Abstract
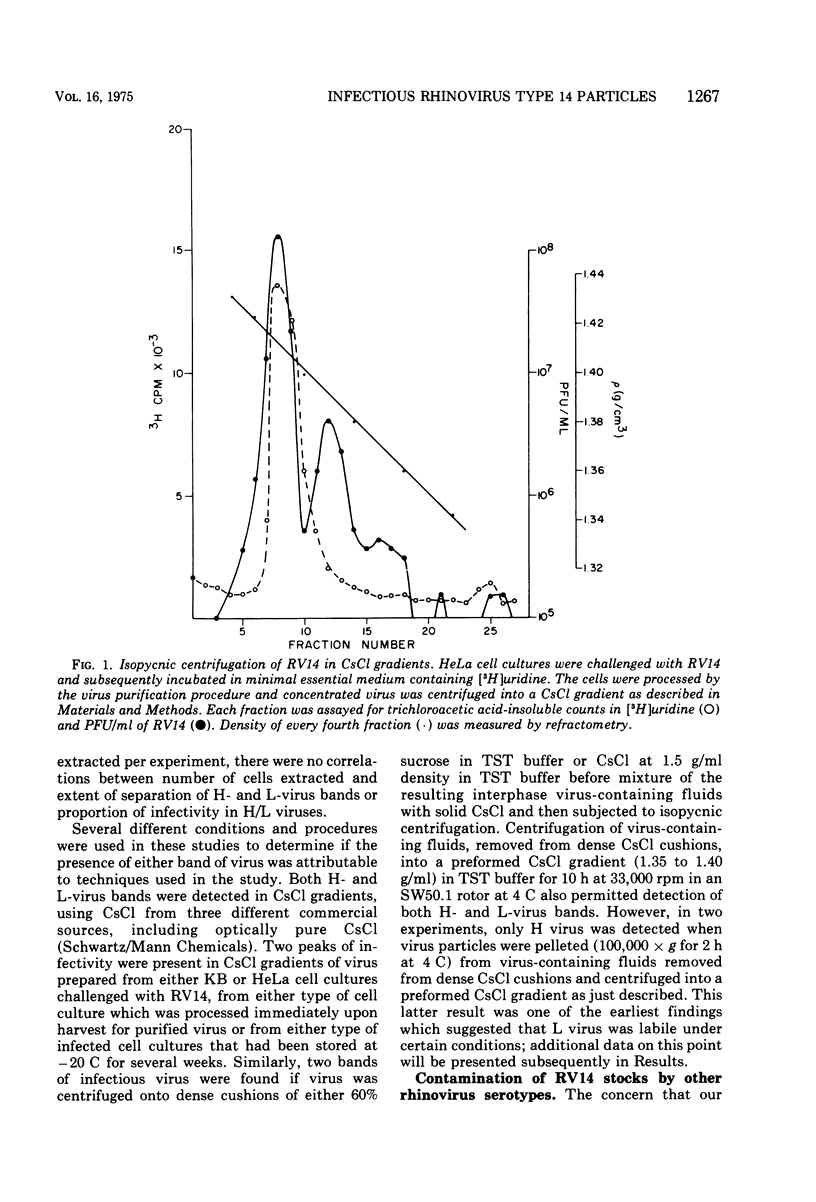
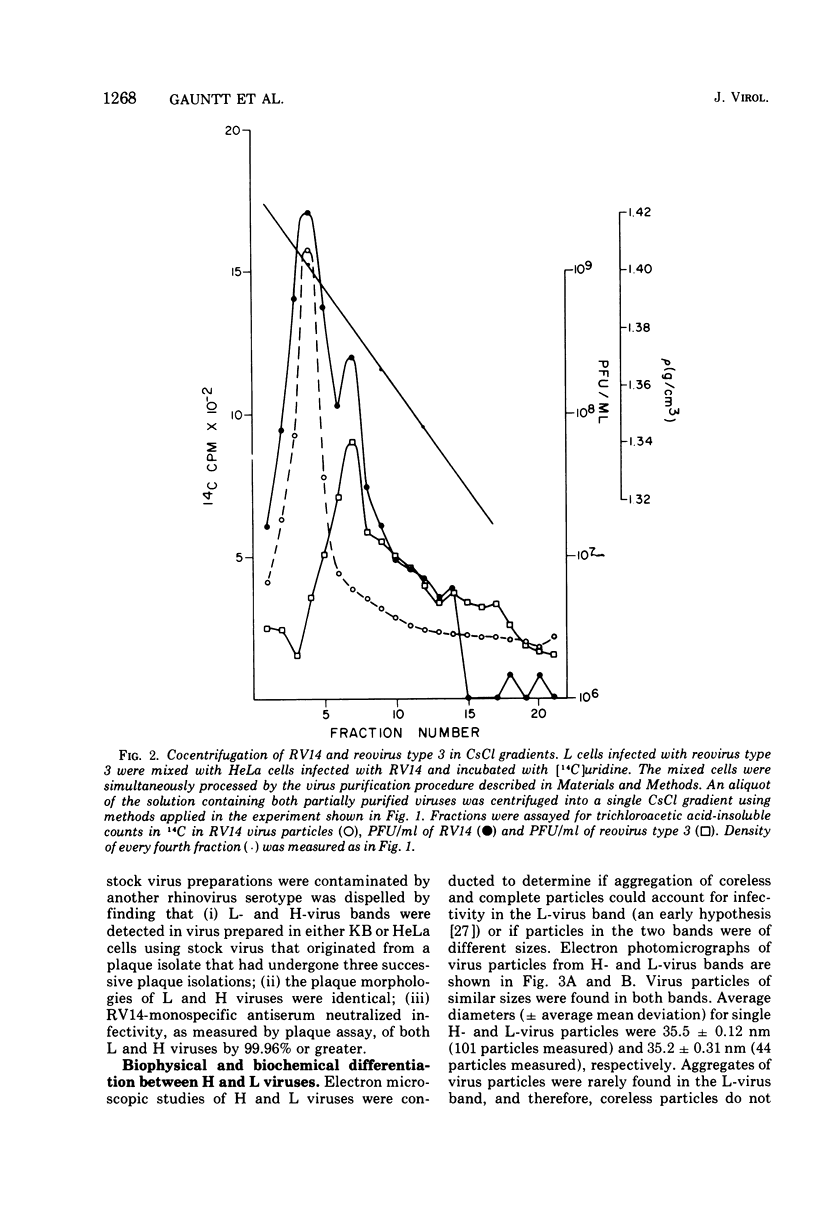
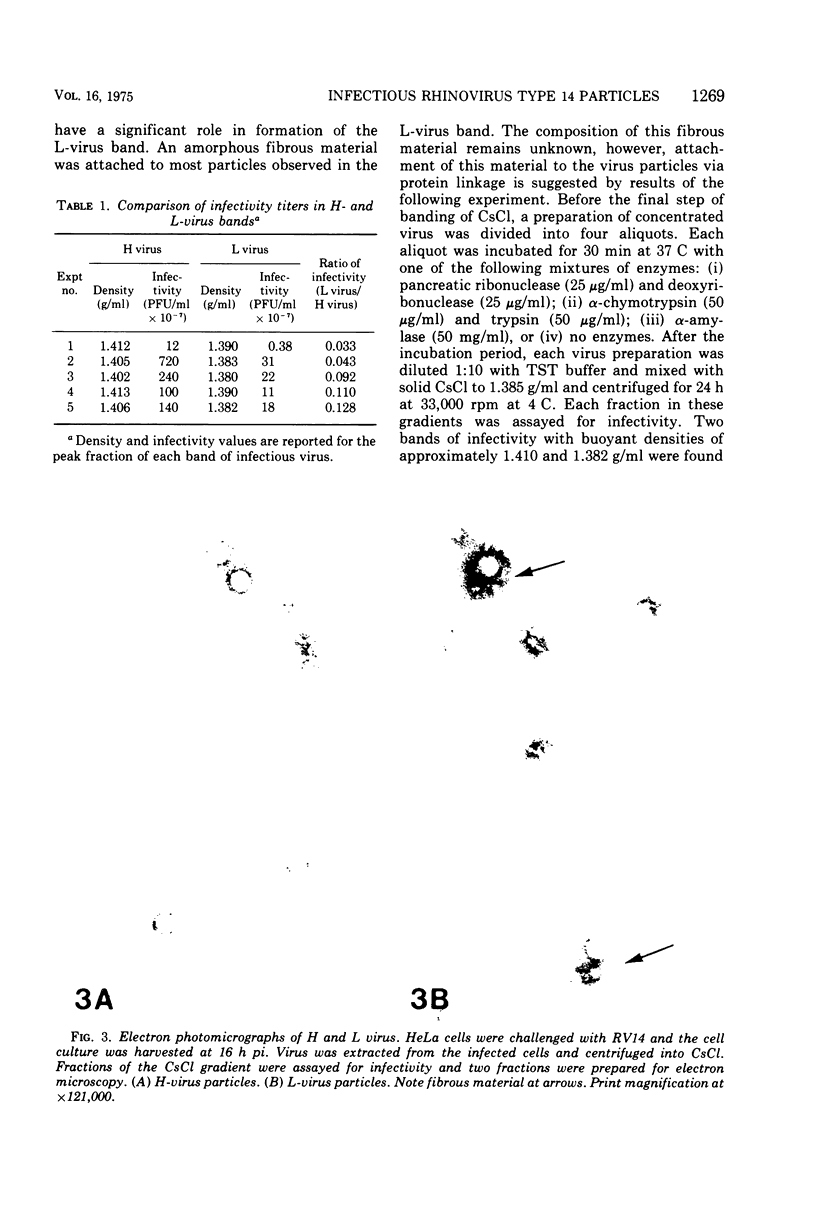
Isopycnic centrifugation of rhinovirus type 14 (RV14), purified from infected HeLa or KB cell cultures, into CsCl gradients resolved two bands of infectious virus particles with buoyant density values of 1.409 +/- 0.007 (H virus) and 1.386 +/- 0.004 (L virus) g/ml. Only H virus was detected by incorporation of radiolabeled uridine into viral RNA, and H virus accounted for the majority of infectivity in gradients. H and L virus could not be differentiated by plaque morphology, extent of neutralization by RV14-specific antiserum, or particle size. Electron microscope studies showed that most L-virus particles were associated with an amorphous material. Treatment of L virus with proteolytic enzymes or rebanding L virus in CsCl gradients resulted in recovery of the majority of infectivity as H virus. Virus purified from cell-free fluids from infected HeLa or KB cell cultures banded only as H virus. HeLa cell cultures challenged with purified H virus and harvested at 3 h postinoculation for virus purification yielded only infectious H virus. Both H and L viruses were detected in cell cultures that had been challenged with purified H virus and harvested at 12 h postinoculation. The data suggest that H virus represents progeny virus, whereas L virus represents sequestered infectious virus particles which become associated with an amorphous material and do not enter into viral replicative processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsa J., Grover J., Chapman J. D. Presence of nucleoside triphosphate phosphohydrolase activity in purified virions of reovirus. J Virol. 1970 Sep;6(3):295–302. doi: 10.1128/jvi.6.3.295-302.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Newman J. F., Stott E. J. Molecular weight of rhinovirus ribonucleic acid. J Gen Virol. 1970 Aug;8(2):145–148. doi: 10.1099/0022-1317-8-2-145. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Compans R. W. The formation of poliovirus particles in association with the RNA replication complexes. J Gen Virol. 1973 Oct;21:99–108. doi: 10.1099/0022-1317-21-1-99. [DOI] [PubMed] [Google Scholar]
- Chapple P. J., Harris W. J. Biophysical studies of a rhinovirus. Ultracentrifugation and electron microscopy. Nature. 1966 Feb 19;209(5025):790–792. doi: 10.1038/209790a0. [DOI] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dans P. E., Forsyth B. R., Chanock R. M. Density of infectious virus and complement-fixing antigens of two rhinovirus strains. J Bacteriol. 1966 Apr;91(4):1605–1611. doi: 10.1128/jb.91.4.1605-1611.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman M., Reynier M., Bucchini D., Girard M. Retarded growth of poliovirus in contact inhibited cells. J Gen Virol. 1974 Apr;23(1):73–82. doi: 10.1099/0022-1317-23-1-73. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J. Synthesis of ribonucleic acids in KB cells infected with rhinovirus type 14. J Gen Virol. 1973 Nov;21(2):253–267. doi: 10.1099/0022-1317-21-2-253. [DOI] [PubMed] [Google Scholar]
- Gauntt C., Sauck J., Carlson E. Growth of rhinovirus type 14. Brief report. Arch Gesamte Virusforsch. 1973;41(4):382–385. doi: 10.1007/BF01250211. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Richter W. R., Fenters J. D., Holper J. C. Use of zonal ultracentrifuge systems for biophysical studies of rhinoviruses. J Virol. 1968 Sep;2(9):937–943. doi: 10.1128/jvi.2.9.937-943.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodheart C. R. Non-transfer of RNA from parent to progeny of encephalomyocarditis virus. J Mol Biol. 1967 Jan 28;23(2):183–190. doi: 10.1016/s0022-2836(67)80025-1. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Doyle M., Perrault J., Kingsbury D. T., Etchison J. Proteinase activity in purified animal viruses. Biochem Biophys Res Commun. 1972 Jan 31;46(2):634–639. doi: 10.1016/s0006-291x(72)80187-6. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K. K., Halperen S. Structural polypeptides of three rhinoviruses. Biochem Biophys Res Commun. 1970 Oct 23;41(2):477–481. doi: 10.1016/0006-291x(70)90530-9. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Stasny J. T. Crystallization of human rhinovirus 1A. Virology. 1973 Oct;55(2):410–417. doi: 10.1016/0042-6822(73)90182-7. [DOI] [PubMed] [Google Scholar]
- Loh P. C., Oie H. K. Role of lysine in the replication of reovirus: I. Synthesis of complete and empty virions. J Virol. 1969 Dec;4(6):890–895. doi: 10.1128/jvi.4.6.890-895.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Noble-Harvey J. Comparison of in vitro and cell-mediated alteration of a human Rhinovirus and its inhibition by sodium dodecyl sulfate. J Virol. 1973 Oct;12(4):819–826. doi: 10.1128/jvi.12.4.819-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL B. THE FATE OF THE INOCULUM IN HELA CELLS INFECTED WITH POLIOVIRUS. Virology. 1965 Jan;25:152–154. doi: 10.1016/0042-6822(65)90264-3. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Rowlands D. J., Brown F. A physico-chemical sub-grouping of the mammalian picornaviruses. J Gen Virol. 1973 Feb;18(2):171–180. doi: 10.1099/0022-1317-18-2-171. [DOI] [PubMed] [Google Scholar]
- Noble J., Lonberg-Holm K. Interactions of components of human rhinovirus type 2 with Hela cells. Virology. 1973 Feb;51(2):270–278. doi: 10.1016/0042-6822(73)90427-3. [DOI] [PubMed] [Google Scholar]
- Prevec L., Watanabe Y., Gauntt C. J., Graham A. F. Transcription of the genomes of type 1 and type 3 reoviruses. J Virol. 1968 Apr;2(4):289–297. doi: 10.1128/jvi.2.4.289-297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. D., Mayor H. D. The effects of hydrogen ions on the morphology and infectivity of rhinovirions. Arch Gesamte Virusforsch. 1973;40(3):325–333. doi: 10.1007/BF01242552. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Sangar D. V., Brown F. Buoyant density of picornaviruses in caesium salts. J Gen Virol. 1971 Oct;13(1):141–152. doi: 10.1099/0022-1317-13-1-141. [DOI] [PubMed] [Google Scholar]
- Stoker D., Kiernat J., Gauntt C. Interferon induction by rhinoviruses and effect of interferon on rhinovirus yields in human cell lines. Proc Soc Exp Biol Med. 1973 May;143(1):23–27. doi: 10.3181/00379727-143-37245. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Killington R. A. Rhinoviruses. Annu Rev Microbiol. 1972;26:503–524. doi: 10.1146/annurev.mi.26.100172.002443. [DOI] [PubMed] [Google Scholar]
- Sutmoller P., McVicar J. W., Cottral G. E. The epizootiological importance of foot-and-mouth disease carriers. I. Experimentally produced foot-and-mouth disease carriers in susceptible and immune cattle. Arch Gesamte Virusforsch. 1968;23(3):227–235. doi: 10.1007/BF01241895. [DOI] [PubMed] [Google Scholar]
- Tang T. T., Sedmak G. V., Siegesmund K. A., McCreadie S. R. Chronic myopathy associated with coxsackievirus type A9. A combined electron microscopical and viral isolation study. N Engl J Med. 1975 Mar 20;292(12):608–611. doi: 10.1056/NEJM197503202921203. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Conant R. M., Hamparian V. V. Rhinovirus replication in suspension cultures of HeLa cells. Proc Soc Exp Biol Med. 1970 Jan;133(1):62–65. doi: 10.3181/00379727-133-34407. [DOI] [PubMed] [Google Scholar]
- Wouters M., Vanden Berghe D., Boeyé A. Composition of poliovirus fibrils. Arch Gesamte Virusforsch. 1973;43(1):25–33. doi: 10.1007/BF01249345. [DOI] [PubMed] [Google Scholar]