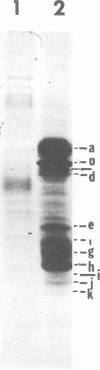
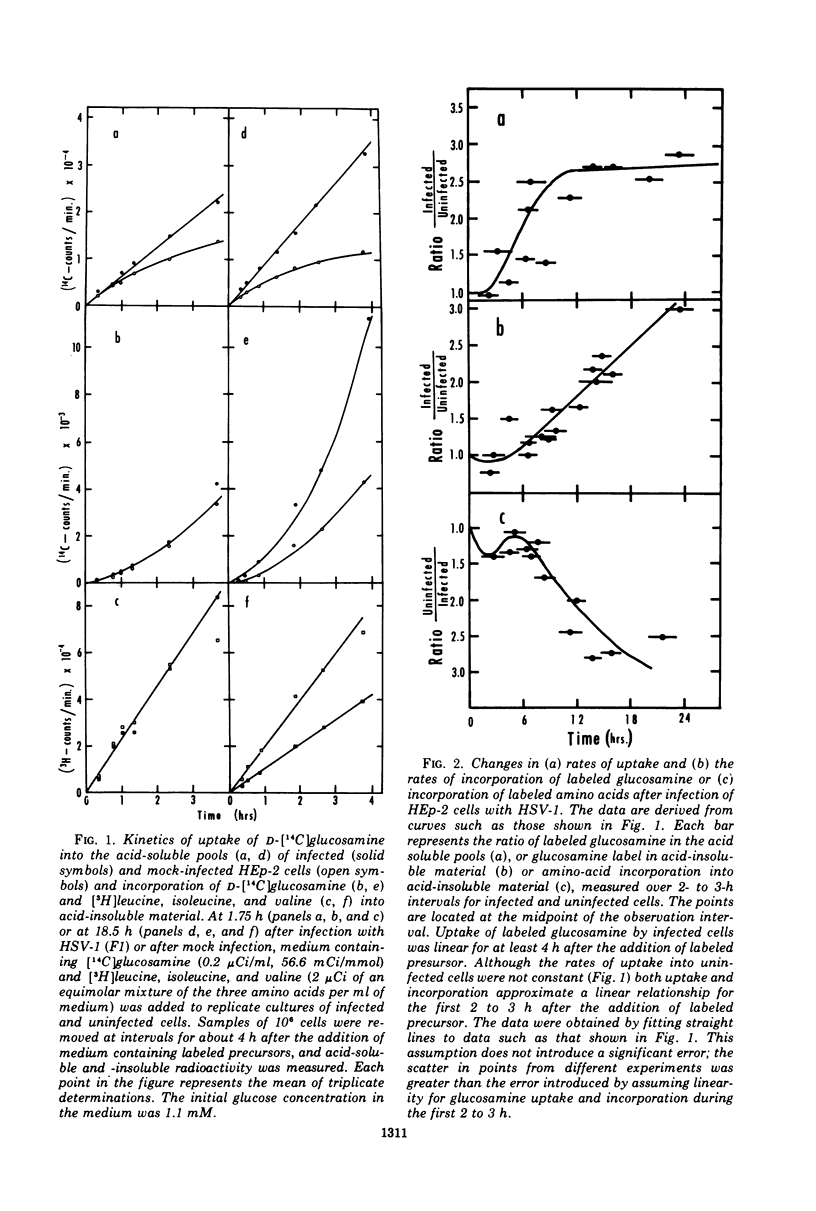
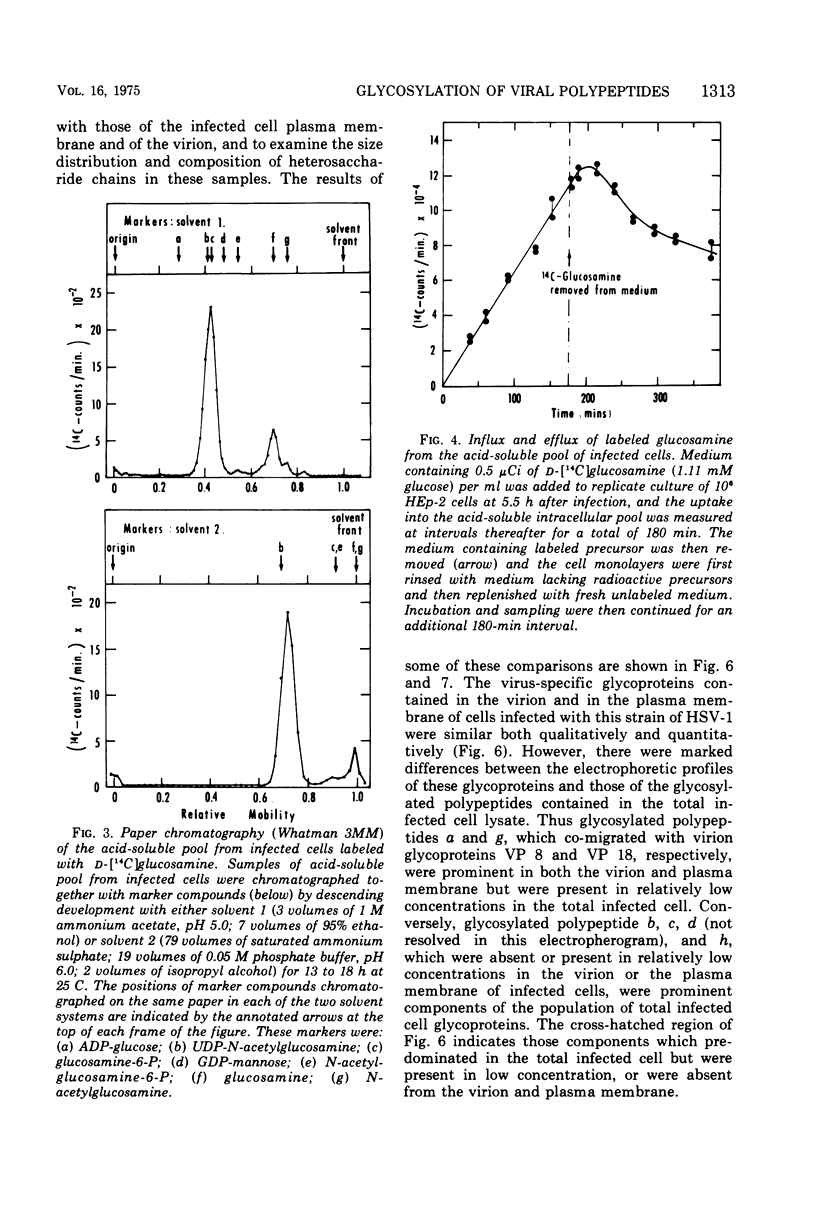
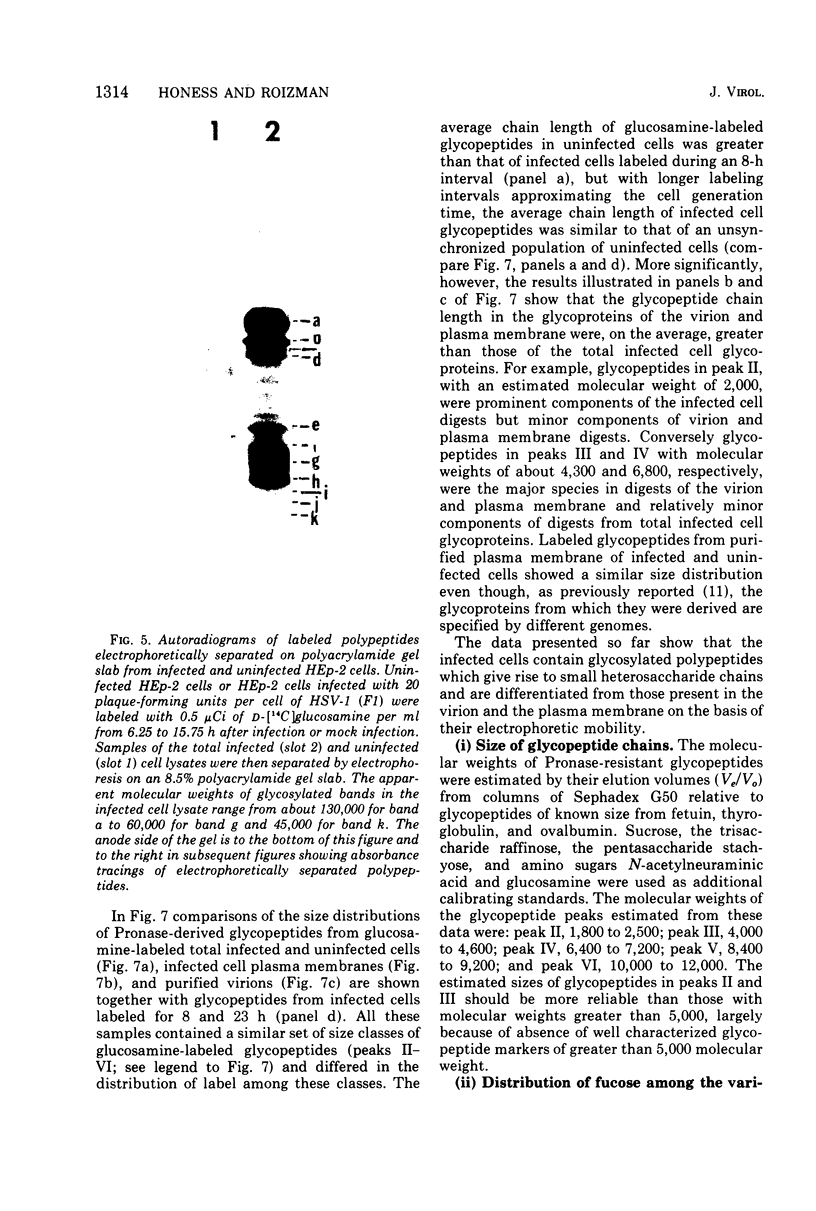
Abstract
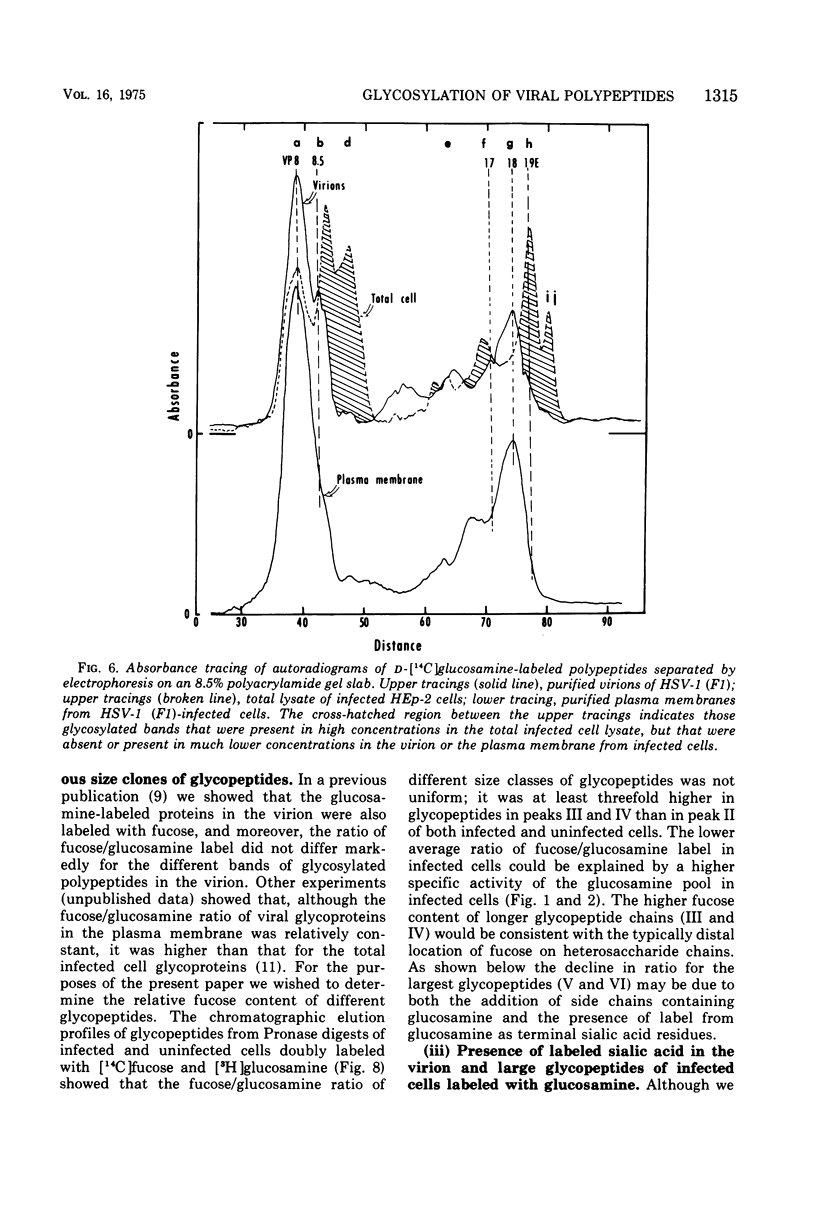
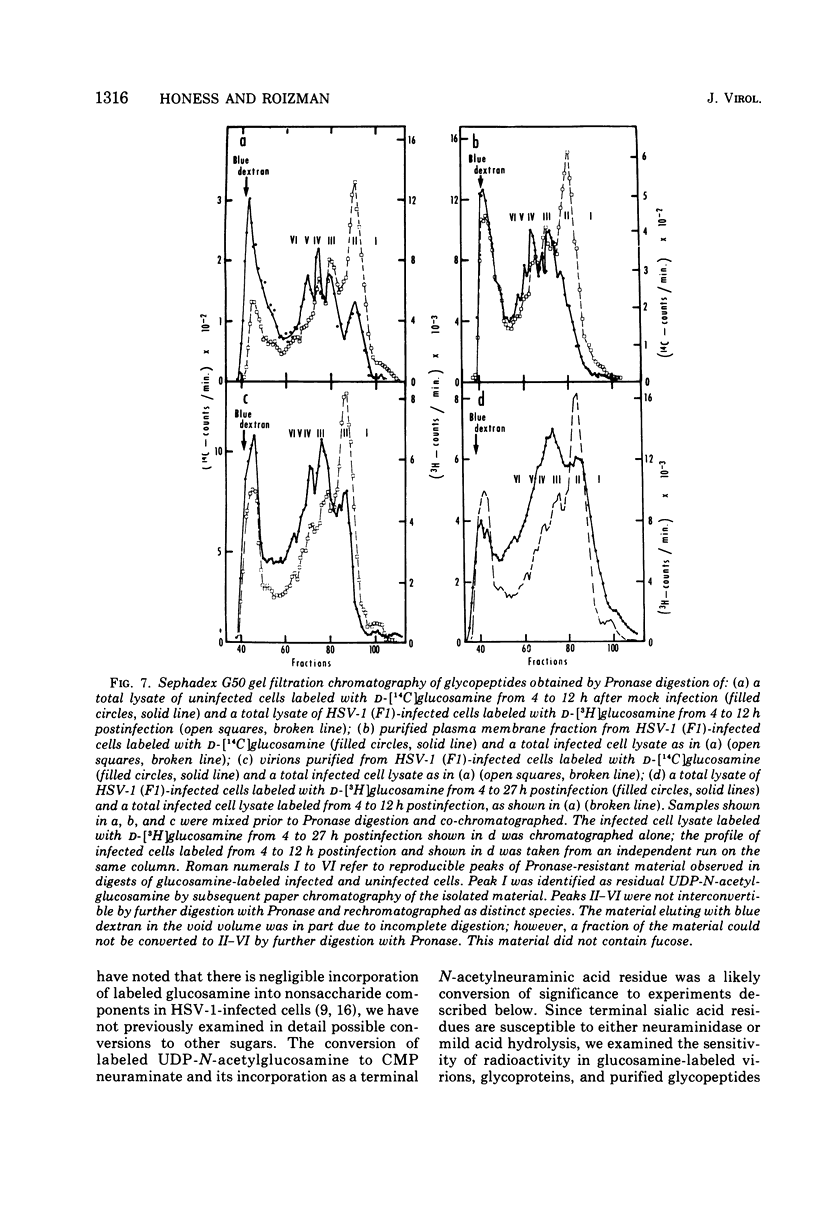
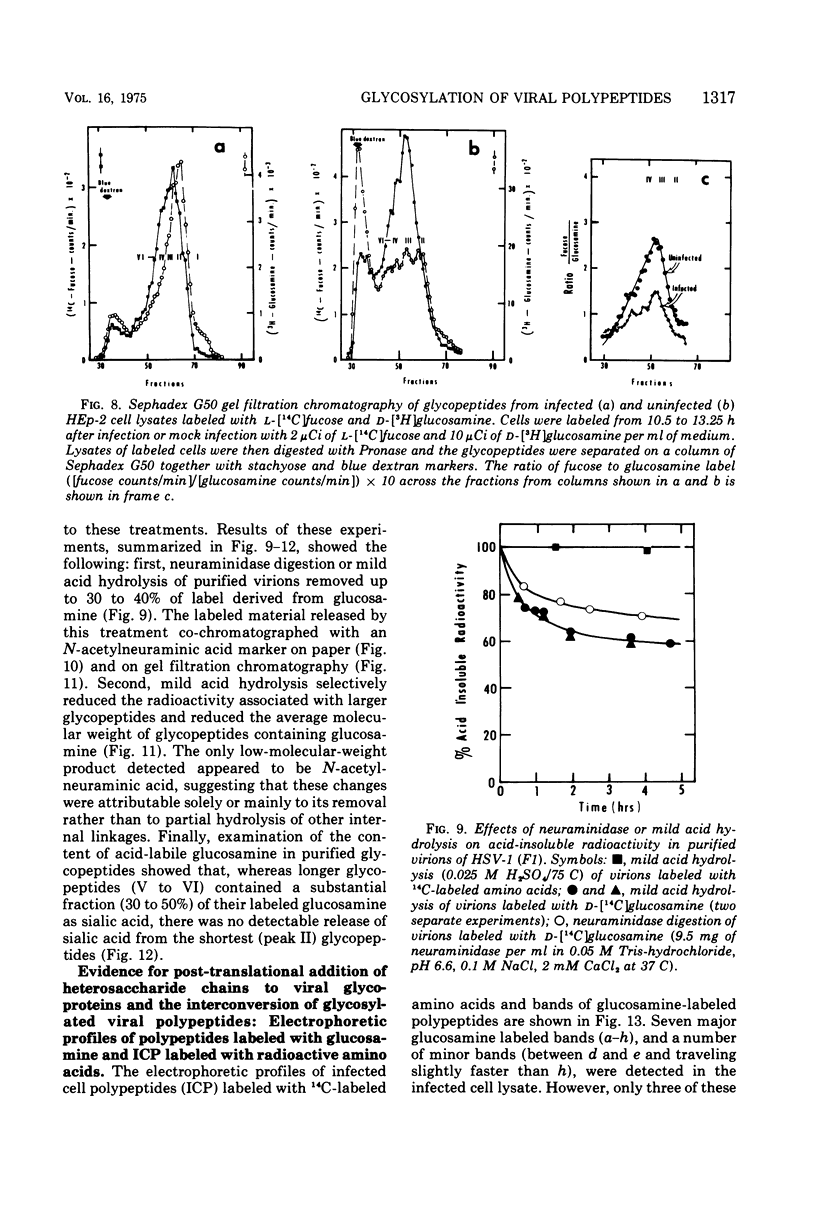
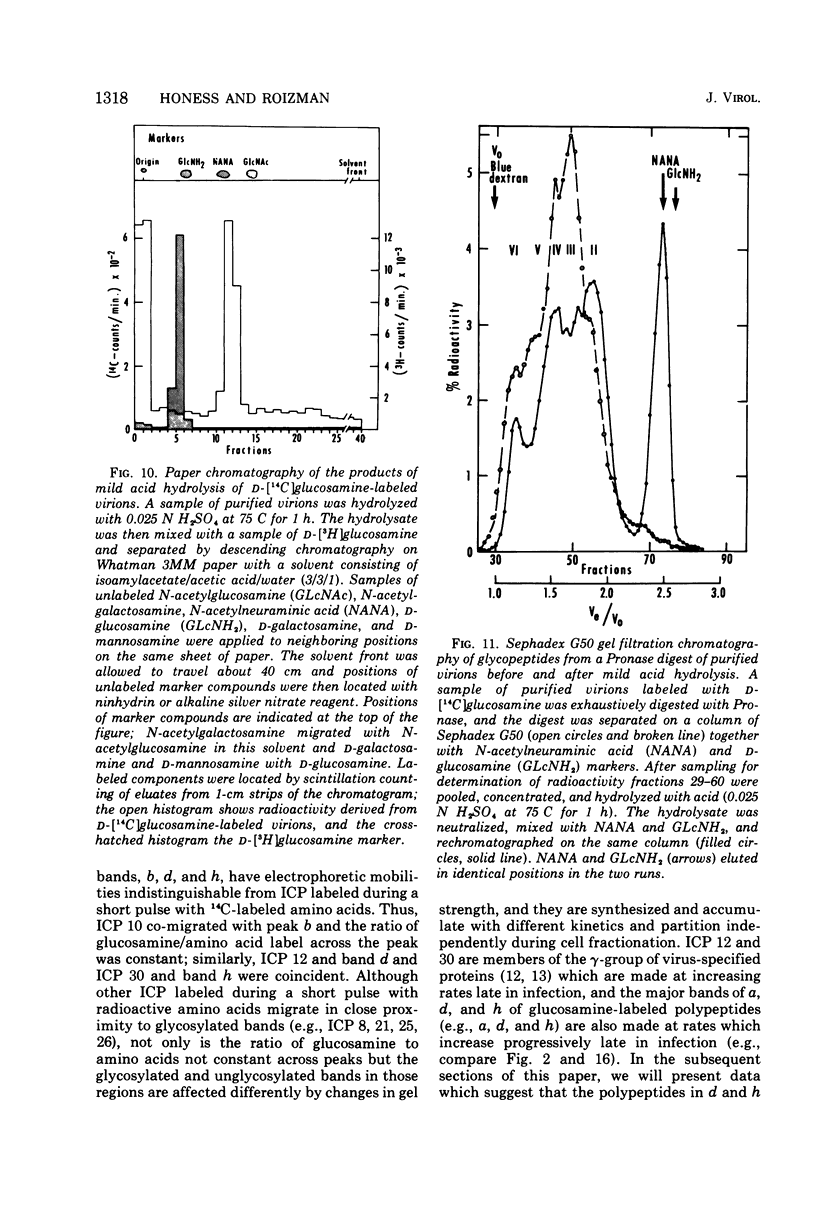
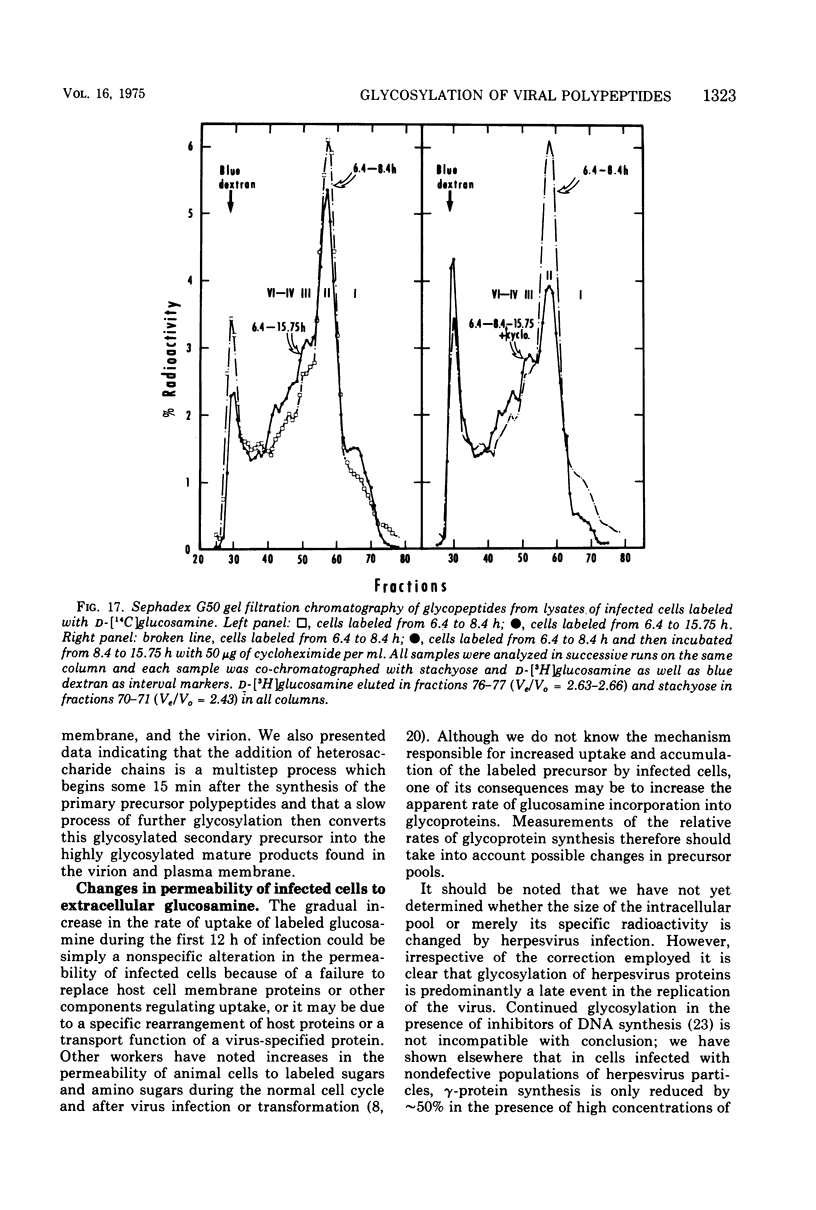
In the course of herpes simplex virus 1 (HSV-1) replication in human epidermoid carcinoma no. 2 cells, the synthesis and glycosylation of host cell proteins ceases and is replaced by the synthesis and glycosylation of virus-specified polypeptides. Analyses of the synthesis of viral glycoproteins show that the glycosylation of viral polypeptides occurs late in the virus growth cycle and that certain of the precursors to major vital glycoproteins are members of the gamma group of polypeptides, i.e., polypeptides synthesized at increasing rates until 12 to 15 h postinfection. Viral glycoproteins are formed by stepwise additions of heterosaccharide chains to completed precursor polypeptides. The precursor and the highly glycosylated product are separable by gel electrophoresis and are localized in different fractions of infected cells. Within 15 min of their synthesis, precursor polypeptides acquire heterosaccharide chains of about 2,000 molecular weight, which contain glucosamine but little or nor fucose or sialic acid. Both precursor and product of this first stage of glycosylation are absent or present in low concentrations in the surface membranes of the infected cell and in the virion. The partially glycosylated product is then conjugated further in a slow, discontinuous process to form the mature glycoprotein of the virion and plasma membrane. These mature products bear large heterosaccharide units with molecular weights greater than 4,000 to 5,000; these contain fucose and sialic acid as well as glucosamine. Heterosaccharide chains from infected and uninfected cells are distributed among discrete size classes and the smallest chains consist of multiple saccharide residues.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Bussell R. H., Robinson W. S. Membrane proteins of uninfected and Rous sarcoma virus- transformed avian cells. J Virol. 1973 Aug;12(2):320–327. doi: 10.1128/jvi.12.2.320-327.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd The envelope of Herpesvirus. Prog Med Virol. 1969;11:16–45. [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Roizman B. Proteins specified by herpes simplex virus. IX. Contiguity of host and viral proteins in the plasma membrane of infected cells. J Virol. 1973 May;11(5):810–813. doi: 10.1128/jvi.11.5.810-813.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- KAMAT V. B., WALLACH D. F. SEPARATION AND PARTIAL PURIFICATION OF PLASMA-MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA MICROSOMES. Science. 1965 Jun 4;148(3675):1343–1345. doi: 10.1126/science.148.3675.1343. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Ginsburg V. The metabolism of glucosamine by tissue culture cells. Exp Cell Res. 1966 Mar;41(3):592–600. doi: 10.1016/s0014-4827(66)80109-x. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Differences between the envelope glycoproteins and glycopeptides of avian tumor viruses released from transformed and from nontransformed cells. Virology. 1972 Nov;50(2):359–372. doi: 10.1016/0042-6822(72)90387-x. [DOI] [PubMed] [Google Scholar]
- Lawford G. R., Schachter H. Biosynthesis of glycoprotein by liver. The incorporation in vivo of 14C-glucosamine into protein-bound hexosamine and sialic acid of rat liver subcellular fractions. J Biol Chem. 1966 Nov 25;241(22):5408–5418. [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M., Hsu K. C. Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol. 1968 Oct;2(10):1172–1184. doi: 10.1128/jvi.2.10.1172-1184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. THE CARBOHYDRATE UNITS OF THYROGLOBULIN. J Biol Chem. 1965 Apr;240:1603–1610. [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus, IV. Site of glycosylation and accumulation of viral membrane proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):730–737. doi: 10.1073/pnas.66.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]