K+ channels have many timing and control functions throughout the body, especially in the nervous system and heart. These vital functions are made possible by several types of “gates” that govern ion flow through the channels (Yellen, 1998). KV channels (K+ selective, voltage activated), exemplified by Drosophila melanogaster Shaker channels, are homotetramers, with each subunit containing six transmembrane helices (TMS1–TMS6); they have an internally located gate (here called the V-gate) formed by the convergence of the S6 transmembrane helices, with one from each of the four subunits. Each of the four TMS6 helices is in turn controlled by a positively charged TMS4 segment, and all four TMS4 segments activate by moving outward to open the V-gate. Other gates are found in both naturally occurring and engineered KV channels. Some channels spontaneously inactivate after conducting for a time after depolarization; conduction ceases even though membrane potential (Vm) is held constant. Of the two well-recognized forms of inactivation in KV channels, N-type and C-type (Kurata and Fedida, 2006), N-type inactivation, especially that in the ShB channel—an alternatively spliced variant of the Shaker channel (Timpe et al., 1988)—is particularly well understood (Hoshi et al., 1990, 1991). N-type inactivation is conferred by a “ball and chain” mechanism, which blocks ion conduction, often in a matter of milliseconds: the N terminus of any one of the four subunits (or, in other channels, a moiety of a β subunit [Rettig et al., 1994], an auxiliary subunit) diffuses in through the open V-gate to a site in the vestibule (or cavity) and blocks conduction (Hoshi et al., 1990; MacKinnon et al., 1993; M. Zhou et al., 2001). Removal of this “N-gate” enzymatically or genetically makes C-type inactivation more readily visible (Hoshi et al., 1991; Kurata and Fedida, 2006). With a few notable exceptions (Smith et al., 1996; Spector et al., 1996), C-type inactivation is slow. Experimentally, it is associated with alterations of the channel’s extracellular mouth (Yellen et al., 1994; Liu et al., 1996), with probable cooperativity between subunits (Ogielska et al., 1995; Panyi et al., 1995; Larsson and Elinder, 2000). Both N-type and C-type inactivation usually occur after activation (Zagotta et al., 1989; Hoshi et al., 1990, 1991), but for C-type inactivation, the mechanisms that translate TMS4 movement or V-gate opening to inactivation remain largely obscure. The two questions considered in this Viewpoint are: (1) how does C-type inactivation in voltage-gated KV channels alter the outer mouth of the pore; and (2) how is the V-gate or the TMS4 segments that control it related to C-type inactivation. For a review of many aspects of inactivation of KV channels, see Kurata and Fedida (2006). Here we put forward a new mechanistic hypothesis of C-type inactivation: pore dilation, not pore constriction, causes C-type inactivation in KV channels.
Functional importance of inactivation of KV channels
Inactivation gating of K+ channels provides a form of short-term memory. It can modulate the firing patterns of neurons on the time scale of seconds (Aldrich et al., 1979; Roeper et al., 1997), which is potentially useful in ways too numerous to count. Impaired inactivation may lead to a variety of neurological and psychiatric disorders (Adelman et al., 1995). The clearest use of C-type inactivation is in human ERG (HERG or KV11) channels in cardiac ventricular cells (Keating and Sanguinetti, 2001). These channels inactivate rapidly on depolarization (Schönherr and Heinemann, 1996; Smith et al., 1996) and perform their main function during repolarization from the plateau of the action potential: they pull Vm back to the resting potential and hold it there, thus preventing arrhythmias, until their V-gates slowly close (Nerbonne and Kass, 2005; Sanguinetti and Tristani-Firouzi, 2006).
Residue numbering
Most of the biophysical and mutational studies cited here have been performed on ShB channels (Timpe et al., 1988). Crystallization and x-ray analysis, on the other hand, has been performed on a chimeric channel, Kv1.2-2.1 (Protein Data Bank accession no. 2R9R) (Long et al., 2007), of almost identical sequence in the regions involved in activation and inactivation. Here we use ShB experiments and residue numbering for biophysical experiments, expressed with italic font, e.g., Y445 for ShB tyrosine 445. For structural illustrations, we use Kv1.2-2.1 numbering with regular font for residues and number, e.g., Y373, which corresponds to Y445 in ShB (and will be referred to as Y373/Y445, or Y445\Y373 when mutations on ShB are the subject). Correspondence between some important residues is shown in Fig. 1.
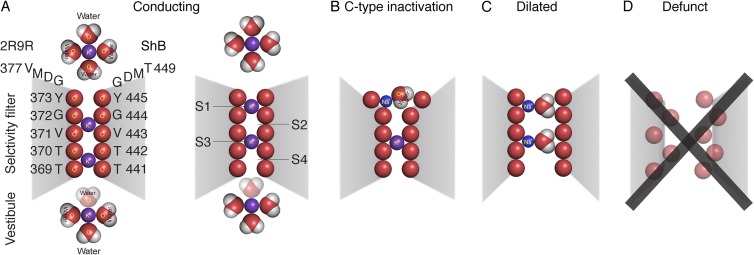
Figure 1.
Postulated occupancy states of the selectivity filter, shown in a section through the pore. (A) Conducting states. Two K+ ions complexed with carbonyl oxygens are thought to be in the filter at any instant, in sites 2 and 4, or sites 1 and 3. Occupancy of sites 2 and 4 is more probable at resting Vm. When the gate opens, IK is normally outward, and occupancy oscillates between sites 4,2 and 3,1. This oscillation is driven by voltage, and outward movement of the ion shown in the vestibule, which sheds its watery coat and enters site 4. The ion in site 1 is driven to the site shown just external to the filter as it rehydrates. The numbering of the amino acid residues in the filter in and just above the filter of 2R9R (left) and ShB (right) are shown. (B) Postulate of a C-type inactivated channel: the outermost site 1 of the selectivity filter dilates and cannot effectively complex a K+ ion, and thus cannot accept an ion moving outward from site 2. A hydrated Na+ ion may be present in site 1, as shown. (C) Dilated state in the complete absence of K+ ions. The selectivity filter dilates from top to bottom when close-fitting K+ ions are not present and the carbonyl oxygens of the filter mutually repel. This allows partially hydrated Na+ and other large cations to permeate. (D) Defunct state. The selectivity filter is hopelessly denatured by carbonyl–carbonyl repulsion. Ion permeation is not possible. Structural details of the defunct state are unknown.
What is the final event in C-type inactivation?
A chain of events that begins with depolarization leads to C-type inactivation; we first consider the final event in the chain. Well before the atomic structure of a K+ channel was elucidated by crystallization, Yellen et al. (1994) explored the idea that this event consisted of a constriction near the pore mouth. They replaced the threonine residue at position 449 in ShB with a cysteine (T449C; see Fig. 1). Cd2+ in the external medium promoted C-type inactivation in this mutant with a potency that suggested (but did not prove) binding to more than one and perhaps to all four of the cysteines (one in each subunit). The crystal structure of the Kv1.2-2.1 chimera shows that these four residues (V377/T449) are 15 Å apart, and it seems unlikely that the proximity required for multiple binding of Cd2+, ∼4 Å (Maher et al., 2004), can be achieved without a very large (and, we think, somewhat improbable) rearrangement. Although ample evidence shows that changes in the reactivity of engineered cysteines at the external mouth of the pore accompany C-type inactivation in KV channels (Yellen et al., 1994; Liu et al., 1996; Schlief et al., 1996; Larsson and Elinder, 2000), there is no direct proof that these changes depend on pore mouth constriction.
With regard to the pore constriction hypothesis of Yellen et al. (1994), crystal structures of the bacterial channel, KcsA, have been investigated (Cuello et al., 2010). These channels are largely voltage independent and thus lack a key feature of C-type inactivation in voltage-gated eukaryotic K+ channels. Nevertheless, currents through KcsA, activated by changes in pH, decline with time (Chakrapani et al., 2007), and this gating behavior may involve structural changes similar to those in C-type inactivation of voltage-gated KV channels. To explore this possibility, Cuello et al. (2010) crystalized a truncated KcsA construct 25 times in identical conditions. Of the 25 trials, 15 are reported and all 15 are somewhat different according to the metric presented. The general structure selected as “open-inactivated” was seen in 4 out of the 25 total trials. Its main characteristics are: (a) a drastic change in K+ occupancy of the selectivity filter with probable rearrangement of the carbonyl groups, which are not resolved; (b) a 2-Å pinch at the position of Gly77, equivalent to G372/G444 (see Fig. 1 A); and (c) a considerable dilation of the selectivity filter at position S3 (see Fig. 1 A). Any of these three changes could render the channel nonconducting. Based, presumably, on the hypothesis of Yellen et al. (1994), Cuello et al. (2010) highlight the pinch at Gly77 in KcsA as a structural model of inactivation of KcsA as well as for C-type inactivation in KV channels. As noted, the pore constriction postulate of Yellen et al. (1994), put forth before the crystallographic atomic structures were available, would require a very large rearrangement of the channel’s outer mouth. Cuello et al. (2010) did not observe a large change in the outer mouth, casting doubts on whether these KcsA structures are directly relevant to the work of Yellen et al. (1994). Y. Zhou et al. (2001) also saw a pinch at Gly77 in KcsA, in a crystal formed in low K+, which, they speculated, might be nonconducting. However, the functional state in this case also remains unresolved. KcsA crystals formed or soaked in Cd2+, which would facilitate C-type inactivation in KV channels, also failed to show a large constriction: the diagonal and adjacent separation of the Cd2+ ions were 16.5 and 11.7 Å, respectively (Raghuraman et al., 2012), versus ∼4 Å required for a Cd2+ bridge. Spin labels attached to KcsA residue Y82C suggested a relatively small constriction, but the cysteines of residue 82 remained too far apart to form a Cd2+ bridge. These experiments illustrate the difficulty inherent in assigning a functional state to crystallized channels, or to channels in the conditions required for a spin label experiment. In our opinion, the crystallization and spin label experiments thus far have failed to yield compelling evidence for the constriction hypothesis of C-type inactivation in KV channels.
Another possibility is that C-type inactivation in fact results from a small dilation of the outermost site in the selectivity filter, destroying the site’s ability to selectively complex and conduct K+ ions. We favor this possibility, based on substantial literature regarding the antagonistic effect of K+ on C-type inactivation, which is an almost defining characteristic (López-Barneo et al., 1993; Baukrowitz and Yellen, 1995, 1996). K+ and its absence, however, have other effects on the K+ channel, some of which may be confused with C-type inactivation. For clarification, Fig. 1 is an outline cartoon of our ideas, with details given later in the text. The two left cartoons are of the KV selectivity filter in physiological K+-containing ionic solutions and show the conducting state (Fig. 1 A), with K+ ions at sites 4 and 2 (4–2 state; left), and 3 and 1 (3–1 state; right) of the selectivity filter (Y. Zhou et al., 2001). The 4–2 state is probably preferred at resting Vm, whereas during conduction, there is alternation between the 3–1 and the 4–2 state. Fig. 1 B is our postulate of the C-type inactivated state, where the outer site of the filter is dilated and unable to complex a K+ ion. Fig. 1 C is a dilated state of the entire filter that occurs transiently after the complete removal of K+ inside and out. In this condition, there is substantial permeability to Na+ (Starkus et al., 1997, 1998; Kiss and Korn, 1998; Ogielska and Aldrich, 1999), and two partially hydrated Na+ ions are shown in the dilated filter. The Na+ permeability can be blocked by 1 mM or less internal K+ (Starkus et al., 1997; Kiss and Korn, 1998), by filling—we postulate—the inner filter sites with their preferred cation, K+. If the period in K+-free solutions is prolonged (greater or equal to ∼200 s), the channels enter a slowly reversible nonconducting state (Fig. 1 D) in which gating charge movement is abnormal (Olcese et al., 1997). Crystal structures of the bacterial voltage-independent K+ channel KcsA in low K+ (Y. Zhou et al., 2001) suggest the possible structural details of this denatured or “defunct” state (Melishchuk et al., 1998), which are otherwise unknown.
Normal K+ binding by the selectivity filter
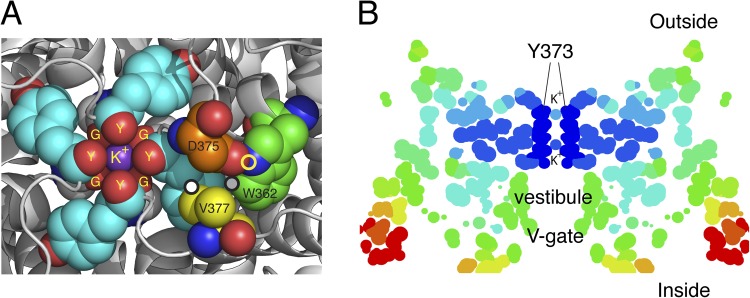
The extracellular mouth of the Kv1.2-2.1 chimera (Long et al., 2007) is shown in Fig. 2 A. The outermost K+ ion in the filter is tightly complexed to site 1: the four carbonyls of Y373/Y445 and the four carbonyls of G372/G444 just below. Several residues important in C-type inactivation are also labeled and will be discussed. The three circles highlight three close contacts relevant to C-type inactivation: a white closed circle between V377/T449 and Y373/Y445, a gray closed circle between W362/W434 and Y373/Y445, and a yellow open circle representing a hydrogen bond between W362/W434 and D375/D447.
Figure 2.
Structural arrangements of the Kv1.2-2.1 chimera (2R9R) near the pore. (A) The outermost K+ ion in site 1 (S1) of the filter is tightly complexed to the four carbonyls of Y373 (Y445) (Y) and the four carbonyls of G372 (G444) (G), just below. Several residues important in C-type inactivation are labeled. The carbon atoms of these residues are rendered with different colors for clarity: gray, G372 (Y444) in ShB; cyan, Y373 (Y445); orange, D375 (D447); yellow, V377 (T449); green, W362 (W434). Y373 (Y445) and G372 (G444) from all four subunits are shown, but only one set of D375 (D447), W362 (W434), and V377 (T449) is shown as spheres. D375/447 and W362/434 residues shown are from one subunit, whereas V377 (T449) is from an adjacent subunit. The circles mark three of the close contacts postulated to be important for C-type inactivation (see text). Prepared using MacPyMol (version 0.99). (B) A “temperature” map of a section obtained from the B-factor values along the pore axis of 2R9R. Dark blue marks regions of high stability, ranging through light blue, yellow, orange, and red as stability decreases progressively away from the selectivity filter. Prepared using RasMol.
Efficient conduction of K+ ions requires that they face no large energy barrier (or deep well) as they move from water to the selectivity filter on one side, and back to water on the other side. In water, they are tightly bound to surrounding dipolar water molecules with a total free energy of ∼73 kCal/mol (∼306 kJ/mole) (Robinson and Stokes, 2002), which approximates the strength of a covalent bond. To achieve a free energy this low in the filter, a dehydrated K+ ion must complex to surrounding carbonyls (Bezanilla and Armstrong, 1972; Morais-Cabral et al., 2001). Significantly, carbonyl groups have a higher dipole moment than water molecules (∼2.7 D in many aldehydes and ketones vs. 1.85 D for water) and thus bind to K+ more tightly than water does. This additional component of binding energy may be necessary to compensate for the relatively low dielectric constant of the channel and its surrounding membrane. The main function of the selectivity filter is to exclude Na+, which is present at high concentration in the extracellular medium and would flood inward, particularly at negative Vm, were it not excluded. The carbonyls of the filter are too far apart to complex a Na+ ion (0.95 Å in radius vs. 1.33 Å for K+) effectively and thus face a high energy barrier to entry.
Fig. 2 B is a slice along the pore axis, with the selectivity filter at top, the vestibule or cavity below, and the open V-gate at bottom. The colors reflect the thermal motions of the atoms inferred from the B-factor information of the crystal structure, with dark blue representing a region of high stability and very restricted thermal motion (root-mean-square deviation of 0.56 Å), ranging to orange and red (root-mean-square deviation of 1.1 Å) for very labile. The most stable region is the selectivity filter. Undoubtedly, a major factor in its stability is the induced fit forced by the tight binding of K+ ions. A similar temperature map of the KcsA channel in 200 mM K+ shows comparable relative stability, whereas the filter in 3 mM K+ is much more labile (Y. Zhou et al., 2001), underscoring the importance of K+ ions in stabilizing the filter structure. Destabilization of the K+ channel was originally shown in squid axon: the combination of K+-free intracellular and extracellular solutions together led to a loss of K+ conductance for the lifetime of the axon (Chandler and Meves, 1970; Almers and Armstrong, 1980). Almers and Armstrong (1980) hypothesized that on withdrawal of K+, the (postulated) carbonyls of the filter, which normally complex K+, repel each other in its absence and destabilize the filter. Experiments with Shaker channels show a similar but slowly reversible loss of K+ conductance (Gómez-Lagunas, 1997; Melishchuk et al., 1998). Remarkably, when the channel’s V-gate is held closed by keeping Vm at −80 mV during zero K+ exposure (no depolarizing pulses), the K+ conductance is preserved for at least 10 min (Gómez-Lagunas, 1997). Depolarization facilitates loss of K+ from the selectivity filter by opening the V-gate, and ∼40% of the conductance is lost with each depolarizing pulse. These results clearly demonstrate the stable binding of K+ in the filter when the V-gate is closed, as well as the necessity for K+ to maintain the filter structure.
K+ and C-type inactivation
External K+ has a marked effect on the C-type inactivation in KV channels (Pardo et al., 1992; López-Barneo et al., 1993; Baukrowitz and Yellen, 1995; Starkus et al., 1997). Increases in extracellular K+ concentration ([K+]o) slow C-type inactivation with an apparent affinity of a few millimolar (Baukrowitz and Yellen, 1996), a physiologically relevant concentration, and accelerate ionic current recovery from C-type inactivation at hyperpolarized potentials (Levy and Deutsch, 1996; Starkus et al., 1997, 1998). Thus, elevations in [K+]o, after a burst of action potentials, have the potential to alter neuronal excitability by affecting C-type inactivation (Pardo et al., 1992; Baukrowitz and Yellen, 1995, 1996). Further, rises in [K+]o can, paradoxically, increase currents through some ShB mutants despite the reduction in driving force for K+ (Pardo et al., 1992). In this case, many channels seem to be in the C-type inactivated state even at the resting Vm when in low [K+]o; higher [K+]o increases the conducting fraction. When extracellular [K+]o is replaced with nonpermeant ions, C-type inactivation is accelerated (López-Barneo et al., 1993; Baukrowitz and Yellen, 1995, 1996; Starkus et al., 1997; Kiss and Korn, 1998). Similar acceleration of C-type inactivation is observed when the supply of K+ ions from the inside is curtailed, either by lowering the intracellular K+ concentration ([K+]i) or when K+ efflux is prevented by channel blockers or by closure of the N-gate (Hoshi et al., 1991; Baukrowitz and Yellen, 1995, 1996; Starkus et al., 1997; Kiss and Korn, 1998). In another illustration of the importance of K+ to C-type inactivation, Cs+ efflux through K channels inhibits recovery from C-type inactivation, apparently by inhibiting K+ entry into the filter from outside (Ray and Deutsch, 2006). Further, Cs+ trapped in the inner vestibule (by hyperpolarizing to −100 mV with a high concentration of Cs+ inside) slows recovery from C-type inactivation, again, presumably, by preventing K+ from entering the selectivity filter to promote recovery.
Complete removal of K+ causes a transient (a few seconds) increase of Na+ permeability in ShB channels (Starkus et al., 1997; Kiss and Korn, 1998). The increase of Na+ permeability is followed by total loss of conduction. This nonconducting denatured or defunct state has abnormalities extending to the gating apparatus: reduced gating charge movement (Qg) and altered gating current (Ig) time course (Olcese et al., 1997; Melishchuk et al., 1998; Loboda et al., 2001). This defunct state is not a C-type inactivated state, which can occur with high [K+]i and does not alter Ig or Qg. Significantly, the ShB T449V and T449Y mutants, which do not undergo C-type inactivation (López-Barneo et al., 1993), become neither Na+ permeable nor denatured/defunct in the absence of K+ (Loboda et al., 2001), demonstrating the close connection of C-type inactivation and filter dilation.
Overall, it seems reasonable to test the suggestion that C-type inactivation is the result of a dilation of the selectivity filter’s outermost site, sufficient to prevent K+ conductance. The dilation is limited to the outer site by the presence of high [K+]i, which keeps the inner filter sites intact. The importance of the structural integrity of the outermost site in the filter maintained in part by the inner sites could readily explain the fact that numerous mutations in the pore domain alter C-type inactivation in KV channels (Kurata and Fedida, 2006). A mutation could impair C-type inactivation directly by altering the outermost site and/or indirectly by altering the inner sites. An example of the latter indirect mechanism is provided by Ogielska and Aldrich (1999), who showed that mutation of A463, which is in the inner pore helix S6 and lies near the innermost site in the filter (S4 in Fig. 1 A), modulates C-type inactivation by changing the affinity of site 4 to K+. A reduction in occupancy of K+ in site 4 weakens electrostatic interactions among the permeating K+ ions in the filter and increases the K+ occupancy in the outermost site, thus protecting the filter’s structural integrity.
C-type inactivation and the S4 voltage sensors
C-type inactivation is more probable after activation of the TMS4 voltage sensors and/or opening of the V-gate (Hoshi et al., 1991), indicative of coupling of C-type inactivation and activation. Such coupling is also suggested by voltage-clamp fluorometry measurements using fluorophores placed near the extracellular end of TMS4 or TMS5; fluorescent changes similar to the C-type inactivation time course have been detected (Loots and Isacoff, 1998; Gandhi et al., 2000). However, the nature of the coupling process between the TMS4 voltage sensors, the V-gate, and the C-type inactivation effector remains mostly obscure.
Mutations in and near the selectivity filter
In addition to the influence of K+ binding on filter stability, steric stabilization from the tightly packed residues around the pore mouth is obviously important. Some of these residues in Kv1.2-2.1 are labeled in Fig. 2 A, with important close contacts marked by circles. The ring of Y373/Y445 is in close contact with W362/W434 on one edge (3.6 Å; Fig. 2 A, gray closed circle) and the γ carbon of V377/T449 on the other (3.7 Å; Fig. 2 A, white closed circle). D375/D447 forms a hydrogen bond to W362/W434 (2.8 Å; Fig. 2 A, open yellow circle). Five results of mutation of these residues in the ShB channel are the following:
(1) In general, mutation of Y445 \Y373 or near neighbors has a profound effect on C-type inactivation without altering Ig (Loboda et al., 2001). This suggests that only the filter is altered, and that S4 motion and the opening of the V-gate are unaffected.
(2) The mutation Y445A\Y373A abolishes conduction and leaves Ig unaffected (Loboda et al., 2001).
(3) With the mutation W434F\W362F, K+ current is visible only at the single-channel level, presumably because the channels are almost permanently in the C-type inactivated state (Yang et al., 1997).
(4) D447E\D375E makes C-type inactivation very fast (Molina et al., 1997), perhaps by weakening the hydrogen bond to W434\W362, thus altering the position of the latter residue, which, in turn, disturbs its near neighbor Y445\Y373 (see Figs. 2 A and 3 A). D447N\D375N, which breaks the hydrogen bond, eliminates ionic currents presumably because the channels are stable in the C-type inactivated state (Loots and Isacoff, 1998).
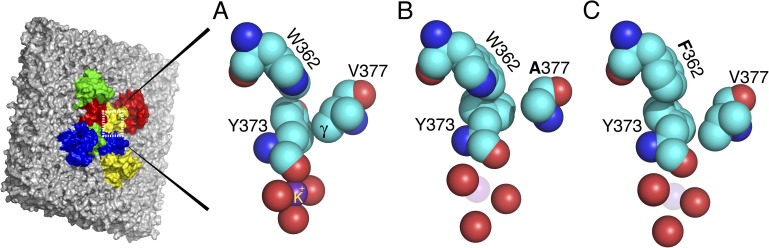
Figure 3.
An expanded view of the outer mouth of the selectivity filter of the Kv1.2-2.1 chimera and two simulated mutations. The four outermost Y373 carbonyl oxygens outline the pore, but only one tyrosine is shown in full. (A) The conducting conformation of the filter mouth, taken from the Kv1.2-2.1 chimera. Y373 (Y445) has close contacts with W362 (W434) and V377 (T449). (B) A simulated mutation of the Kv1.2-2.1 chimera that replaces V377 (T449) with V377A (T449A), allowing Y373 (Y445) to rotate and dilate the filter mouth. The mutation in effect deletes the two γ carbons of V377 (T449), removing a contact that locks Y373 (Y445) in the conducting, nondilated position. (C) Simulated mutation W362 (W434) of the Kv1.2-2.1 chimera that replaces W362 (W434) with F362 (F434). Relative to W362 (W434), F362 (F434) is rotated clockwise in this view; it presses against the ring of Y373 (Y445), causing Y373 (Y445) to rotate counterclockwise, dilating the filter mouth. The angle of view is the same throughout the figure. The images were prepared using MacPyMol (version 0.99).
(5) Mutation at T449 can either speed up C-type inactivation (T449A, C, K, E, S) or virtually abolish it (T449Y, V, I) (López-Barneo et al., 1993). It is probably significant that the Val and Iso branch at the Cβ, placing a Cγ in close contact with Y445\Y373. We believe this contact makes unlikely the rotation of Y445\Y373 as shown in the homology models below.
Technical difficulties in studying C-type inactivation
From the forgoing, it is clear that C-type inactivation is associated with the pore mouth, and that many of the neighboring residues are in some way involved. The existing biophysical and mutational evidence is plentiful but has not yet provided a clear mechanism (Kurata and Fedida, 2006). We believe that our hypothesis invoking dilation of the selectivity filter’s outermost site provides a plausible mechanism for C-type inactivation, consistent with existing evidence in the literature. How could this hypothesis be experimentally tested? Ideally, high resolution atomic structures of a KV channel determined at controlled values of Vm and in various ionic solutions could be assigned to the conducting and C-type inactivated states. This is, however, a formidable challenge because Vm is difficult or impossible to manipulate during crystallization, and a very high K+ concentration (e.g., 0.2 M) seems to be required in the final crystallization solution. It is unlikely that C-type inactivation could occur at such high K+ concentration. Finally, it is possible that important functional changes may not be detected by the currently available structural methods (Gonzalez-Gutierrez et al., 2012). Useful but less definitive would be molecular dynamics simulations, but the slowness of typical C-type inactivation makes it unsuitable to this technique, which so far is limited, at best, to gating events occurring in the microsecond range, even when the events are accelerated by unphysiologically extreme voltages (e.g., from −750 to 750 mV; Jensen et al., 2012).
In view of these technical limitations, we have used homology modeling to provide insight into the possible effects of mutating some residues that affect C-type inactivation. The starting point is Kv1.2-2.1 chimera structure analyzed by Long et al. (2007). This channel has a valine at position 377/449, and this, in combination with the high K+ concentration in the crystallization mix, makes the crystallized channel unlikely to be C-type inactivated even at 0 mV (see above). Consistent with this, the Kv1.2-2.1 channel shows no evidence of any obstruction to its ability to complex and conduct K+. Homology models were prepared using the MODELLER algorithm (Eswar et al., 2008), which uses statistical knowledge-based potentials from known structures to predict interatomic distances, rather than relying on force field–based molecular dynamics calculations. A single “mutation,” e.g., V377A, was introduced into the Kv1.2-2.1 structure, and the resulting “mutant” was optimized with the MODELLER algorithm. The result gives only the steady state of the mutant channel, equivalent to a depolarization to 0 mV of long duration.
Fig. 3 A shows the positions of three important residues in the wild-type channel, with an angle of view (constant throughout the figure) selected to make clear the changes caused by mutation. The outer ring of carbonyl oxygens of Y373/Y445 surrounds a K+ ion and marks the filter mouth. The ShB equivalent of residue V377 is T449, perhaps the most studied of all residues involved in C-type inactivation. One of the γ carbons of V377/T449 is in close contact with the lower edge of the phenyl ring of Y373/Y445, whereas W362/W434 is in close contact with the other edge of the ring.
Fig. 3 B shows the result of a mutation in which the valine at position 377/449 is changed to an alanine. This mutation in ShB (T449A) makes C-type inactivation very fast and complete in the steady state at 0 mV (López-Barneo et al., 1993). In the simulated mutant structure, contact between residues A377/A449 and Y373/Y445 is completely lost. This appears to allow Y373/Y445 (in each subunit) to rotate ∼60° (counterclockwise in the view in Fig. 3 B), dilating the filter mouth as each of the Y373/Y445 carbonyl oxygens moves away from the pore axis by ∼1 Å. This, we believe, is the condition of the filter mouth in the C-type inactivated state. It is clear that K+ cannot be well complexed in the simulated mutant, and the probability of K+ occupancy would be decreased by a large factor. With certainty, a dehydrated K+ would not enter this site. One can speculate that a valine at position 377/449 hinders C-type inactivation because one of its γ carbons locks Y373/Y445 so that its carbonyl oxygen remains near the central axis of the pore. Consistent with this idea, the mutation T449V in ShB completely prevents C-type inactivation (López-Barneo et al., 1993).
Fig. 3 C is the simulated mutation W362F/W434F (Perozo et al., 1993). Again, cause and effect are hard to determine, but four points can be noted. (1) The phenyl ring of F362 is rotated clockwise by ∼15° relative to the W362 ring in Fig. 3 A, and the point of contact with the ring of Y373 has moved down and to the left in the figure. (2) The tyrosine ring is rotated counterclockwise in the mutant by ∼20°. This appears to be the result of the altered position of residue 362. (3) Y373/Y445 appears to rotate approximately as a rigid body, and the carbonyl oxygen in all four subunits moves ∼1 Å away from the pore axis, destroying the ability to complex K+. (4) Further rotation seems to be prevented by the presence of the γ carbon of V377/T449. It is worth noting that in ShB, when W434F is combined with T449V, the channel is transformed from nonconducting (virtually complete C-type inactivation with W434F alone) to a conducting channel with fast C-type inactivation (Yang et al., 2002). Overall, the simulated mutations support the idea that the outer selectivity filter site is dilated in the C-type inactivation state.
Cooperativity among subunits
The ShB W434F mutation of even a single subunit strongly predisposes the whole channel to C-type inactivation (Panyi et al., 1995; Yang et al., 1997; Larsson and Elinder, 2000). The reason is evident from the preceding discussion. A mutation that disturbs the support structure of a single tyrosine in the filter mouth could predispose its carbonyl to recede from the pore axis. This would cause a chain reaction: mutual repulsion between the remaining tyrosine carbonyls during intervals of low K+ occupancy would lead to further dilation, still lower K+ occupancy, and full C-type inactivation. Thus, the likelihood and rate of C-type inactivation would increase with the number of subunits mutated. Cooperativity thus arises where the four subunits come together to complex a K+ ion.
Coupling of activation and C-type inactivation
Experimentally, C-type inactivation is more probable after channel opening (Hoshi et al., 1991) and presumably coupled to TMS4 motion. In the Kv1.2-2.1 structure, it is possible to suggest a coupling path from the “foot,” R293/R365, through the “knee,” T380/G452, and along the “thigh” to V377/T449, and thence to the filter mouth (Fig. 4). In ShB experiments, all of the residues on this path have been experimentally implicated in C-type inactivation. Upon activation of the channel, R293/R365 in TMS4 is thought to move outward (toward the reader) from its resting position, assuming the position shown in the figure, in close contact with F344/F416 at the extracellular end of S5, and only two residues away from E346/E418. The mutations E418C and E418Q in ShB strongly predispose the channel to C-type inactivation (Larsson and Elinder, 2000), probably by disturbing hydrogen bonds from E418 to V451 and G452 (in Kv1.2-2.1, which is shown in Fig. 4, E346 is hydrogen bonded to T379 and T380). Further, the double mutation E418C:V451C in ShB leads to formation of a disulfide bond that stabilizes the C-type inactivated state, whereas the double mutation E418C:G452C yields a disulfide bond that stabilizes the noninactivated state (Larsson and Elinder, 2000). Thus it seems reasonable to suppose that TMS4 motion influences the position of E418/E346, V451/T379, and G452/T380.
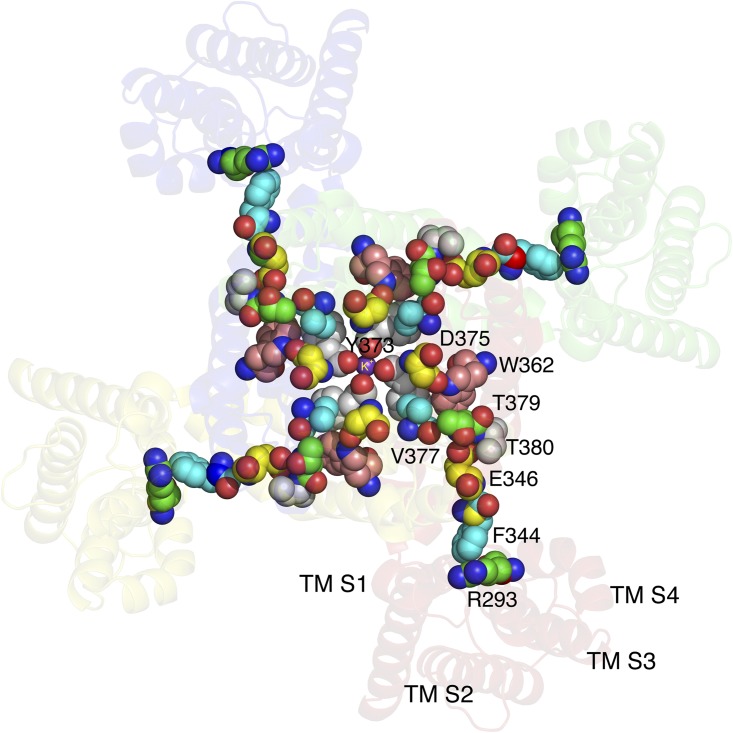
Figure 4.
“Four-legged runner” model of the coupling of S4 activation and C-type inactivation. A Kv1.2-2.1 chimera (2R9R) is viewed from the extracellular side. Consistent with the literature, a conformational wave begins with contact between F344 in TMS5 and R293 in TMS4, when the latter is driven outward by voltage. The wave spreads through the illustrated residues to the region of the pore mouth, causing, in susceptible mutants, dilation of the pore mouth and C-type inactivation. The Kv1.2-2.1 chimera does not undergo C-type inactivation because V377 locks Y373 in position; the pore mouth here is not dilated. All of the residues presented as spheres have been shown to affect C-type inactivation. The carbon atoms of these residues are shown using different colors for clarity. TMS1–TMS4 identify the respective transmembrane segments. Prepared using MacPyMol (version 0.99).
Proceeding from T379/V451 toward the pore mouth along the thigh, all of the ShB residues, from P450 through D447, are known either to change conformation upon C-type inactivation and/or to have a strong effect on the rate of C-type inactivation (Liu et al., 1996; Molina et al., 1997). The presumptive coupling path thus stretches from R293/R365 in S4 to residues immediately adjacent to the filter mouth. We postulate that perturbation of these residues by a conformational change spreading from contact between R293/R365 and F344/F416 destabilizes the filter mouth, causing transient partial dilation and decreased K+ occupancy, which, if sufficiently prolonged, leads to full lasting dilation of the pore mouth and C-type inactivation.
We recognize that experimental elucidation of the coupling pathway may prove to be difficult. One possible approach has been suggested by Yifrach and colleagues (Sadovsky and Yifrach, 2007; Azaria et al., 2010), which focuses on revealing the nature of the transition state separating two kinetically distinguishable states. Required isolation of one transition out of the known numerous states in KV channels (Zagotta et al., 1994; Schoppa and Sigworth, 1998) will be undoubtedly challenging. Alternatively/additionally, various computational methods could be deployed (Pan et al., 2011).
A final note on nomenclature
In the absence of a defined mechanism, the name “C-type inactivation” has been applied to K+ channels with zero K+ inside and out that have altered Ig and Qg (see Kurata and Fedida, 2006), and “P-type inactivation” has been applied to what had formerly been called C-type inactivation, making a right mess. As noted, we believe altered Ig and Qg in the absence of K+ is a mark of defunct (denatured) channels (Fig. 1 D). Hence, we reserve the name C-type inactivation for the state portrayed in Fig. 1 B, consistent with the original nomenclature. U-type inactivation (Kurata and Fedida, 2006) is undefined mechanistically. We believe that it may result from occupancy changes of the outermost K+-binding site in the selectivity filter (Fig. 1, site 1) as voltage is made very positive.
Acknowledgments
This work was supported in part by the public through the National Institutes of Health. The authors declare no conflict of interest.
Edward N. Pugh Jr. served as editor.
References
- Adelman J.P., Bond C.T., Pessia M., Maylie J. 1995. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron. 15:1449–1454 10.1016/0896-6273(95)90022-5 [DOI] [PubMed] [Google Scholar]
- Aldrich R.W., Jr, Getting P.A., Thompson S.H. 1979. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J. Physiol. 291:531–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Armstrong C.M. 1980. Survival of K+ permeability and gating currents in squid axons perfused with K+-free media. J. Gen. Physiol. 75:61–78 10.1085/jgp.75.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaria R., Irit O., Ben-Abu Y., Yifrach O. 2010. Probing the transition state of the allosteric pathway of the Shaker Kv channel pore by linear free-energy relations. J. Mol. Biol. 403:167–173 10.1016/j.jmb.2010.08.041 [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. 1995. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 15:951–960 10.1016/0896-6273(95)90185-X [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. 1996. Two functionally distinct subsites for the binding of internal blockers to the pore of voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 93:13357–13361 10.1073/pnas.93.23.13357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C.M. 1972. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J. Gen. Physiol. 60:588–608 10.1085/jgp.60.5.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S., Cordero-Morales J.F., Perozo E. 2007. A quantitative description of KcsA gating II: Single-channel currents. J. Gen. Physiol. 130:479–496 10.1085/jgp.200709844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W.K., Meves H. 1970. Sodium and potassium currents in squid axons perfused with fluoride solutions. J. Physiol. 211:623–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello L.G., Jogini V., Cortes D.M., Perozo E. 2010. Structural mechanism of C-type inactivation in K+ channels. Nature. 466:203–208 10.1038/nature09153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar N., Eramian D., Webb B., Shen M.Y., Sali A. 2008. Protein structure modeling with MODELLER. Methods Mol. Biol. 426:145–159 10.1007/978-1-60327-058-8_8 [DOI] [PubMed] [Google Scholar]
- Gandhi C.S., Loots E., Isacoff E.Y. 2000. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron. 27:585–595 10.1016/S0896-6273(00)00068-4 [DOI] [PubMed] [Google Scholar]
- Gómez-Lagunas F. 1997. Shaker B K+ conductance in Na+ solutions lacking K+ ions: a remarkably stable non-conducting state produced by membrane depolarizations. J. Physiol. 499:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gutierrez G., Lukk T., Agarwal V., Papke D., Nair S.K., Grosman C. 2012. Mutations that stabilize the open state of the Erwinia chrisanthemi ligand-gated ion channel fail to change the conformation of the pore domain in crystals. Proc. Natl. Acad. Sci. USA. 109:6331–6336 10.1073/pnas.1119268109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538 10.1126/science.2122519 [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. 1991. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 7:547–556 10.1016/0896-6273(91)90367-9 [DOI] [PubMed] [Google Scholar]
- Jensen M.Ø., Jogini V., Borhani D.W., Leffler A.E., Dror R.O., Shaw D.E. 2012. Mechanism of voltage gating in potassium channels. Science. 336:229–233 10.1126/science.1216533 [DOI] [PubMed] [Google Scholar]
- Keating M.T., Sanguinetti M.C. 2001. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 104:569–580 10.1016/S0092-8674(01)00243-4 [DOI] [PubMed] [Google Scholar]
- Kiss L., Korn S.J. 1998. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys. J. 74:1840–1849 10.1016/S0006-3495(98)77894-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata H.T., Fedida D. 2006. A structural interpretation of voltage-gated potassium channel inactivation. Prog. Biophys. Mol. Biol. 92:185–208 10.1016/j.pbiomolbio.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Larsson H.P., Elinder F. 2000. A conserved glutamate is important for slow inactivation in K+ channels. Neuron. 27:573–583 10.1016/S0896-6273(00)00067-2 [DOI] [PubMed] [Google Scholar]
- Levy D.I., Deutsch C. 1996. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys. J. 70:798–805 10.1016/S0006-3495(96)79619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jurman M.E., Yellen G. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867 10.1016/S0896-6273(00)80106-3 [DOI] [PubMed] [Google Scholar]
- Loboda A., Melishchuk A., Armstrong C. 2001. Dilated and defunct K channels in the absence of K+. Biophys. J. 80:2704–2714 10.1016/S0006-3495(01)76239-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.B., Tao X., Campbell E.B., MacKinnon R. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Loots E., Isacoff E.Y. 1998. Protein rearrangements underlying slow inactivation of the Shaker K+ channel. J. Gen. Physiol. 112:377–389 10.1085/jgp.112.4.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S.H., Aldrich R.W. 1993. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71 [PubMed] [Google Scholar]
- MacKinnon R., Aldrich R.W., Lee A.W. 1993. Functional stoichiometry of Shaker potassium channel inactivation. Science. 262:757–759 10.1126/science.7694359 [DOI] [PubMed] [Google Scholar]
- Maher M., Cross M., Wilce M.C., Guss J.M., Wedd A.G. 2004. Metal-substituted derivatives of the rubredoxin from Clostridium pasteurianum. Acta Crystallogr. D Biol. Crystallogr. 60:298–303 10.1107/S090744490302794X [DOI] [PubMed] [Google Scholar]
- Melishchuk A., Loboda A., Armstrong C.M. 1998. Loss of shaker K channel conductance in 0 K+ solutions: role of the voltage sensor. Biophys. J. 75:1828–1835 10.1016/S0006-3495(98)77624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A., Castellano A.G., López-Barneo J. 1997. Pore mutations in Shaker K+ channels distinguish between the sites of tetraethylammonium blockade and C-type inactivation. J. Physiol. 499:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral J.H., Zhou Y., MacKinnon R. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42 10.1038/35102000 [DOI] [PubMed] [Google Scholar]
- Nerbonne J.M., Kass R.S. 2005. Molecular physiology of cardiac repolarization. Physiol. Rev. 85:1205–1253 10.1152/physrev.00002.2005 [DOI] [PubMed] [Google Scholar]
- Ogielska E.M., Aldrich R.W. 1999. Functional consequences of a decreased potassium affinity in a potassium channel pore. Ion interactions and C-type inactivation. J. Gen. Physiol. 113:347–358 10.1085/jgp.113.2.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogielska E.M., Zagotta W.N., Hoshi T., Heinemann S.H., Haab J., Aldrich R.W. 1995. Cooperative subunit interactions in C-type inactivation of K channels. Biophys. J. 69:2449–2457 10.1016/S0006-3495(95)80114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese R., Latorre R., Toro L., Bezanilla F., Stefani E. 1997. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J. Gen. Physiol. 110:579–589 10.1085/jgp.110.5.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A.C., Cuello L.G., Perozo E., Roux B. 2011. Thermodynamic coupling between activation and inactivation gating in potassium channels revealed by free energy molecular dynamics simulations. J. Gen. Physiol. 138:571–580 10.1085/jgp.201110670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G., Sheng Z., Deutsch C. 1995. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys. J. 69:896–903 10.1016/S0006-3495(95)79963-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo L.A., Heinemann S.H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. 1992. Extracellular K+ specifically modulates a rat brain K+ channel. Proc. Natl. Acad. Sci. USA. 89:2466–2470 10.1073/pnas.89.6.2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., MacKinnon R., Bezanilla F., Stefani E. 1993. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 11:353–358 10.1016/0896-6273(93)90190-3 [DOI] [PubMed] [Google Scholar]
- Raghuraman H., Cordero-Morales J.F., Jogini V., Pan A.C., Kollewe A., Roux B., Perozo E. 2012. Mechanism of Cd2+ coordination during slow inactivation in potassium channels. Structure. 20:1332–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray E.C., Deutsch C. 2006. A trapped intracellular cation modulates K+ channel recovery from slow inactivation. J. Gen. Physiol. 128:203–217 10.1085/jgp.200609561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J., Heinemann S.H., Wunder F., Lorra C., Parcej D.N., Dolly J.O., Pongs O. 1994. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature. 369:289–294 10.1038/369289a0 [DOI] [PubMed] [Google Scholar]
- Robinson R.A., Stokes R.H. 2002. Electrolyte Solutions. Dover Publications, Mineola, NY: 571 pp [Google Scholar]
- Roeper J., Lorra C., Pongs O. 1997. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J. Neurosci. 17:3379–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovsky E., Yifrach O. 2007. Principles underlying energetic coupling along an allosteric communication trajectory of a voltage-activated K+ channel. Proc. Natl. Acad. Sci. USA. 104:19813–19818 10.1073/pnas.0708120104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M.C., Tristani-Firouzi M. 2006. hERG potassium channels and cardiac arrhythmia. Nature. 440:463–469 10.1038/nature04710 [DOI] [PubMed] [Google Scholar]
- Schlief T., Schönherr R., Heinemann S.H. 1996. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflügers Arch. 431:483–493 10.1007/BF02191894 [DOI] [PubMed] [Google Scholar]
- Schönherr R., Heinemann S.H. 1996. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J. Physiol. 493:635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa N.E., Sigworth F.J. 1998. Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111:313–342 10.1085/jgp.111.2.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.L., Baukrowitz T., Yellen G. 1996. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 379:833–836 10.1038/379833a0 [DOI] [PubMed] [Google Scholar]
- Spector P.S., Curran M.E., Zou A.R., Keating M.T., Sanguinetti M.C. 1996. Fast inactivation causes rectification of the IKr channel. J. Gen. Physiol. 107:611–619 10.1085/jgp.107.5.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J.G., Kuschel L., Rayner M.D., Heinemann S.H. 1997. Ion conduction through C-type inactivated Shaker channels. J. Gen. Physiol. 110:539–550 10.1085/jgp.110.5.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J.G., Kuschel L., Rayner M.D., Heinemann S.H. 1998. Macroscopic Na+ currents in the “nonconducting” Shaker potassium channel mutant W434F. J. Gen. Physiol. 112:85–93 10.1085/jgp.112.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpe L.C., Schwarz T.L., Tempel B.L., Papazian D.M., Jan Y.N., Jan L.Y. 1988. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 331:143–145 10.1038/331143a0 [DOI] [PubMed] [Google Scholar]
- Yang Y., Yan Y., Sigworth F.J. 1997. How does the W434F mutation block current in Shaker potassium channels? J. Gen. Physiol. 109:779–789 10.1085/jgp.109.6.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yan Y., Sigworth F.J. 2002. The Shaker mutation T449V rescues ionic currents of W434F K+ channels. Biophys. J. 82:234e [Google Scholar]
- Yellen G. 1998. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31:239–295 10.1017/S0033583598003448 [DOI] [PubMed] [Google Scholar]
- Yellen G., Sodickson D., Chen T.Y., Jurman M.E. 1994. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys. J. 66:1068–1075 10.1016/S0006-3495(94)80888-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. 1989. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with Shaker mRNA. Proc. Natl. Acad. Sci. USA. 86:7243–7247 10.1073/pnas.86.18.7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. 1994. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 103:321–362 10.1085/jgp.103.2.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Morais-Cabral J.H., Mann S., MacKinnon R. 2001. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661 10.1038/35079500 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Morais-Cabral J.H., Kaufman A., MacKinnon R. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48 10.1038/35102009 [DOI] [PubMed] [Google Scholar]