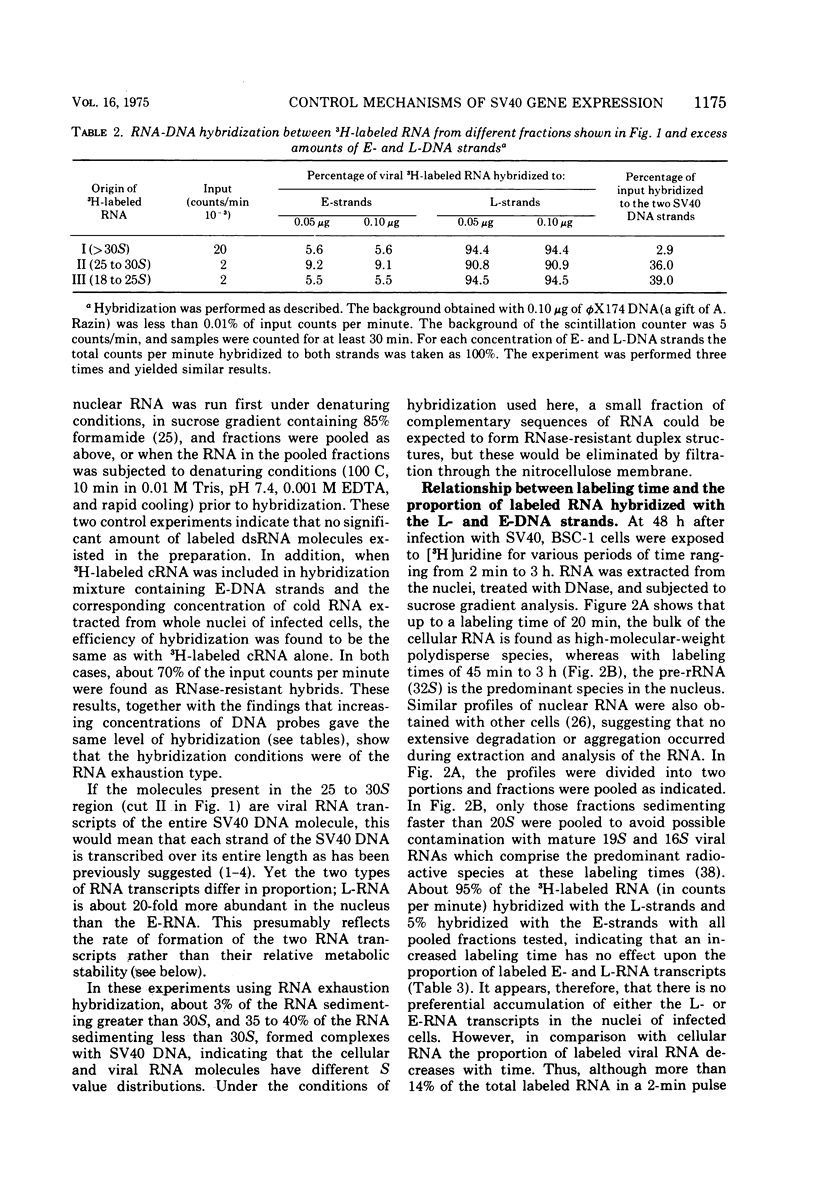
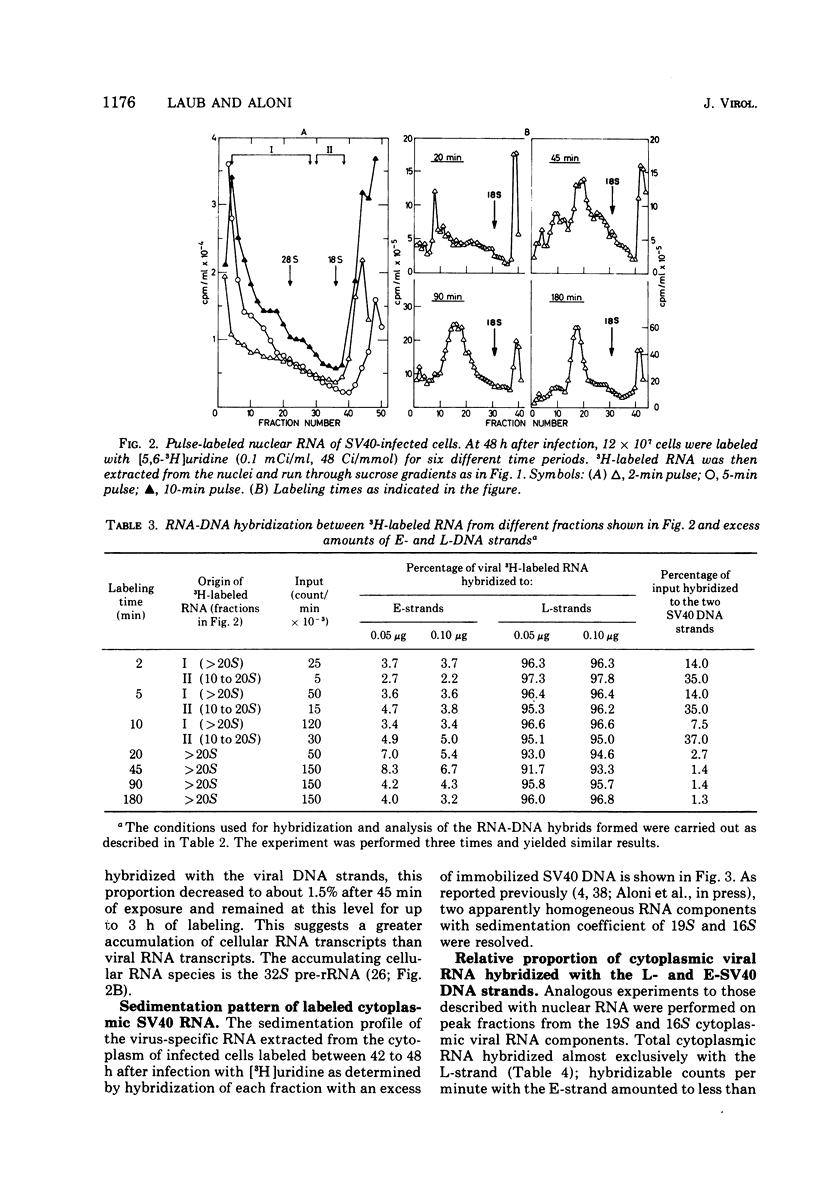
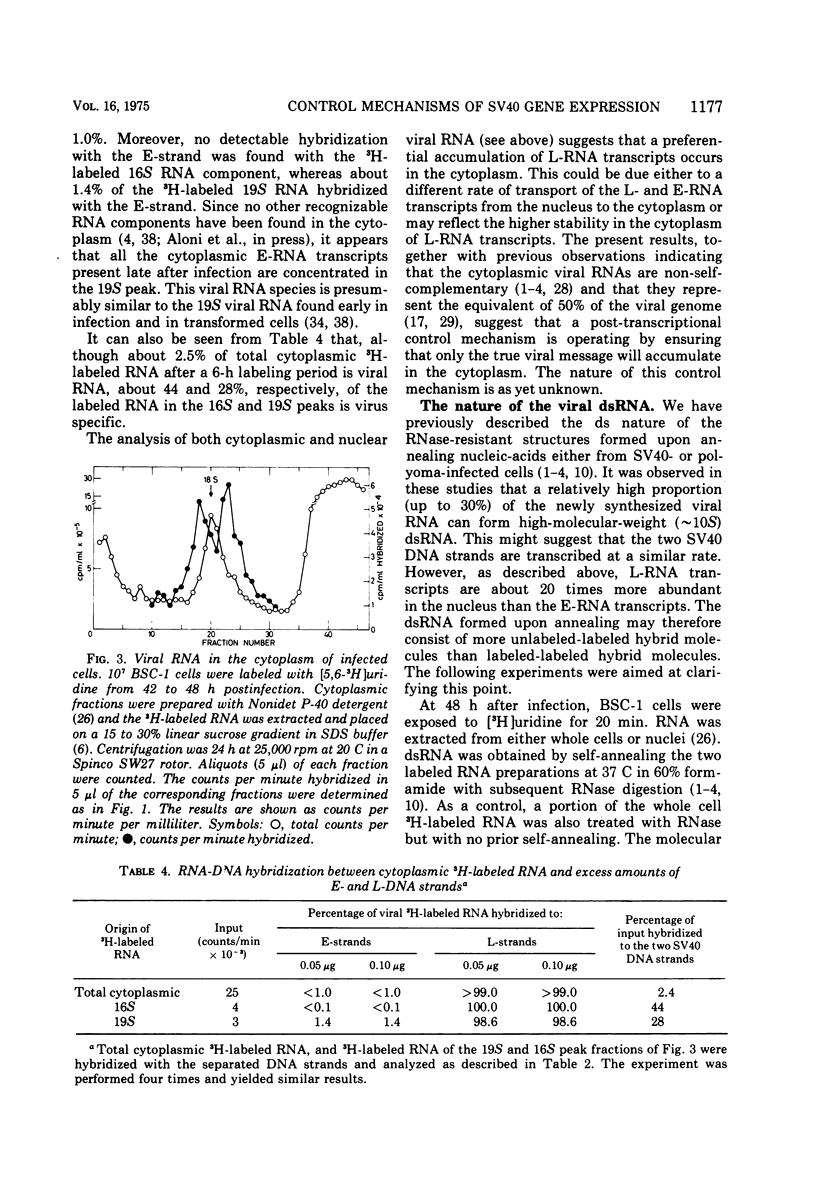
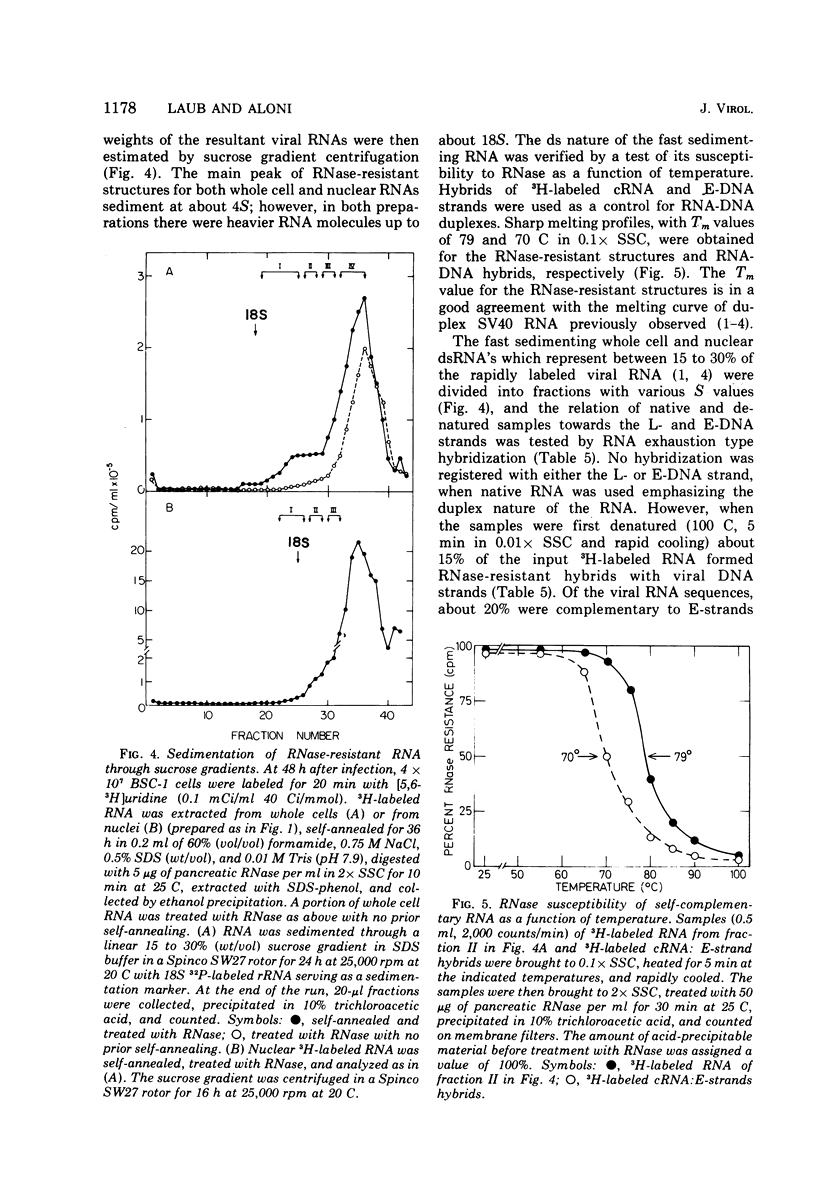
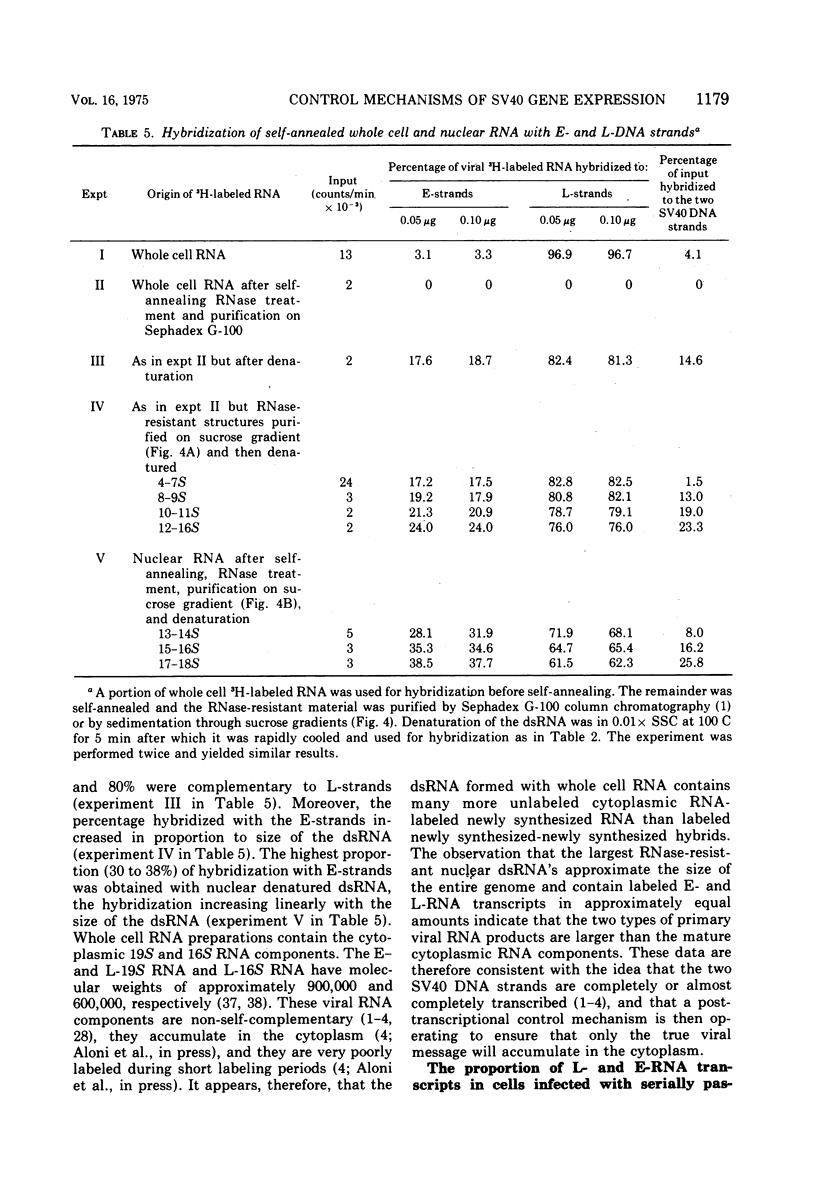
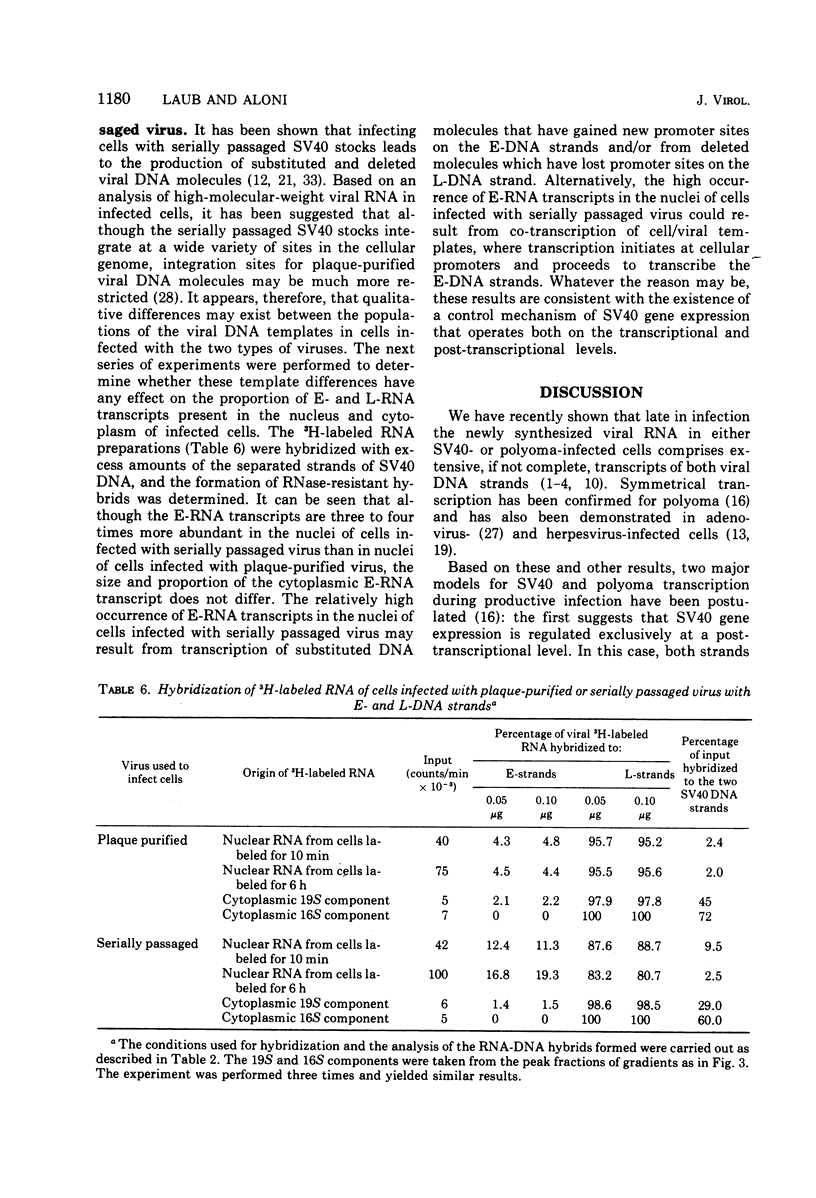
RNA "exhaustion type" hybridization was used to measure the complementarity of nuclear and cytoplasmic viral RNA to the early (E) and late (L) simian virus 40 (SV40) DNA strands. This type of hybridization measures the amount of labeled RNA complementary to each of the two DNA strands, rather than the fraction of each SV40 DNA strand that is homologous to SV40 RNA. At 48 h after infection, about 5% of the nuclear newly synthesized viral RNA was complementary to the E-strand (- strand) and 95% was complementary to the L-strand (+ strand). This proportion was independent of the labeling time, indicating similar accumulation of the E- and L-RNA transcripts in the nucleus. The nuclear E- and L-viral RNA transcripts sedimented in a similar manner on sucrose gradients. Of the cytoplasmic viral RNA only about 1% was complementary to the E-strand, these molecules sedimenting at 19S, whereas 99% were complementary to the L-strand and sedimented at 19S and 16S. The abundance of E-RNA transcripts in nuclei of cells infected with serially passaged virus was about four times higher than that in nuclei of cells infected with plaque-purified virus; however, the size and proportion of the corresponding cytoplasmic E- and L-RNA transcripts was independent of the type of virus used to infect the cells. According to these results at least two control mechanisms regulate viral gene expression in productively infected cells, one operates at the trnascriptional level and the second at the post-transcriptional level.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. II. Evidence for complete transcription of mitochondrial DNA. J Mol Biol. 1971 Jan 28;55(2):251–267. doi: 10.1016/0022-2836(71)90195-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. XI. Isolation and characterization of transcription complexes of mitochondrial DNA. J Mol Biol. 1972 Sep 28;70(2):363–373. doi: 10.1016/0022-2836(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. XII. Relationship between mitochondrial fast-sedimenting RNA components and ribosomal and 4 s RNA. J Mol Biol. 1972 Sep 28;70(2):375–381. doi: 10.1016/0022-2836(72)90546-3. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1757–1761. doi: 10.1073/pnas.68.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y. Biogenesis and characterization of SV40 and polyoma RNAs in productively infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):165–178. doi: 10.1101/sqb.1974.039.01.023. [DOI] [PubMed] [Google Scholar]
- Aloni Y. Extensive symmetrical transcription of Simian Virus 40 DNA in virus-yielding cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2404–2409. doi: 10.1073/pnas.69.9.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Locker H. Symmetrical in vivo transcription of polyoma DNA and the separation of self-complementary viral and cell RNA. Virology. 1973 Aug;54(2):495–505. doi: 10.1016/0042-6822(73)90159-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y. Methylated SV40 mRNAs. FEBS Lett. 1975 Jul 1;54(3):363–367. doi: 10.1016/0014-5793(75)80940-9. [DOI] [PubMed] [Google Scholar]
- Aloni Y. Poly A and symmetrical transcription of SV40 DNA. Nat New Biol. 1973 May 2;243(122):2–6. [PubMed] [Google Scholar]
- Aloni Y. Symmetrical transcription in animal cells and viruses. Adv Exp Med Biol. 1974 Jun;44(1):45–70. doi: 10.1007/978-1-4684-3246-6_6. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- Ben Zeev A., Becker Y. Symmetrical transcription of herpes simplex virus DNA in infected BSC-1 cells. Nature. 1975 Apr 24;254(5502):719–722. doi: 10.1038/254719a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Roizman B. RNA synthesis in cells infected with herpes simplex virus. IX. Evidence for accumulation of abundant symmetric transcripts in nuclei. J Virol. 1975 Jan;15(1):36–40. doi: 10.1128/jvi.15.1.36-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H., Schäfer K. P. Mitochondrial RNA polymerase from Neurospora crassa. Nat New Biol. 1971 Jun 30;231(26):265–269. doi: 10.1038/newbio231265a0. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Lindstrom D. M., Dulbecco R. Strand orientation of simian virus 40 transcription in productively infected cells. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1517–1520. doi: 10.1073/pnas.69.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Synthesis of complementary RNA sequences during productive adenovirus infection. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4887–4891. doi: 10.1073/pnas.71.12.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Winocour E. Covalently linked cell and SV40-specific sequences in an RNA from productively infected cells. Virology. 1972 Nov;50(2):558–566. doi: 10.1016/0042-6822(72)90407-2. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sugden B., Keller W., Sharp P. A. Transcription of simian virus 40. 3. Mapping of "early" and "late" species of RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3711–3715. doi: 10.1073/pnas.70.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Keller W. Mammalian deoxyribonucleic acid-dependent ribonucleic acid polymerases. I. Purification and properties of an -amanitin-sensitive ribonucleic acid polymerase and stimulatory factors from HeLa and KB cells. J Biol Chem. 1973 Jun 10;248(11):3777–3788. [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Simian virus 40 transcription in productively infected and transformed cells. J Virol. 1974 Jun;13(6):1263–1273. doi: 10.1128/jvi.13.6.1263-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


