Abstract
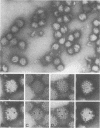
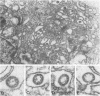
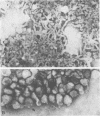
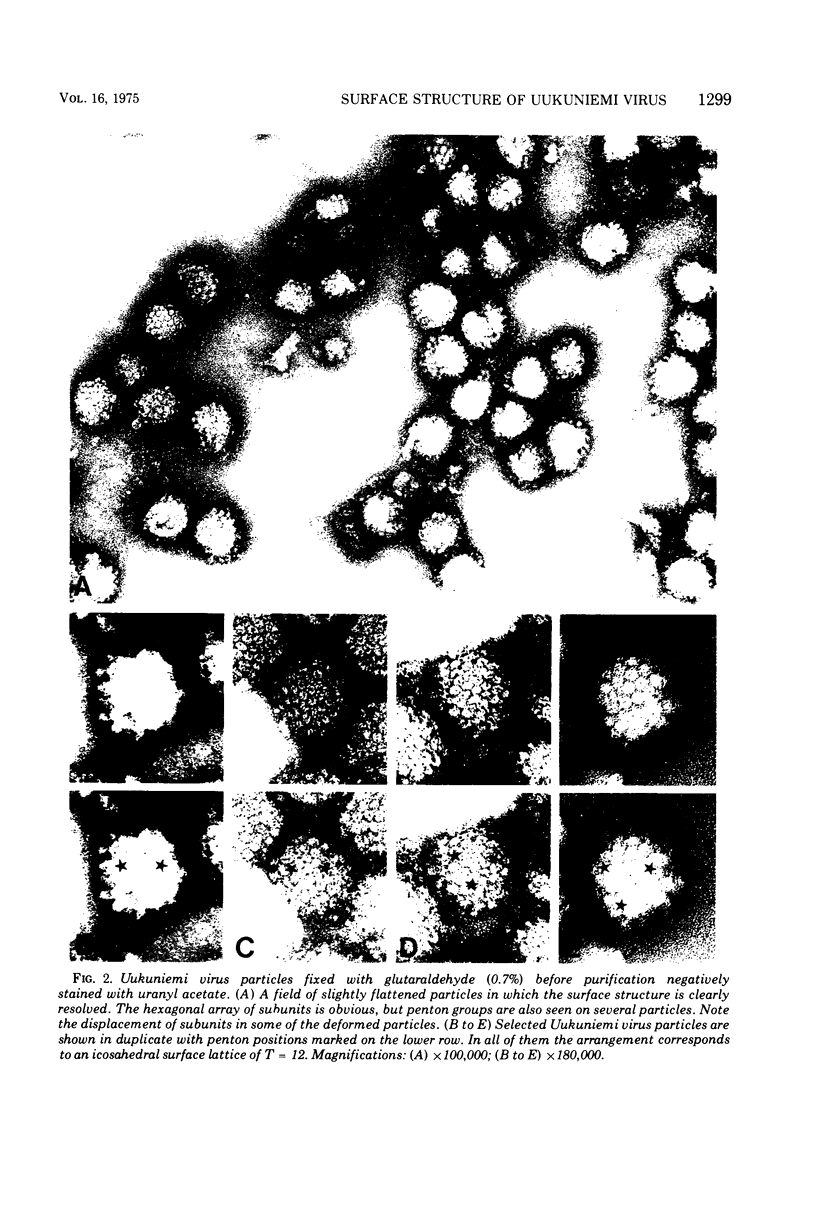
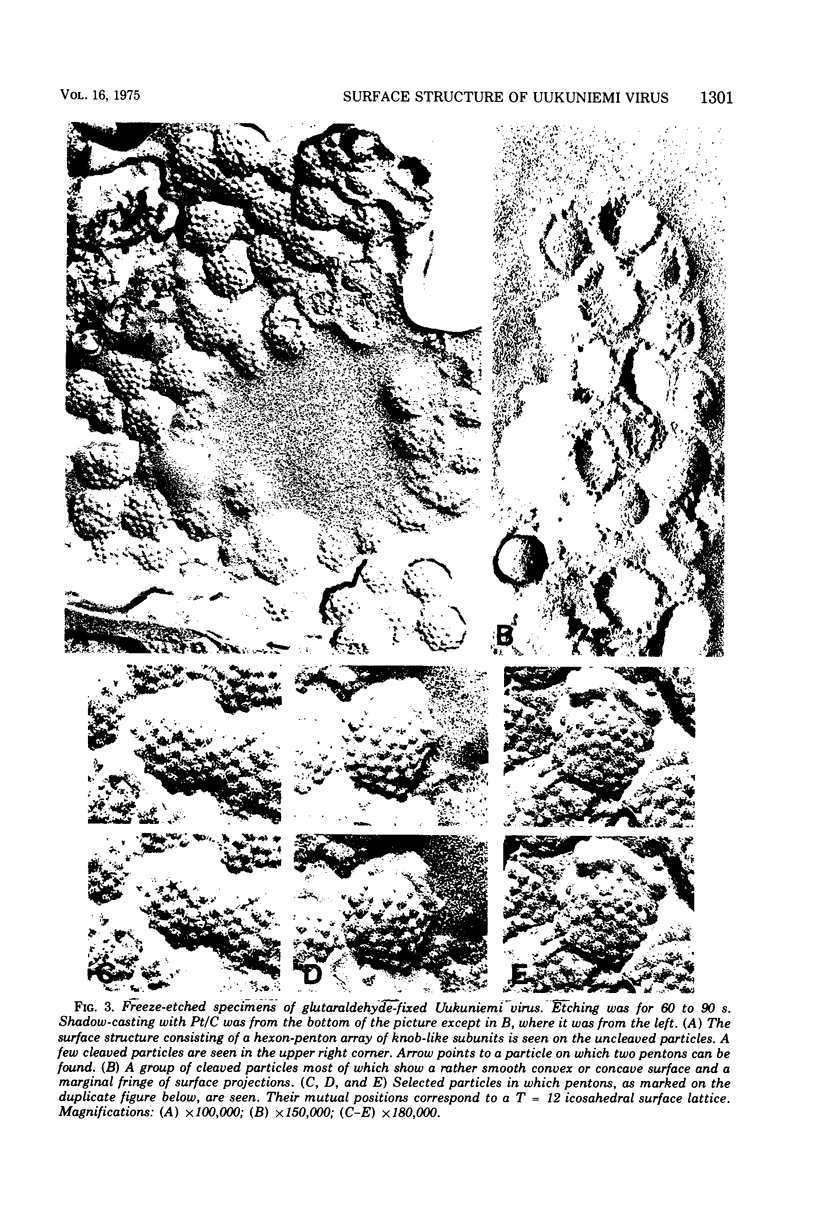
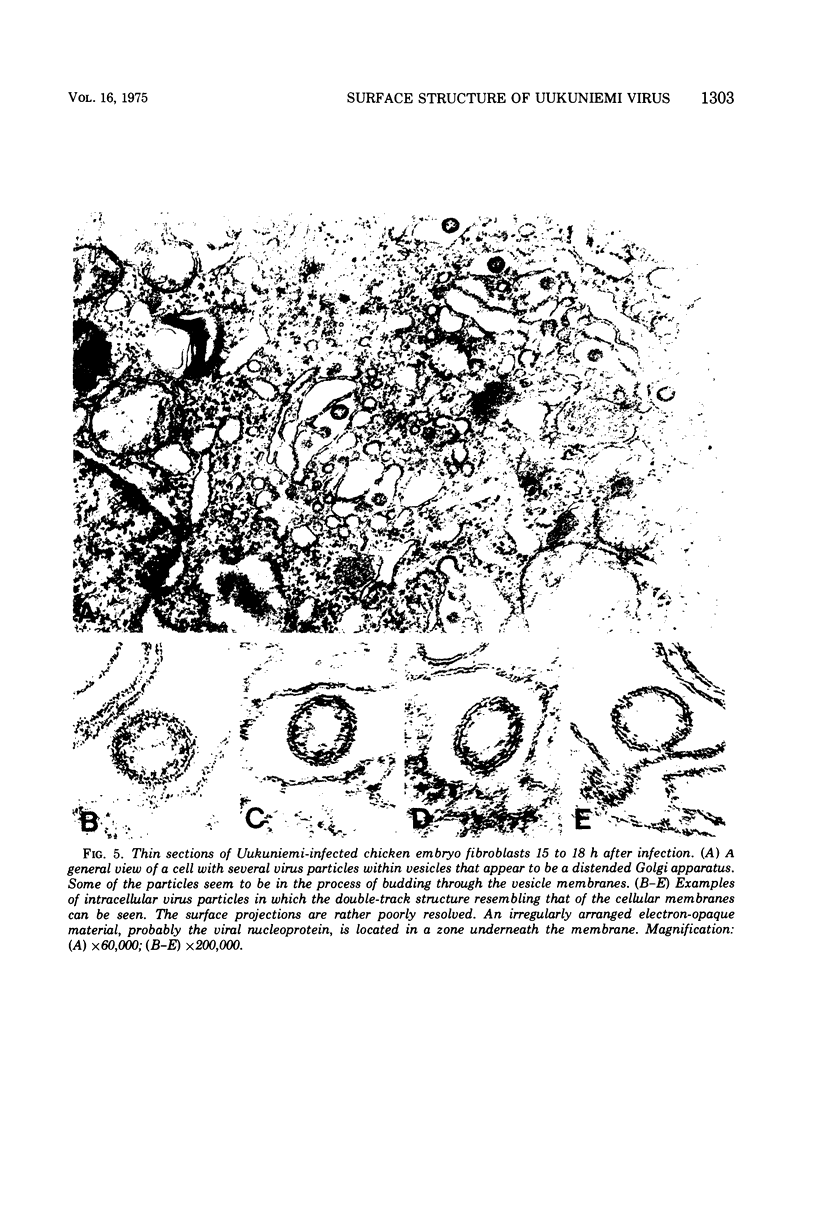
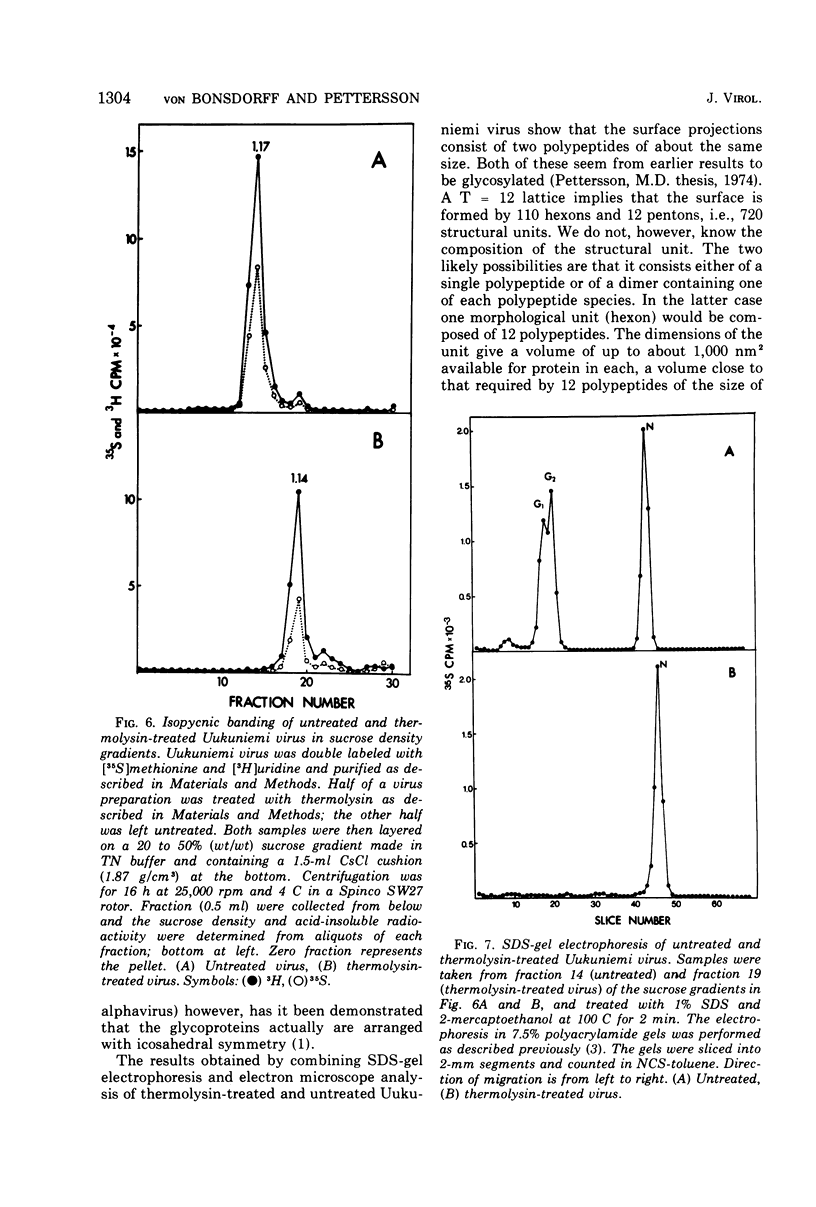
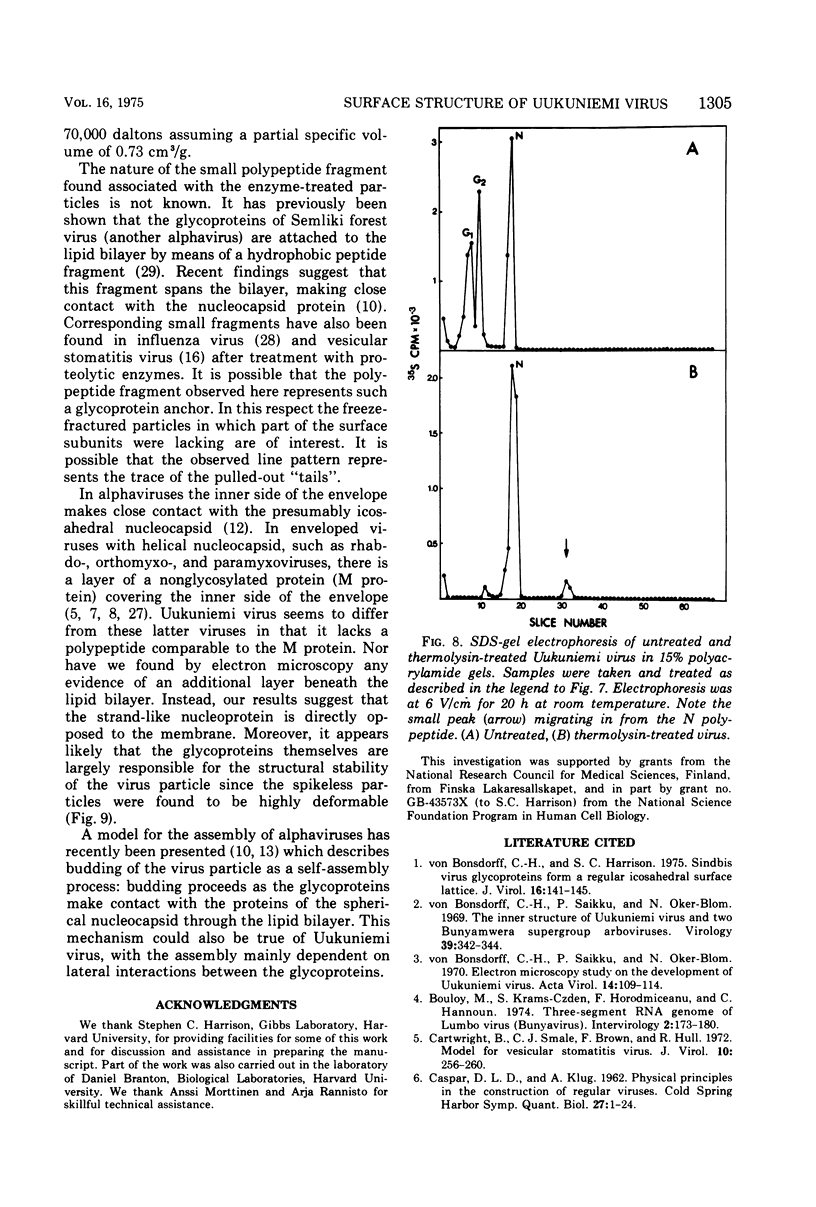
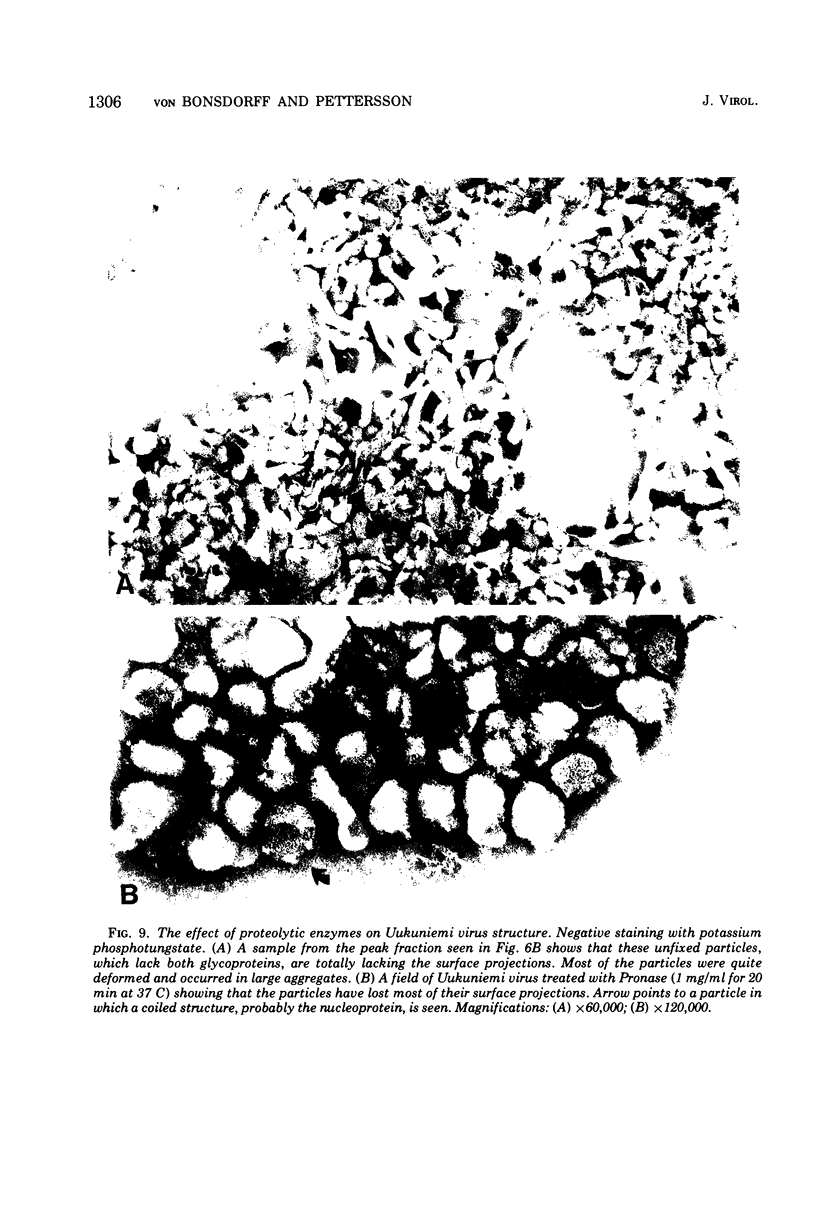
Uukuniemi virus, grown in chicken embryo fibroblasts, has been studied by electron microscopy using negative staining, thin sectioning, and freeze-etching techniques. The spherical virus particle measures about 95 nm in diameter. Its envelope consists of a 5-nm thick membrane covered by 8- to 10-nm long surface projections. These are composed of two polypeptides species of about the same size. Both of them can be removed by digestion with the proteolytic enzyme thermolysin except for a small fragment. The enzyme-treated particles are smooth surfaced and extremely deformable. The glycopolypeptides are clustered to form hollow cylindrical morphological units, 10 to 12 nm in diameter, with a 5-nm central cavity. Both negative staining and freeze-etching suggest that these units are penton-hexon clusters arranged in a T = 12, P = 3, icosahedral surface lattice. The membrane to which the surface subunits are attached is probably a lipid bilayer as evidenced by its double-track appearance in thin sections and the tendency of the freeze fracturing to occur within it. The strand-like nucleoprotein appears from thin-sectioning results to be to a large part located in a zone underneath the membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouloy M., Krams-Ozden S., Horodniceanu F., Hannoun C. Three-segment RNA genome of Lumbo virus (Bunyavirus). Intervirology. 1973;2(3):173–180. doi: 10.1159/000149420. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Compans R. W., Choppin P. W. Parainfluenza virus surface projections: glycoproteins with haemagglutinin and neuraminidase activities. J Gen Virol. 1971 Apr;11(1):53–58. doi: 10.1099/0022-1317-11-1-53. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C., Caspar D. L., Camerini-Otero R. D., Franklin R. M. Lipid and protein arrangement in bacteriophage PM2. Nat New Biol. 1971 Feb 17;229(7):197–201. doi: 10.1038/newbio229197a0. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Jack A., Goodenough D., Sefton B. M. Structural studies of spherical viruses. J Supramol Struct. 1974;2(2-4):486–495. doi: 10.1002/jss.400020233. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Bunyaviridae: morphologic and morphogenetic similarities of Bunyamwera serologic supergroup viruses and several other arthropod-borne viruses. Intervirology. 1973;1(4):297–316. doi: 10.1159/000148858. [DOI] [PubMed] [Google Scholar]
- OKER-BLOM N., SALMINEN A., BRUMMER-KORVENKONTIO M., KAEAERIAEINEN L., WECKSTROEM P. ISOLATION OF SOME VIRUSES OTHER THAN TYPICAL TICK-BORNE ENCEPHALITIS VIRUSES FROM IXODES RICINUS TICKS IN FINLAND. Ann Med Exp Biol Fenn. 1964;42:109–112. [PubMed] [Google Scholar]
- Pettersson R. F., von Bonsdorff C. H. Ribonucleoproteins of Uukuniemi virus are circular. J Virol. 1975 Feb;15(2):386–392. doi: 10.1128/jvi.15.2.386-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973 Dec;56(2):608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L., von Bonsdorff C. H., Oker-Blom N. Structural components of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1971 Dec;46(3):721–729. doi: 10.1016/0042-6822(71)90074-2. [DOI] [PubMed] [Google Scholar]
- Porterfield J. S., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Holmes I. H., Horzinek M. C., Mussgay M., Russell P. K. Bunyaviruses and bunyaviridae. Intervirology. 1974;2(4):270–272. doi: 10.1159/000149433. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Käriäinen L., Pettersson R., Oker-Blom N. The phospholipid composition of Uukuniemi virus, a non-cubical tick-borne arbovirus. Virology. 1972 Dec;50(3):899–901. doi: 10.1016/0042-6822(72)90443-6. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Von Bonsdorff C. H., Oker-Blom N. The structure of Uukuniemi virus. Acta Virol. 1970 Mar;14(2):103–107. [PubMed] [Google Scholar]
- Saikku P., von Bonsdorff C. H. Electron microscopy of the Uukuniemi virus, an ungrouped arbovirus. Virology. 1968 Apr;34(4):804–806. doi: 10.1016/0042-6822(68)90104-9. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972 Jan;47(1):181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bonsdorff C. H., Saikku P., Oker-Blom N. Electron microscope study on the development of Uukuniemi virus. Acta Virol. 1970 Mar;14(2):109–114. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J Virol. 1975 Jul;16(1):141–145. doi: 10.1128/jvi.16.1.141-145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Saikku P., Oker-Blom N. The inner structure of Uukuniemi and two Bunyamwera supergroup arboviruses. Virology. 1969 Oct;39(2):342–344. doi: 10.1016/0042-6822(69)90057-9. [DOI] [PubMed] [Google Scholar]