Abstract
In skeletal muscle, excitation leads to increased [Na+]i, loss of K+, increased [K+]o, depolarization, and Cl− influx. This study quantifies these changes in rat extensor digitorum longus (EDL) muscles in vitro and in vivo using flame photometric determination of Na+ and K+ and 36Cl as a tracer for Cl−. In vitro, 5-Hz stimulation for 300 s increased intracellular Na+ content by 4.6 ± 1.2 µmol/g wet wt (P < 0.002) and decreased intracellular K+ content by 5.5 ± 2.3 µmol/g wet wt (P < 0.03). This would increase [K+]o by 28 ± 12 mM, sufficient to cause severe loss of excitability as the result of inactivation of Na+ channels. In rat EDL, in vivo stimulation at 5 Hz for 300 s or 60 Hz for 60 s induced significant loss of K+ (P < 0.01), sufficient to increase [K+]o by 71 ± 22 mM and 73 ± 15 mM, respectively. In spite of this, excitability may be maintained by the rapid and marked stimulation of the electrogenic Na+,K+ pumps already documented. This may require full utilization of the transport capacity of Na+,K+ pumps, which then becomes a limiting factor for physical performance. In buffer containing 36Cl, depolarization induced by increasing [K+]o to 40–80 mM augmented intracellular 36Cl by 120–399% (P < 0.001). Stimulation for 120–300 s at 5–20 Hz increased intracellular 36Cl by 100–188% (P < 0.001). In rats, Cl− transport in vivo was examined by injecting 36Cl, where electrical stimulation at 5 Hz for 300 s or 60 Hz for 60 s increased 36Cl uptake by 81% (P < 0.001) and 84% (P < 0.001), respectively, indicating excitation-induced depolarization. Cl− influx favors repolarization, improving K+ clearance and maintenance of excitability. In conclusion, excitation-induced fluxes of Na+, K+, and Cl− can be quantified in vivo, providing new evidence that in working muscles, extracellular accumulation of K+ is considerably higher than previously observed and the resulting depression of membrane excitability may be a major cause of muscle fatigue.
INTRODUCTION
It is well established that in skeletal muscle, excitation leads to a rapid influx of Na+. This is blocked by tetrodotoxin, a specific inhibitor of Na+ channels (Gissel and Clausen, 1999) and is immediately followed by an efflux of K+ leading to a rise in [K+]o (Sejersted and Sjøgaard, 2000). The ensuing depolarization is counterbalanced by stimulation of active electrogenic Na+,K+ transport and influx of Cl−. However, as emphasized in a review (Allen et al., 2008), too little is known about the time course and magnitude of these ion fluxes and the changes in local concentrations of Na+, K+, and Cl− during low-frequency stimulation in vivo. A recent study indicates that during electrical stimulation (60 Hz for 30 s) of isolated intact rat extensor digitorum longus (EDL) muscle, the excitation-induced net loss of cellular K+, measured by flame photometry, leads to a considerably higher level (52 mM) in the mean [K+]o (Clausen, 2008a) than previously recorded in working muscles using K+-sensitive microelectrodes (Hník et al., 1976) or microdialysis probes (Nielsen et al., 2004). Moreover, after 150 s of intermittent stimulation at a mean frequency of 15 Hz, [K+]o of isolated rat EDL muscle reached 74 mM (Clausen, 2011). This would severely impair excitability and raised questions about how K+ could be adequately cleared from the extracellular space in working muscles. However, because these experiments were performed in vitro, it could be argued that the excitation-induced net loss of K+ and the calculated rise in [K+]o were underestimated because of the lower temperature used for the incubation (30°C) and the higher extracellular space found in vitro compared with in vivo (Sheff and Zacks, 1982). Therefore, the primary purpose of the present study is to develop procedures allowing the quantification of the effects of excitation on [K+]o and [Na+]o in EDL muscles of the anaesthetized rat in vivo. It has repeatedly been proposed that the excitation-induced elevation of [K+]o is an important cause of muscle fatigue (Fenn, 1940; Sjøgaard, 1990; Cairns et al., 1997; Clausen 2003), mainly because K+ induces slow inactivation of Na+ channels (Ruff et al., 1988). At variance with the classical fast inactivation, which is not operative around the resting membrane potential, slow inactivation is operative around the resting potential and may partially explain why small depolarizations, e.g., those occurring in some patients with periodic paralysis, can reduce excitability. This effect is likely to depend on the excitation-induced rise in [K+]o and the frequency of action potentials. In keeping with this, the rate of fatigue (determined as force decline) in human muscle is closely correlated to the frequency of stimulation (Sacco et al., 1994). In isolated rat soleus and EDL muscles, force decline during continuous stimulation shows a highly significant correlation to the excitation-induced rise in extracellular K+ (Clausen, 2008b).
It is well established that excitation increases intracellular Cl− in skeletal muscle of animals (Krnjevic and Miledi, 1958; Lindinger et al., 1987) and human subjects (Sahlin et al., 1978), and it is generally accepted that Cl− ions play an important role in the stabilization of the membrane potential (Hodgkin and Horowicz, 1959; Cairns et al., 2004; Pedersen et al., 2005; Dutka et al., 2008). The depolarization induced by the post-excitatory accumulation of K+ in the extracellular space may favor the influx of Cl− ions along their transmembrane concentration gradient. Cl− influx induces repolarization, which favors the uptake of K+ via inward rectifier K+ channels. Thus, Cl− is essential for the maintenance of excitability. Lack of Cl− channel function leads to myotonia (Gurnett et al., 1995). In keeping with this, it was observed that the omission of Cl− from the incubation medium caused a 14-fold increase in the rate of force decline measured during the first 10 s of continuous 60-Hz stimulation in rat EDL muscle (Clausen, 2011). As a follow up on this observation, and a supplement to the abovementioned studies of excitation-induced Na+,K+ fluxes, the present study quantifies the excitation-induced influx of 36Cl and its intracellular concentration during and after electrical stimulation at varying frequencies and durations. These values were compared with the changes in intracellular Cl− induced by elevated extracellular K+ in rat EDL muscle. The excitation-induced uptake of 36Cl was also quantified in vivo. The following working hypotheses were examined: (a) the excitation-induced net loss of K+ and gain of Na+ in rat EDL muscle can be quantified in vivo, allowing calculation of the changes in extracellular concentrations of K+ and Na+ at varying frequencies and durations of electrical stimulation; (b) elevation of [K+]o, leading to depolarization of the muscle cells, induces a rise in intracellular 36Cl content in vitro; and (c) excitation induces 36Cl uptake that can be quantified in vivo and compared with similar data obtained in vitro. This is important for repolarization during and after work.
MATERIALS AND METHODS
Background for incubation procedures
The present experiments were performed using rat EDL muscles, which contain predominantly fast-twitch fibers. In a recent study (Clausen, 2008a), it was examined whether the net loss of K+ from EDL was higher when the muscles were stimulated in buffer than in air, where loss of K+ into the surroundings of the muscle could be excluded. After 30 s of continuous stimulation at 60 Hz in Krebs-Ringer bicarbonate buffer (KR buffer) or in air, extracellular Na+ and K+ were removed by washing the muscles four times for 15 min in ice-cold Na+-free Tris-sucrose buffer, blotting, weighing, and flame photometric determination of total K+ contents. The difference between K+ contents of resting and contralateral stimulated muscles in the muscle pairs kept in air (9.1 ± 1.7 µmol/g wet wt) and those kept in buffer (9.5 ± 1.4 µmol/g wet wt) was not significantly different (n = 9 vs. 7; P = 0.87). This shows that during the 30 s of stimulation in KR buffer, the mean concentration of K+ in the extracellular spaces of EDL muscles reaches the same level as in the muscles stimulated in air (48.6 mM and 46.7 mM, respectively). In keeping with this, the muscles incubated in KR buffer and in air for 300 s showed close similarity in endurance and force (Fig. 3 in Clausen, 2008a). These results indicate that even when EDL muscles are incubated during continuous gassing in standard KR buffer, the net release of K+ from the extracellular space into the buffer is modest within the first 5 min. Thus, it was reasonable to assume that the amount of K+ released into the extracellular space could be quantified by measuring the K+ released into the incubation medium during a subsequent much longer (four times for 15 min) washout in ice-cold, Na+-free buffer, where there is no loss of intracellular K+ (see below). The values for net loss of K+ obtained from measuring the increase in the K+ content of the washing buffer were not significantly different from those obtained measuring the net loss of K+ from the muscle by deducting the K+ content of the stimulated muscles from that of the resting contralateral muscles (Clausen et al., 2004). Measurements of the K+ residing in the extracellular space provide information about the mean extracellular K+ ([K+]o) reached at the end of electrical stimulation.
Animals, preparation, and incubation of muscles
All handling and use of rats complied with Danish animal welfare regulations, including the euthanasia, which in addition was approved by the Animal Welfare Officer of Aarhus University. All experiments were performed using 4–5-wk-old Wistar rats bred at the Department of Biomedicine, Aarhus University, weighing 60–80 g. The animals were fed ad libitum and kept in a thermo-stated environment at 21°C with a 12/12-h light/dark cycle. Animals were killed by cervical dislocation, followed by decapitation. Intact EDL muscles weighing 20–30 mg were immediately dissected out during wash with 154 mM NaCl at room temperature, mounted at resting length, and equilibrated at 30°C in standard KR buffer containing the following (in mM): 122.2 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2, and 5.0 d-glucose. The pH was maintained at 7.4 by continuous gassing with a humidified mixture of 95% O2 and 5% CO2. In some experiments, where the concentration of K+ was augmented to 40, 60, or 80 mM, osmolarity was maintained by replacing NaCl with equimolar amounts of KCl.
In most experiments, the isolated muscles were stimulated via platinum wire electrodes placed around the mid-portion of the muscle so as to obtain optimal excitation. Trains of 10-V pulses of 0.05–1.0-ms duration were used. Immediately after the cessation of the stimulation, the muscles were washed with ice-cold Na+-free Tris-sucrose buffer to remove extracellular Na+ and K+. The contralateral control muscles were not stimulated but exposed to a similar positioning of electrodes around the muscle and the same washing with ice-cold Na+-free Tris-sucrose buffer. Finally, all muscles were blotted on dry filter paper, weighed, and taken for flame photometric determination of Na+ and K+ contents.
Measurements of Na+,K+ contents, Cl− content, 36Cl uptake, [14C]sucrose space, and water content
The total contents of Na+ and K+ were measured by soaking the muscles overnight in ice-cold 0.3 M trichloroacetic acid (TCA). Samples of this extract were taken for counting of isotopes and flame photometry (Radiometer). In several experiments, the Na+,K+ contents of the muscles were determined after rest or stimulation in KR buffer or in air. This was primarily done by washing the muscles during continuous gassing with air four times for 15 min in ice-cold Na+-free Tris-sucrose buffer, followed by blotting on dry filter paper and wet weight determination. The Na+-free Tris-sucrose buffer had the following composition (in mM): 263 sucrose, 10 Tris-HCl, 2.8 KCl, 1.3 CaCl2, 1.2 MgSO4, and 1.2 KH2PO4, pH 7.4. We have previously shown that during wash in this buffer, the K+ content of EDL muscles remains constant for 150 min, whereas the intracellular Na+ content undergoes a slow decrease that can be corrected for by multiplying with a constant, 1.59 per hour of washout (Murphy et al., 2006). Thus, by removing extracellular Na+, as well as the K+ released from the working muscle cells, without causing further loss of intracellular K+, this procedure allows the determination of the total cellular contents of Na+ and K+. In the present study, it was confirmed that during 60 or 120 min of washout in Na+-free Tris-sucrose buffer, EDL muscles showed no significant net loss of K+ (100.4 ± 1.3 µmol/g wet wt [n = 11 muscles] before washout and after 60 or 120 min of washout 100.6 ± 1.4 µmol/g wet wt [n = 14 muscles] and 103.3 ± 2.0 µmol/g wet wt [n = 4 muscles], respectively). The excitation-induced release of intracellular K+ into the extracellular space is calculated from the difference between the K+ contents of resting and stimulated muscle. In some control experiments, the excitation-induced loss of K+ was determined by measuring the K+ content of the ice-cold washout buffer at the end of washout. The values are expressed as µmol/g wet wt and converted to the mean concentration of K+ in the extracellular space measured using [14C]sucrose (Clausen, 2008a). In vitro [14C]sucrose space was determined by preincubating muscles for 90 min in KR buffer containing 1 mM unlabeled sucrose (as a carrier) and 0.2 µCi/ml [14C]sucrose. Then the muscles were either resting or stimulated as indicated in Results, blotted, weighed, and soaked overnight in 0.3 M TCA. This extract was taken for counting of 14C activity. The [14C]sucrose space was calculated on the basis of the [14C]sucrose activity in the tissue and in the KR buffer. Dry weight and total water content as determined by drying muscles overnight at 60°C to constant weight were 24.2% and 75.8%, respectively (n = 33 muscles). In these in vitro experiments, intracellular water space of resting muscles was calculated by deducting [14C]sucrose space (see Table 4) from total water content (75.8% − 24.2% = 51.6%).
Table 1.
Effect of electrical stimulation on total contents of K+ and Na+ in isolated rat EDL muscle in vitro
| Experimental conditions | Total K+ content | Total Na+ content |
| µmol/g wet wt | µmol/g wet wt | |
| Resting muscles | 103.7 ± 2.1 (9) | 13.6 ± 1.0 (9) |
| 5-Hz stimulation, 300 s | 98.2 ± 0.9 (9)a | 18.2 ± 0.6 (9)b |
| Resting muscles | 102.8 ± 1.9 (5) | 11.8 ± 0.6 (5) |
| 60-Hz stimulation, 60 s | 92.8 ± 1.9 (5)c | 21.6 ± 0.4 (5)d |
The muscles were mounted in force transducers for measurement of isometric contractions, adjusted to optimal length, and equilibrated for 30 min in KR buffer at 30°C during gassing with a mixture of 95% O2 and 5% CO2. Continuous direct stimulation was performed using 10-V, 1-ms pulses at 5 or 60 Hz. After stimulation, all muscles were immediately washed four times for 15 min in ice-cold Na+-free Tris-sucrose buffer, blotted, weighed, and taken for flame photometric determination of Na+ and K+ contents. The contralateral muscles from the same animals were allowed to rest, and their content of Na+ and K+ was compared with that of the stimulated muscles. All values are given as means ± SEM, with the number of muscles in parentheses.
P < 0.03 compared with resting muscles.
P < 0.002 compared with resting muscles.
P < 0.006 compared with resting muscles.
P < 0.001 compared with resting muscles.
To assess whether 36Cl is a valid and representative tracer for Cl− in the muscles, Cl− was measured by titration using an ABU 91 autoburette (Radiometer) and compared with the uptake of 36Cl in EDL during 90-min incubation in KR buffer, sufficient to achieve equilibration of 36Cl in rat EDL (Hayward and Barchi, 1980). The uptake of 36Cl in EDL as measured by counting in a β-counter (Tri-Carb 2100 TR; Packard) corresponded to 96.8% of the total Cl− content measured in the same extract by titration (n = 8 vs. 8 muscles).
The excitation-induced uptake of 36Cl was measured in muscles equilibrated for 90 min in KR buffer containing 0.4–1.0 µCi/ml 36Cl. After this equilibration, the muscles were transferred to empty incubation chambers thermostatically maintained at 30°C, and the drips of buffer adhering to the surface of the muscle and the inner walls of the incubation chamber were carefully removed using thin folded strips of dry filter paper. Thus, it could be assumed that during the subsequent electrical stimulation, intracellular uptake of 36Cl could only take place from the interstitial water space of the muscle. Incubation in the absence of buffer was earlier shown to allow maintenance of contractile performance for at least 8 min at 30°C (Clausen, 2008a). During the incubation in the absence of buffer, the muscles were stimulated or allowed to rest. The muscles were then washed for the indicated intervals (10–60 min) in ice-cold Na+-free Tris-sucrose buffer, blotted, weighed, and taken for counting of 36Cl and flame photometric determination of Na+ and K+.
In vivo experiments
These were performed to quantify the excitation-induced gain of Na+ and loss of K+ in EDL muscles in the intact animal (rats weighing 60–80 g). Parallel to these experiments, the extracellular space was measured by giving an intraperitoneal injection of [14C]sucrose (2–5 µCi and 10 µmol unlabeled sucrose [as a carrier] per animal) and allowing 60 min for equilibration of this marker. The rats were then anaesthetized by giving an intraperitoneal injection of 0.11 ml of a solution containing 0.55 mg fluanisone, 0.0173 mg fentanyl citrate, and 0.275 mg midazolam, causing full analgesia in 10–15 min. The animals were placed on their back, and the legs fixed with sports tape. When analgesia was fully developed, a blood sample of 0.5 ml was taken from a tail vein using a thin cannula (0.5 mm × 16 mm Microlance; BD) and centrifuged, and plasma was withdrawn for sedimentation of proteins with 0.3 M TCA and counting of 14C activity in the clear supernatant obtained by the centrifugation. Then the EDL muscle was dissected out, blotted on dry filter paper, and weighed, and the 14C activity was extracted in 0.3 M TCA. It can be assumed that after 60 min [14C]sucrose is equilibrated in the extracellular space and plasma of the rat in vivo (Law and Phelps, 1966), allowing calculation of the water space available to this marker in the whole EDL muscle. In contrast to the muscles used for measuring Na+,K+ and 36Cl− content, the muscles used for measuring [14C]sucrose space in vivo were not washed in the cold. In 19 resting muscles, the [14C]sucrose space was 18.2 ± 1.1% and in 20 muscles stimulated at 60 Hz for 60 s 17.7 ± 1.0%, which is not significantly different from that of the resting muscles (P = 0.70). Dry weight and total water content were 24% and 76%, respectively. The wet weight of the 19 resting muscles was 29.1 ± 0.7 mg and that of the 20 stimulated muscles 29.7 ± 0.5 mg (P = 0.53). In another experiment, there was no significant difference between the wet weight of resting muscles (29.2 ± 1.2 mg) and muscles stimulated at 5 Hz for 300 s in vivo (30.9 ± 1.7 mg; n = 7 vs. 7; P = 0.43). Intracellular water space in vivo was calculated by deducting [14C]sucrose space from total water content (76% − 18% = 58%). The muscles used for measuring [14C]sucrose space were taken for determination of total Na+ and K+ content. There was no significant difference between the Na+ content of resting muscles (26.3 ± 0.8 µmol/g wet wt) and muscles stimulated at 60 Hz for 60 s (27.6 ± 0.7 µmol/g wet wt; n = 19 vs. 20; P = 0.22). Neither was there any significant difference between the K+ content of resting muscles (103.2 ± 2.7 µmol/g wet wt) and stimulated muscles (101.0 ± 2.6 µmol/g wet wt; n = 19 vs. 20; P = 0.57). This shows that the differences in cellular Na+ and K+ contents between resting and stimulated muscles shown in Table 3 require removal of extracellular Na+ and K+ by washout in the cold to be detected. These results also indicate that the net loss of K+ observed in stimulated muscles is not the outcome of a more direct and efficient clearance of K+ via the capillaries.
Table 3.
Effect of electrical stimulation on total contents of Na+ and K+ in rat EDL muscle in vivo
| Experimental conditions | Total K+ content | Total Na+ content |
| µmol/g wet wt | µmol/g wet wt | |
| Resting muscles | 98.2 ± 2.2 (8) | 13.5 ± 1.0 (8) |
| 5-Hz stimulation, 300 s | 85.6 ± 3.3 (8)a | 21.7 ± 1.0 (8)b |
| Resting muscles | 90.8 ± 1.9 (6) | 10.7 ± 0.5 (6) |
| 60-Hz stimulation, 60 s | 77.7 ± 1.8 (7)b | 17.1 ± 0.5 (7)b |
As described in Materials and methods, rats were anaesthetized and the EDL muscles stimulated at resting length using platinum wire electrodes and 1-ms pulses at 10 V applied at the durations and frequencies indicated. After stimulation, the muscles were immediately washed four times for 15 min in ice-cold Na+-free Tris-sucrose buffer, blotted, weighed, and taken for flame photometric determination of Na+ and K+ content. Values are given as mean ± SEM, with the number of muscles in parentheses.
P < 0.007 compared with resting muscles.
P < 0.001 compared with resting muscles.
For the study of the effects of electrical stimulation on the distribution of Na+ and K+, the skin covering the frontal surface of the hind leg was opened, and the tendon of the tibialis anterior muscle was grasped using a surgical forceps. The muscle was gently and slowly drawn aside so that the EDL muscle could be reached. The two branches of a platinum wire electrode were placed around the mid-portion of the EDL, and the muscle was stimulated at its resting length using pulse trains of 10 V at the duration and frequency indicated in the legends and text. Immediately after the cessation of the stimulation, ice-cold Na+-free Tris-sucrose buffer was dripped from a pipette on the muscle to obtain rapid cooling, which markedly inhibits the efflux of K+ and Na+,K+ pump–mediated reaccumulation of K+ (the Na+,K+ pumps in rat skeletal muscle have a Q10 of 2.3 [Clausen, 2003]). 5 s later, the muscle was cut free across the tendons, carefully avoiding damage to the muscle fibers. Then the muscle was transferred to a perforated polyethylene cylinder that was washed four times for 5 min or four times for 15 min in ice-cold Na+-free Tris-sucrose buffer during continuous gassing with air. Henceforth, the muscles were blotted on dry filter paper, weighed, and soaked overnight in ice-cold 0.3 M TCA for extraction of Na+ and K+. The TCA extract was then taken for flame photometric determination of Na+ and K+ contents.
The excitation-induced uptake of 36Cl in EDL in vivo was measured by injecting 200 µl of a 154 mM NaCl solution containing 4 µCi 36Cl intraperitoneally. The isotope was allowed to equilibrate in the water space of the animal for 90 min (like in the in vitro experiments with 36Cl). 15 min before the end of this period, the rat was anaesthetized by intraperitoneal injection of analgesics as described above. A blood sample was withdrawn from a tail vein for measurement of the content of 36Cl in plasma. At the end of the equilibration period, EDL was exposed and stimulated as indicated above. Immediately after the stimulation, the muscle was washed with ice-cold Na+-free Tris-sucrose buffer, cut free, and washed four times for 5 min in ice-cold Tris-sucrose, blotted, weighed, and soaked in 0.3 M TCA, and the extract was taken for counting of 36Cl.
Chemicals and isotopes
All chemicals used were of analytical grade. [14C]sucrose was obtained from GE Healthcare and 36Cl from the Hevesy Laboratory, Risø National Laboratory.
Statistics
All data are presented as means with SEM. The statistical significance of a difference between two groups was ascertained using the Student’s two-tailed t test for nonpaired observations.
RESULTS
Effects of electrical stimulation on Na+,K+ content and extracellular concentration of K+ ([K+]o) and Na+ ([Na+]o) in vitro
As shown in Table 1, electrical stimulation of isolated EDL muscles induces a significant increase in total Na+ content and a significant decrease in total K+ content. After 5- and 60-Hz stimulation, the net loss of K+ is 5.5 ± 2.3 µmol/g wet wt and 10.0 ± 2.7 µmol/g wet wt, respectively. The excitation-induced net loss of intracellular K+ takes place into the extracellular space, causing a rise in [K+]o. This rise can be calculated by dividing the net loss of K+ by the extracellular volume (ECV), which as measured in vitro using [14C]sucrose amounts to 19.4% of muscle wet weight or 0.194 ml/g muscle wet wt in the stimulated muscles (see Table 4). From the data shown in Table 1, the calculated rise in [K+]o in the extracellular space after 5- and 60-Hz stimulation is 28 ± 12 mM (5.5 ± 2.3/0.194) and 52 ± 14 mM (10.0 ± 2.7/0.194), respectively. Stimulation at 5 or 60 Hz increases total intracellular Na+ content by 4.6 ± 1.2 µmol/g wet wt and 9.8 ± 0.7 µmol/g wet wt, respectively. Because these increases reflect a loss of Na+ from the extracellular space, it can be calculated that stimulation at 5 and 60 Hz reduces the extracellular Na+ concentration by 24 ± 6 mM (4.6 ± 1.2/0.194) and 51 ± 4 mM (9.8 ± 0.7/0.194), respectively. When the washout time is reduced from 60 to 20 min, there is no change in the measured excitation-induced loss of K+, indicating that this is not taking place during the washout in the ice-cold Na+-free Tris-sucrose buffer, in keeping with the results of the longer lasting washout experiments described in Materials and methods. The gain in intracellular Na+ must happen during the electrical stimulation in KR buffer because it cannot take place during the washout in the Na+-free buffer. Most of the excitation-induced loss of K+ was already observed after 15 s of stimulation at 60 Hz, amounting to 9.9 ± 1.4 µmol/g wet wt, sufficient to increase [K+]o by 51 ± 7 mM (9.9 ± 1.4/0.194; n = 6 vs. 6 muscles; P < 0.001).
Table 4.
Effect of electrical stimulation on the relative uptake of [14C]sucrose and 36Cl in rat EDL muscle in vitro
| Experimental conditions | [14C]sucrose space | 36Cl space |
| % | % | |
| Rest | 24.7 ± 1.8 (6) | 23.6 ± 0.4 (6) |
| 60-Hz stimulation, 120 s | 19.4 ± 0.4 (6) | 22.6 ± 0.6 (6) |
Isolated rat EDL muscles were mounted in force transducers for isometric contractions and preequilibrated for 15 min in KR buffer at 30°C, followed by 90 min of incubation in KR buffer with 0.4 µCi/ml 36Cl or 0.2 µCi/ml [14C]sucrose and 1 mM of unlabelled sucrose (as a carrier). Finally, six of the muscles were stimulated for 120 s at 60 Hz using 0.2-ms, 10-V pulses. Six other muscles were resting. The muscles were blotted on dry filter paper, weighed, and taken for counting of 36Cl and 14C activity. On the basis of the 36Cl and 14C activity of the incubation medium, the relative uptake of the isotopes was expressed as a percentage and given as mean ± SEM, with the number of muscles in parentheses. For example, a 36Cl uptake of 23.6% means that 1 g of muscle (wet weight) has taken up 36Cl corresponding to that contained in 0.236 ml of incubation medium.
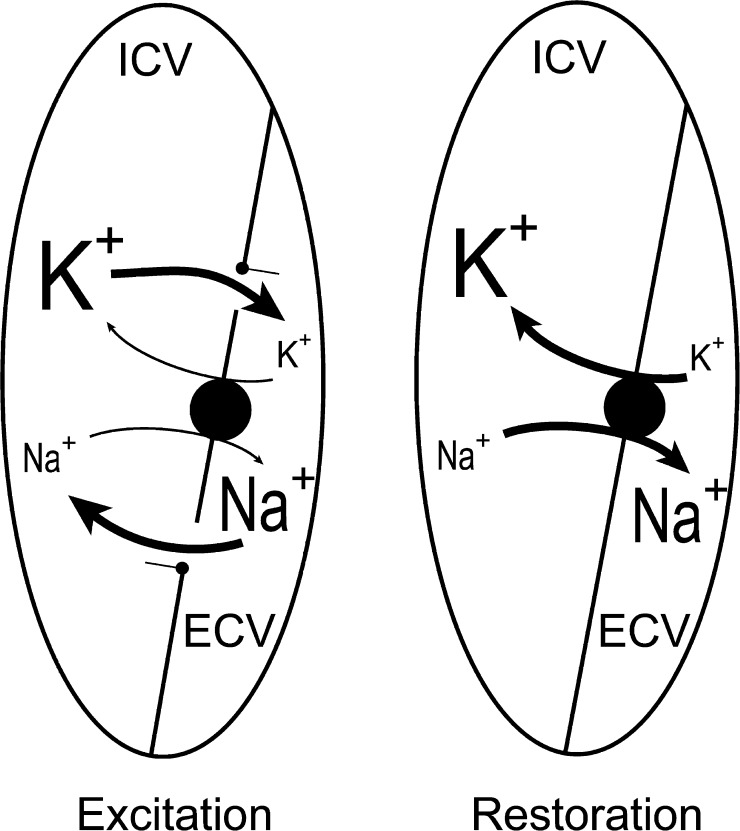
As a test of muscle integrity and the reversibility of the excitation-induced loss of K+ and gain of Na+, the effects of 60 s of 60-Hz stimulation with the muscles mounted in empty incubation chambers were examined without or with a subsequent recovery period of 600 s. As shown in Table 2, this stimulation caused a net loss of K+ of 11.0 ± 2.0 µmol/g wet wt, sufficient to cause a rise in [K+]o of 57 ± 10 mM. When the muscles after the stimulation were allowed to stay in the empty chambers and rest for 600 s, K+ contents as well as Na+ contents were completely restored to those of the unstimulated muscles. This indicates that all the K+ lost during the 60 s of stimulation into the ECV (0.194 ml/g wet wt) was returning to the muscle cells during the 600 s of poststimulation rest. Simultaneously, the Na+ taken up into the cells was completely reextruded into the ECV. As illustrated in the diagram in Fig. 1, a muscle mounted in air contains two defined compartments, the intracellular volume (ICV) and the ECV, and may be used to study the exchange of Na+ and K+ between these two volumes, undisturbed of exchange with an incubation buffer.
Table 2.
Reversibility of the effects of 60-Hz stimulation on the contents of Na+ and K+ in rat EDL in vitro
| Experimental conditions | Total K+ content | Total Na+ content |
| µmol/g wet wt | µmol/g wet wt | |
| Resting muscles | 102.7 ± 1.7 (3) | 12.9 ± 0.4 (3) |
| 60-Hz stimulation, 60 s | 91.7 ± 1.1 (6)a | 24.1 ± 0.9 (6)b |
| 60-Hz stimulation, 60 s + 600-s rest | 105.8 ± 0.8 (3)c | 12.3 ± 0.4 (3)c |
The muscles were mounted in force transducers for measurement of isometric contractions, adjusted to optimal length, and equilibrated for 30 min at 30°C during gassing with a mixture of 95% O2 and 5% CO2. Then all muscles were moved to empty tubes, and the last drips of the buffer adhering to the muscle surface were carefully removed. One group of three muscles was left resting for 60 s, another group of six muscles was stimulated for 60 s at 60 Hz using 0.2-ms pulses at 10 V, and a third group of three muscles was stimulated for 60 s at 60 Hz using 0.2-ms pulses at 10 V and then left resting for 600 s in the empty tubes, allowing reaccumulation of the K+ lost to the extracellular space into the muscle cells (see Fig. 1). Finally, all muscles were washed four times for 15 min in ice-cold Na+-free Tris-sucrose buffer, blotted, weighed, and taken for flame photometric determination of Na+ and K+ contents. All values are given as means ± SEM, with the number of muscles in parentheses.
P < 0.001 compared with resting muscles.
P < 0.01 compared with resting muscles.
P < 0.001 compared with 60-Hz stimulation, 60 s.
Figure 1.
Diagram of excitation-induced exchange of Na+ and K+ between ECV and ICV. See Table 2. Excitation (left) is induced by a rapid influx of Na+ (11.2 µmol/g wet wt) from ECV into ICV, followed by an equivalent efflux of K+ (11.0 µmol/g wet wt) from ICV into the ECV. This takes place while the muscles are in air, and during the following washout in ice-cold Na+-free Tris-sucrose, 11 µmol/g wet wt of K+ is removed from the ECV as can be seen from the loss of 11 µmol/g wet wt while the Na+ taken up into ICV stays in the ICV, leading to a reduction in total K+ content and a similar increase in total Na+. As shown in Table 2, the Na+,K+ contents are restored during the 600-s incubation without buffer by stimulation of the Na+,K+ pumps. When the muscles are incubated in air, these movements of Na+ and K+ may be followed undisturbed of exchange with a surrounding incubation medium.
Previous experiments (Clausen, 2011) showed that when EDL muscles were stimulated at 60 Hz in air, force declined by 80% in 30 s and the net loss of K+ reached 9.5 ± 3.0 µmol/g wet wt, sufficient to cause a rise in extracellular K+ of 46 ± 14 mM (9.5 ± 3.0/0.207; please note that in those experiments, ECV was 0.207 ml/g wet wt). Very similar results were obtained with EDL muscles incubated and stimulated in KR buffer, where it could be calculated that during 30 s of 60-Hz stimulation, [K+]o increased by 46 ± 7 mM, sufficient to cause almost complete suppression of excitability as well as further loss of K+. During the last 15 s of the 30-s stimulation, the increase in [K+]o was only one-fifth of the increase observed during the first 15 s (Clausen, 2011). The general implication is that in these stimulation experiments, most of the excitation-induced loss of K+ takes place in the early phase, indicating that as the result of progressive extracellular accumulation of K+, excitation is a self-limiting process.
Effect of electrical stimulation on Na+,K+ contents, [14C]sucrose space, and [K+]o in vivo
As shown in Table 3, direct stimulation of EDL muscles in vivo using 10-V pulses of 1 ms at 5 Hz for 300 s caused a net loss of K+ of 12.6 ± 3.9 µmol/g wet wt (n = 8 vs. 8 muscles; P < 0.007). With the extracellular space of 17.7% (0.177 ml/g wet wt) measured after stimulation in vivo, this would be sufficient to cause an increase in mean [K+]o of 71 ± 22 mM (12.6 ± 3.9/0.177). The concomitant increase in Na+ content was 8.2 ± 1.5 µmol/g wet wt. Because this Na+ is lost from the extracellular space into the muscle cells, [Na+]o can be expected to decrease by 46 ± 8 mM (8.2 ± 1.5/0.177). As shown in Table 3, when stimulating in vivo at 60 Hz for 60 s, the net loss of K+ from the muscles reached 13.1 ± 2.6 µmol/g wet wt (6 vs. 7 muscles; P < 0.001), sufficient to cause an increase in [K+]o of 74 ± 15 mM (13.1 ± 2.6/0.177). The concomitant increase in Na+ content of the EDL muscle was 6.4 ± 0.7 µmol/g wet wt (P < 0.001). Because this Na+ is lost from the extracellular space into the muscle cells, [Na+]o can be expected to decrease by 36 ± 4 mM (6.5 ± 0.7/0.177). Hence, like in the in vitro experiments, the excitation-induced increase in intracellular Na+ content is somewhat smaller than the decrease in K+ content. In an earlier study, the excitation-induced increase in Na+ content was more similar to the rise in K+ content (Clausen, 2011), in keeping with the well-established selective one for one exchange of Na+ and K+ causing the action potentials.
In another in vivo experiment, 1-ms pulses given at 60 Hz for 60 s induced a net loss of K+ of 13.2 ± 1.6 µmol/g wet wt (P < 0.001), which could increase [K+]o by 75 ± 9 mM (13.2 ± 1.6/0.177; n = 7 vs. 8 muscles). In the same experiment, the net loss of K+ into the ice-cold Tris-sucrose buffer during the quadruple 15-min washout was also measured. A value of 9 ± 1.6 µmol/g wet wt was obtained, corresponding to 68% recovery of the measured net decrease in the K+ content of the muscles. Within the 60 s of stimulation, this quantity of K+ could have increased [K+]o by 51 ± 9 mM (9 ± 1.6/0.177). It is reasonable to assume that the incomplete recovery is caused by inevitable and partial washout of extracellular K+ taking place during the cooling of the muscles by dripping with ice-cold Tris-sucrose buffer for 5 s before the quadruple 15-min washout in Tris-sucrose. As already mentioned, this rapid cooling was necessary to reduce post-excitatory reaccumulation of K+ into the muscle cells.
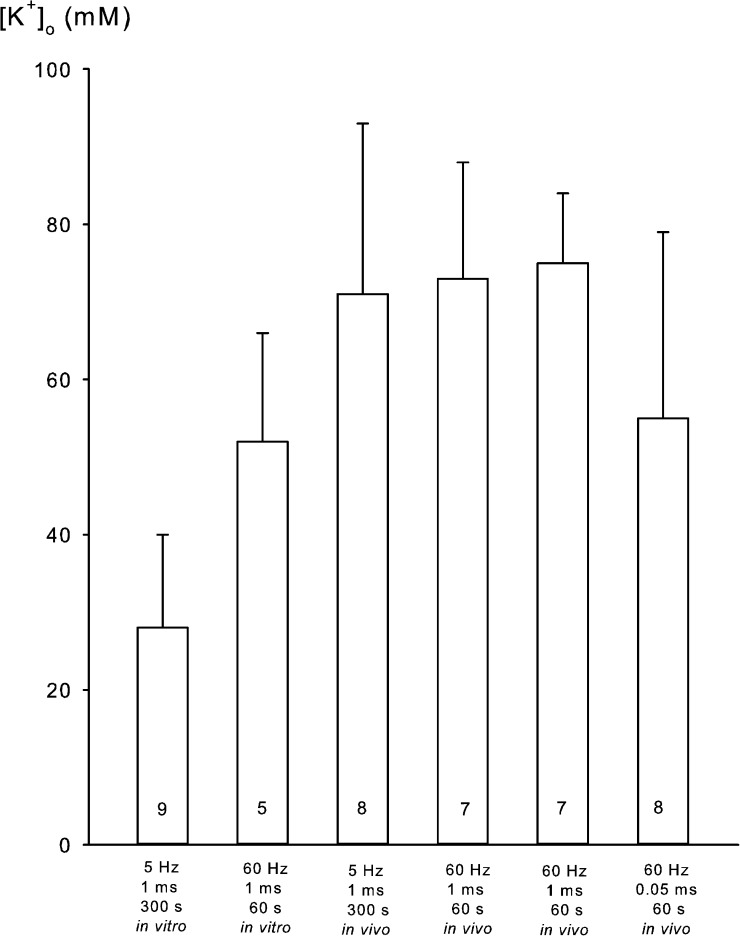
To examine whether these relatively high values for K+ loss reflected the release of K+ via cellular leaks caused by the use of relatively long pulses (1 ms), which might happen, albeit after longer duration of the stimulation (Mikkelsen et al., 2004), the same in vivo experiments were also performed using 10-V pulses of 0.05 ms at 60 Hz for 60 s, sufficient to cause full indirect stimulation of contractile force. This indirect stimulation caused a net decrease in K+ content of the muscles of 9.8 ± 4.1 µmol/g wet wt (P = 0.03; 8 vs. 8 muscles). Assuming the mean extracellular space of 17.7% measured in vivo, this could cause an increase in [K+]o of 55 ± 23 mM (9.8 ± 4.1/0.177), 26% lower than stimulation with 1-ms pulses. The effects of electrical stimulation in vitro and in vivo on [K+]o in EDL are summarized in Fig. 2. The SEM values are large because the K+ loss is calculated as the difference between the relatively large values for K+ content (Tables 1 and 3). However, it should be noted that in all experiments, the excitation-induced loss of K+ was statistically significant. The experiments with [14C]sucrose space measurements in vivo showed that the excitation-induced net uptake of Na+ and net loss of K+ cannot be detected in muscles not undergoing washout in the cold. The washout makes the excitation-induced changes visible and documents that they are significant.
Figure 2.
Stimulation-induced expected rise in [K+]o in EDL muscles in six different experiments. Experiments were performed as described in Materials and methods and the legends to Tables 1 and 3. Mean values are obtained by dividing the net loss of K+ by the extracellular space measured in the stimulated muscles in vitro (0.194 ml/g wet wt) and in vivo (0.177 ml/g wet wt). Values are given with bars denoting SEM and the number of muscles inside the columns. The frequency and duration of stimulation are given below each column.
Effect of electrical stimulation on the uptake of [14C]sucrose and 36Cl in vitro
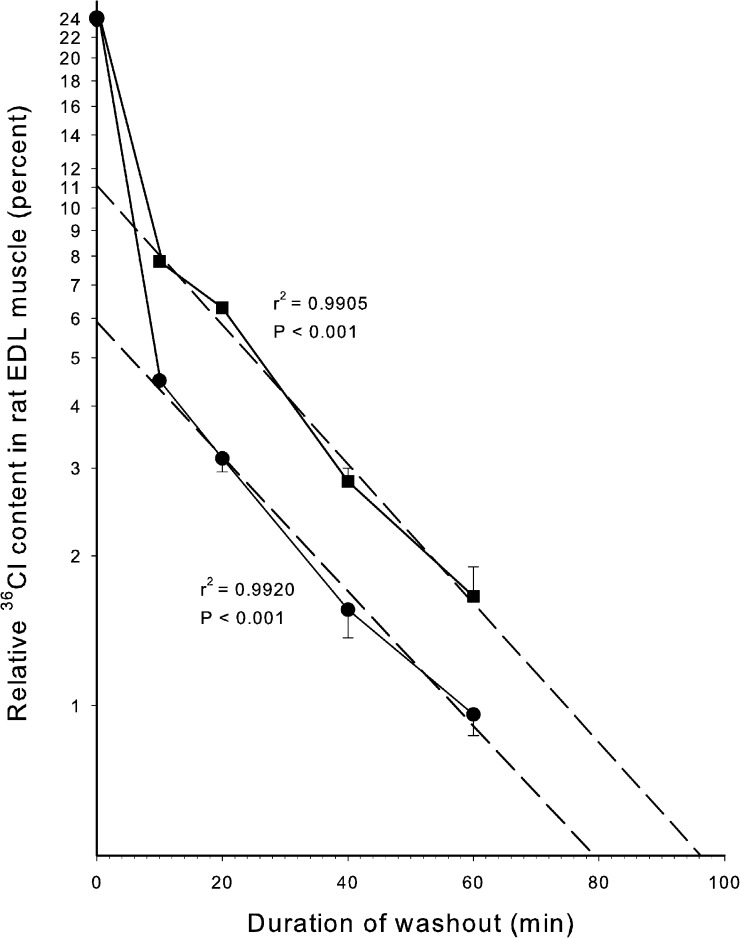
As shown in Table 4, the tissue water space available to 36Cl is not very different from that available to [14C]sucrose. Electrical stimulation caused a minor reduction (21%) in [14C]sucrose space (P = 0.04) and no significant change in 36Cl space (P = 0.17). There was no significant difference between 36Cl− space and [14C]sucrose space at rest (P = 0.59), and after electrical stimulation, 36Cl− space was slightly lower than [14C]sucrose space (P = 0.05). Thus, the intracellular uptake of 36Cl cannot be measured with satisfactory accuracy by comparing the spaces available to [14C]sucrose and 36Cl. Therefore, after incubation with 36Cl, the relatively large extracellular pool of 36Cl was reduced by repeated washout, allowing more accurate quantification of the cellular 36Cl remaining in the muscles after washout. Because the efflux of 36Cl from the intracellular space of rat EDL is markedly reduced at low temperatures (Palade and Barchi, 1977; Hayward and Barchi, 1980), the present washout was performed at 0°C. To allow comparison between intracellular 36Cl content and Na+,K+ contents, ice-cold Na+-free Tris sucrose was used, a procedure earlier shown to allow the determination of excitation-induced changes in intracellular contents of Na+ and K+ (Everts and Clausen, 1992; Murphy et al., 2006, 2008). Fig. 3 shows the time course of the washout of 36Cl from EDL muscles preloaded with 36Cl for 90 min in KR buffer without or with a subsequent 60 s of electrical stimulation at 60 Hz. As shown at the onset of washout (0 time), this caused no significant change in total content of 36Cl. Only when washout is started, clearing the extracellular 36Cl, does it become visible that the stimulated muscles contain more 36Cl than the resting muscles, which must have been taken up during the stimulation. When illustrated in a semilogarithmic plot, the wash at 0°C caused a rapid early loss of 36Cl, assumedly reflecting clearance of 36Cl from the extracellular phase, followed by a considerably slower phase reflecting efflux of 36Cl from the intracellular pool. This is in keeping with an earlier study on isolated EDL muscles from young rats showing that at 5°C, 36Cl efflux has an early rapid component lasting 3–4 min, followed by a much slower washout (Hayward and Barchi, 1980). By extrapolation of the linear phase (dashed lines) to the onset of washout shown in Fig. 3, the magnitude of the slowly exchanging pool can be read off from the intercepts with the ordinate. In the resting muscles, the intercept value is 5.8%. Because the Cl− concentration in the KR buffer is 127.5 mM, this corresponds to a 36Cl content of 7.4 µmol/g wet wt or an intracellular concentration of Cl− of 14.5 mM, based on the aforementioned value for intracellular water space in vitro of 51%. In the stimulated muscles, the intercept value per gram of muscle wet weight is 5.2% larger, corresponding to an excitation-induced increase of 6.63 µmol/g wet wt or reaching an intracellular concentration of Cl− of 27.5 mM. This implies that during the 60 s of 60-Hz stimulation (3,600 pulses), 36Cl influx corresponds to 6.63 µmol/g wet wt/min or 1.84 nmol 36Cl/g wet wt/pulse. In these experiments with 36Cl, the resting and the contralateral stimulated muscles showed no significant difference in wet weight (resting muscles weighing 26.74 ± 0.62 mg wet wt vs. stimulated weighing 26.66 ± 0.60 mg, n = 21 vs. 21; P = 0.93). Therefore, the higher 36Cl uptake in the stimulated muscles shown in Fig. 3 cannot be attributed to excitation-induced reduction in wet weight.
Figure 3.
The effects of electrical stimulation on the washout of 36Cl from isolated EDL muscles. Muscles were mounted in holders for isometric contractions, preequilibrated for 15 min in KR buffer at 30°C, and preloaded with 36Cl for 90 min in KR buffer containing 0.3–1.0 µCi/ml 36Cl. Then all muscles were moved into empty tubes and carefully blotted to remove the last drips of buffer adhering to the tissue using thin folded strips of dry filter paper. Then, while staying in the empty thermo-stated tubes, contralateral muscles were either stimulated for 60 s at 60 Hz using 0.2-ms, 10-V pulses or left resting for the same interval of time. This would allow the 36Cl residing in the extracellular space to enter the muscle cells. Then resting and stimulated muscles were taken for counting of 36Cl (results shown at 0 time), and all the other muscles were washed for periods of twice for 5 min, four times for 5 min, four times for 10 min, or four times for 15 min in ice-cold Na+-free Tris-sucrose buffer, blotted on dry filter paper, weighed, and taken for counting (results shown at the indicated time for sampling). On the basis of the 36Cl activity of the buffer used for the 90-min loading, the relative uptake of 36Cl was plotted at the onset of washout and at the time points indicated from 10 to 60 min. The content of 36Cl is given in relative values as percentage of the total uptake per gram of wet weight reached during the 90-min loading period and during the following wash (e.g., at 0 time, the 36Cl taken up in 1 g wet wt corresponds to the 36Cl content in 0.24 ml KR buffer or 24%). The dashed regression lines were plotted for the resting and the stimulated muscles, respectively, and values for r2 and the significance of the correlation (P) are given. Each point in this figure represents the mean of observations with SEM on three to six muscles. The intercepts of the dashed line with the ordinate gave values of 5.8% and 11%, corresponding to 7.4 µmol and 14 µmol 36Cl for the resting controls and the stimulated muscles, respectively.
As shown in Table 5, when applied at lower and more physiological frequencies (5–20 Hz) and longer duration (120–300 s), electrical stimulation also induces a highly significant (P < 0.001) increase (100–188%) in 36Cl content in EDL muscles. This increase depends on the frequency and duration of stimulation and may arise during the depolarization caused by the excitation-induced rise in [K+]o.
Table 5.
Effect of electrical stimulation on the uptake of 36Cl in isolated rat EDL muscle in vitro
| Experimental conditions | 36Cl uptake | Intracellular 36Cl | Relative increase | P-values |
| µmol/g wet wt | mM | % | ||
| Resting controls | 7.1 ± 0.6 (5) | 13.9 ± 1.2 | ||
| 60-Hz stimulation, 60 s | 12.2 ± 1.8 (5) | 23.9 ± 3.6 | 71 | <0.03 |
| Resting controls | 7.6 ± 0.3 (9) | 14.9 ± 0.6 | ||
| 5-Hz stimulation, 300 s | 16.4 ± 0.7 (3) | 32.2 ± 1.4 | 116 | <0.001 |
| 10-Hz stimulation, 120 s | 15.2 ± 1.0 (3) | 29.9 ± 2.0 | 100 | <0.001 |
| 10-Hz stimulation, 300 s | 19.5 ± 0.4 (3) | 38.2 ± 7.8 | 157 | <0.001 |
| 20-Hz stimulation, 120 s | 21.9 ± 4.2 (3) | 42.9 ± 8.4 | 188 | <0.001 |
Experimental conditions as described in the legend to Fig. 3, except that stimulation was also performed using the indicated lower frequencies and longer durations before the quadruple 15-min washout in Na+-free Tris-sucrose buffer at 0°C. All values were corrected for the loss of 36Cl taking place during the washout in the cold and are given as µmol/g wet wt ± SEM, with the number of muscles in parentheses.
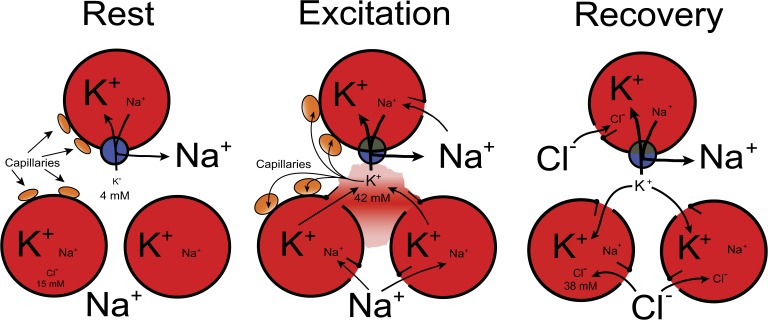
To examine the effect of depolarization on 36Cl uptake, EDL muscles were exposed to KR buffer containing 40–80 mM K+. As shown in Table 6, incubation for 90 min with 40–80 mM K+ induced a marked (120–399%) and highly significant (P < 0.001) increase in 36Cl content as measured after 60 min of washout in the ice-cold Na+-free Tris-sucrose buffer. After incubation with 40 mM K+, intracellular 36Cl concentration reached 38 ± 6 mM, identical to the level reached after electrical stimulation at 10 Hz for 300 s (38 ± 8 mM; Table 5). In the same experiment, the net loss of K+ from the stimulated muscles was 7.45 ± 2.6 µmol/g wet wt, sufficient to cause a rise in [K+]o of 38 ± 13 mM. As shown in Fig. 4, when the 4 mM K+ already present in the extracellular phase is added, [K+]o will reach 42 mM, close to the 40 mM K+ tested for its effect on 36Cl content. This suggests that the effects of excitation on intracellular 36Cl concentration can be mimicked by exposure to elevated K+. In contrast, the increase in intracellular 36Cl concentration induced by 60 s of electrical stimulation at 60 Hz is clearly lower than that reached by long-term exposure to 60 or 80 mM K+. This might be the result of inadequate time to reach full uptake of 36Cl. Anyhow, measurement of 36Cl uptake in vitro allows the detection of depolarization caused by longer-lasting electrical stimulation.
Table 6.
Effects of elevated [K+]o on 36Cl uptake in isolated rat EDL muscle in vitro
| Buffer composition | 36Cl uptake | Intracellular 36Cl | Relative increase | P-values |
| µmol/g wet wt | mM | % | ||
| KR with 4 mM K+ | 8.8 ± 1.3 (5) | 17.3 ± 2.5 (5) | ||
| KR with 40 mM K+ | 19.4 ± 3.0 (5) | 38.0 ± 5.9 (5) | 120 | 0.011 |
| KR with 60 mM K+ | 29.7 ± 1.1 (3) | 58.2 ± 2.2 (3) | 238 | <0.001 |
| KR with 80 mM K+ | 43.9 ± 2.3 (3) | 86.1 ± 4.5 (3) | 399 | <0.001 |
The muscles were mounted in force transducers for measurement of isometric contractions, adjusted to optimal length, and equilibrated for 15 min in KR buffer at 30°C during gassing with a mixture of 95% O2 and 5% CO2. Then the muscles were incubated for 90 min in KR buffer in which NaCl had been replaced by KCl at the concentrations indicated and 0.4 µCi/ml 36Cl added. All muscles were washed four times for 15 min in Na+-free Tris-sucrose buffer to remove extracellular tracer, blotted, weighed, and taken for scintillation counting of 36Cl. For each muscle, the content of 36Cl was measured and expressed as µmol/g wet wt using the specific activity of 36Cl in the loading medium. The data were corrected for the loss of 36Cl during the washout and are given as µmol/g wet wt ± SEM, with the number of muscles in parentheses.
Figure 4.
Diagram describing the sequence of events in excitation-induced redistribution of Na+, K+, and Cl− in skeletal muscle with values for [K+]o and [Cl−]i induced by electrical stimulation for 300 s at 10 Hz and obtained in the present study. (a) Na+ influx inducing depolarization and increased [Na+]i. (b) The depolarization activates K+ efflux, increasing [K+]o from 4 to 42 mM. (c) The increases in [Na+]i and [K+]o activates the Na+,K+ pumps. (d) This is a major mechanism for the clearing of extracellular K+. (e) The increase in [K+]o induces depolarization. (f) This is counterbalanced by the electrogenic action of the Na+,K+ pumps. (g) Elevated [K+]o favors vasodilatation, improving the clearance of [K+]o via the capillaries (Lo et al., 2004; Armstrong et al., 2007). (h) As the result of depolarization, Cl− influx is promoted, inducing repolarization and increase in [Cl−]i from 15 to 38 mM (data from Table 5). (i) As the result of this repolarization, K+ influx via inward rectifiers is increased. (j) This favors the clearance of [K+]o.
Effect of electrical stimulation on 36Cl uptake and intracellular 36Cl concentration in vivo
As described in Materials and methods, 36Cl was injected and allowed to equilibrate for 90 min. After anesthesia, the EDL muscles were made accessible with preserved circulation, and platinum wire electrodes were positioned allowing stimulation. After stimulation at 5 Hz for 300 s, 20 Hz for 300 s, or 60 Hz for 60 s, using 1-ms, 10-V pulses, the muscles were washed four times for 5 min with ice-cold Na+-free Tris-sucrose buffer, blotted, weighed, and taken for counting of 36Cl content and measurement of Na+,K+ contents by flame photometry.
As shown in Table 7, 36Cl content was 8.8 ± 0.3 µmol/g wet wt in the unstimulated EDL and 15.9 ± 0.6 µmol/g wet wt (P < 0.001) in the stimulated EDL at 5 Hz for 300 s using 1-ms, 10-V pulses (n = 4 vs. 4 muscles). The specific activity of 36Cl in these experiments was based on the plasma Cl− of 110 mM measured in the rat (Kotchen et al.,1978). As mentioned above, intracellular water space in vivo amounts to 61.4%, and using this value, the 36Cl content corresponds to mean intracellular 36Cl concentrations of 14.3 ± 0.6 mM and 25.9 ± 0.9 mM in resting and stimulated EDL, respectively. This indicates that in vivo, stimulation at 5 Hz for 300 s increases 36Cl− uptake by 7.1 µmol/g wet wt in 60 s, augmenting intracellular 36Cl− content by 81%. When stimulated at 20 Hz for 300 s or 60 Hz for 60 s, about the same increases in 36Cl uptake or contents were seen. These data are similar to those obtained with stimulation in vitro (Fig. 3 and Table 5). Total water content in vivo showed no significant change with electrical stimulation neither at 5 Hz for 300 s nor 60 Hz for 60 s.
Table 7.
Effect of electrical stimulation on the uptake of 36Cl in rat EDL muscle in vivo
| Experimental conditions | 36Cl uptake | Intracellular 36Cl | Relative increase | P-values |
| µmol/g wet wt | mM | % | ||
| Resting controls | 8.8 ± 0.3 (4) | 14.3 ± 0.6 (4) | ||
| 5-Hz stimulation, 300 s | 15.9 ± 0.6 (4) | 25.9 ± 0.9 (4) | 81 | <0.001 |
| Resting controls | 9.4 ± 0.4 (4) | 15.3 ± 0.7 (4) | ||
| 20-Hz stimulation, 300 s | 16.0 ± 0.5 (4) | 26.1 ± 0.8 (4) | 70 | <0.001 |
| Resting controls | 7.7 ± 0.3 (4) | 12.5 ± 0.6 (4) | ||
| 60-Hz stimulation, 60 s | 14.1 ± 0.6 (4) | 23.0 ± 1.0 (4) | 84 | <0.001 |
200 µl of a 154 mM NaCl solution containing 4 µCi 36Cl was injected intraperitoneally. The isotope was allowed to equilibrate in the water space of the animal for 90 min (like in the experiments with isolated EDL). 15 min before the end of this period, the rats were anaesthetized by intraperitoneal injection of analgesics as described above. A blood sample was withdrawn from a tail vein for measurement of the content of 36Cl in plasma. At the end of the equilibration period, EDL was exposed and stimulated at resting length via platinum wire electrodes at the indicated frequency and duration using 1-ms pulses at 10 V. Immediately after the stimulation, the muscles were washed with ice-cold Na+-free Tris-sucrose buffer, cut free, and washed four times for 5 min in ice-cold Na+-free Tris-sucrose, blotted, weighed, and soaked in 0.3 M TCA, and the extract was taken for the counting of 36Cl. The uptake of 36Cl is given as µmol/g wet wt ± SEM and intracellular 36Cl as mM ± SEM, with the number of muscles in parentheses. All values were corrected for the loss of 36Cl taking place during the washout in the cold.
DISCUSSION
The major novel mechanistic insight provided by the present study is that 300 s of 5-Hz or 60 s of 60-Hz direct electrical stimulation of rat EDL muscles in vivo induces a net loss of K+, which is sufficient to increase [K+]o by up to around 70 mM (Fig. 2). This is accompanied by a somewhat smaller net uptake of Na+, which is likely to lead to a drop in extracellular Na+ (46 mM), amplifying the inhibitory action of the excitation-induced elevation of [K+]o on contractile performance (Bouclin et al., 1995; Overgaard et al., 1997). In humans, voluntary biking, which is assumed to be associated with a mean stimulation frequency of around 6 Hz (Hallén et al., 1994), leads to a net loss of K+ from the working muscles (Sjøgaard, 1990). When stimulating the rat EDL muscles at a more physiological frequency (5 Hz) for 300 s in vivo, the net loss of K+ and gain of Na+ were similar to those induced by 60-Hz stimulation for 60 s. This indicates that under physiological conditions, the excitation-induced increase in [K+]o in working muscles is sufficient to cause at least transient loss of excitability and fatigue. Moreover, the Na+,K+ pumps may occasionally be saturated, leading to full utilization of the Na+-K+ pump capacity (for details see Clausen et al., 1987; Everts and Clausen, 1994; Nielsen and Clausen, 1997; Sejersted and Sjøgaard, 2000). Because of the narrowness and tortuosity of the t-tubules, the concentration of K+ in their lumen is likely to be higher than in the interstitial water space (for details see Clausen, 2003). Measurements of [3H]ouabain binding in rat EDL muscles in this laboratory indicate that 52% of the total population of Na+,K+ pumps are situated in the t-tubular lumen.
Excessive muscular activity may lead to loss of muscle cell integrity and contractile force. In rat muscles that have undergone excitation-induced cell damage, stimulation of the Na+,K+ pumps induces repolarization and improves force recovery as the result of the electrogenic action of the Na+,K+ pumps (Mikkelsen et al., 2006). This mechanism is important for the restoration of muscle function after intense exercise or muscle cell damage. Muscle cell damage, also caused by crush lesions, often causes hyperkalemia, which may induce cardiac arrest. The most important and fatal medical complication in crush syndrome arising during earthquakes is hyperkalemia (Sever et al., 2003). Thus, by clearing K+ from plasma, a high content of Na+,K+ pumps in the skeletal muscles has survival value during exhaustive exercise or muscle lesions.
The present experiments showed that excitation-induced net loss of K+ and net uptake of Na+ are highly significant both in vitro and in vivo, both at low (5 Hz) and high (60 Hz) frequency (Tables 1 and 3). Obviously, the net loss of K+ is faster during stimulation at 60 than at 5 Hz, reflecting that at 5 Hz, there is more time (300 s) for reaccumulation of K+ in the muscle cells. Still, at 60 and 5 Hz in vivo, the rise in [K+]o is not very different, sufficient to induce complete loss of excitability. These observations are in keeping with an earlier study (Murphy et al., 2008) showing that after 30 min of treadmill running, cellular K+ content of rat soleus muscle was reduced by 6 µmol/g wet wt (P < 0.001). This in vivo exercise-induced loss of K+ was increased by reducing the transport capacity of the Na+,K+ pumps in the muscles by injection of ouabain or prior K+ depletion of the rats (Nørgaard et al., 1981).
As shown in Fig. 2, the excitation-induced rise in [K+]o induced by 5- and 60-Hz stimulation is clearly larger in vivo (55–75 mM) than in vitro (28–52 mM). This is likely to be caused by the slightly smaller extracellular space in vivo (17.7%) than in vitro (19.4%) as well as the higher temperature in vivo (37°C) than in vitro (30°C). These large changes in Na+,K+ content and extracellular Na+ and K+ might reflect unspecific muscle cell damage induced by the use of 1-ms stimulation pulses. However, this seems unlikely because the changes are reversible in vitro (Table 2) and are also seen using indirect stimulation with much shorter pulses (0.05 ms) in vivo.
Before gaining access to the capillaries, the net loss of K+ from the muscle cells has to start by an efflux from the intracellular space into the extracellular space, causing a mean increase in extracellular K+ concentration of 50–75 mM. The exact time course of this increase is not yet known, but it is reasonable to assume that it reaches at least this mean level for an interval of 60 s in the experiments with 60-Hz stimulation and probably longer in the experiments with 5-Hz stimulation. An alternative possibility is a short-lasting, even higher early rise in K+ concentration, followed by another phase with a smaller rise in extracellular K+. When averaged, this would reach the values recorded. However, this does not rule out that excitation in vivo causes a large, albeit transient rise in extracellular K+.
The other novel observation of the present study is that electrical stimulation both in vitro and in vivo increases the net uptake of 36Cl in EDL. This effect is rapid in onset and highly significant from 60 Hz for 60 s, down to more physiological frequencies (5–20 Hz in vitro for 120–300 s). At the lower frequencies, 36Cl uptake reached higher levels than at 60 Hz, probably because the depolarizing effect of elevated [K+]o was maintained for a longer period. Thus, after 300-s stimulation at 10 Hz, intracellular 36Cl reached 38 mM, 2.56-fold larger than that of the resting controls (15 mM). In the same experiment, the net loss of K+ from the muscle cells amounted to 7.45 µmol/g wet wt, sufficient to increase [K+]o by 38 mM; 3 vs. 3 muscles). The influx of Cl− contributes to repolarization and thus favors the clearance of K+ via inward rectifier K+ channels during or after a series of contractions. In vitro experiments showed that by elevating [K+]o, 36Cl uptake is markedly stimulated (120–399%). This is likely to reflect depolarization, supporting the idea that the increase in 36Cl uptake induced by electrical stimulation is related to depolarization, possibly also in vivo. The in vivo measurements of 36Cl uptake offers a new method allowing the monitoring of general depolarization in all the cells of a muscle.
Fig. 4 shows a diagram of three muscle cells with extracellular space, capillaries, the Na+,K+ pumps, and ion channels in the plasma membrane. The figure summarizes some of the major factors and mechanisms influencing excitability in contracting muscle. The sequence of events (a–j) in excitation-induced redistribution of Na+, K+, and Cl− is listed in the legend. Each of these events is important for the level of [K+]o and excitability. A previous in vitro study showed that in rat EDL, continuous stimulation at 90 Hz augments [K+]o by 14 mM in 5 s (Clausen et al., 2004). During continuous stimulation, the rate of force decline is closely correlated to the excitation-induced increase in [K+]o (Clausen, 2008b). As shown in another study, 60 s of 20-Hz stimulation may lead to ∼10-fold increase in [K+]o (Clausen, 2011). This will be associated with depolarization and reduced excitability, which has repeatedly been proposed as an important cause of fatigue (Sjøgaard, 1990; Sejersted and Sjøgaard, 2000; Clausen, 2003). It is important, therefore, that K+ can be cleared rapidly from the extracellular space. This happens by diffusion into the extracellular space and the capillaries, which undergo dilatation, allowing more efficient removal of K+ by increased blood flow (Kjellmer, 1965; Lo et al., 2004; Armstrong et al., 2007). As proposed by Lo et al. (2004), the dilatation may be caused by capillaries surrounding the excited muscle cells sensing the increase in K+ release and stimulating arteriolar dilatation via upstream conducted responses. The dilatation depends on the frequency of stimulation and the concentration of K+, and it is suppressed by glibenclamide (Dua et al., 2009). Calcitonin gene-related peptide (CGRP) is one of the most potent vasodilatatory compounds. In rat skeletal muscle, 50–100 mM K+ has been shown to induce marked stimulation of the local release of CGRP (Sakaguchi et al., 1991). The excitation-induced increases in [K+]o of comparable magnitude detected in the present study indicate that CGRP could contribute to the excitation-induced vasodilatation.
In addition, K+ clearance is promoted by stimulation of the Na+,K+ pumps, an effect which can be detected within 10 s of electrical stimulation (Nielsen and Clausen 1997; Buchanan et al., 2002). Thus, after 15 s of stimulation of rat EDL at 60 Hz, reextrusion of Na+ as measured during the following 60 s of rest is 7.3-fold faster than basal Na+ efflux (Clausen, 2011). This activation of the Na+,K+ pumps is not only caused by increased intracellular Na+, but also reflects that the affinity of the Na+,K+ pumps for intracellular Na+ is augmented (Nielsen and Clausen 1997; Buchanan et al., 2002), possibly as the result of activation of protein kinase C (Thomassen et al., 2011). This Na+,K+ pump stimulation not only directly accelerates clearing of extracellular K+, but because the Na+,K+ pumps are electrogenic, the plasma membrane is further polarized, promoting the removal of K+. The total content of Na+,K+ pumps in the EDL muscle from 4-wk-old rats as measured in vivo amounts to 669 ± 82 pmol/g wet wt (mean ± SEM of data from 10 muscles), corresponding to a maximum transport capacity at 37°C of 2 × 8,000 × 669 pmol/g wet wt = 10.7 µmol K+/g wet wt/min (Murphy et al., 2008). This is calculated with the assumptions that the Na+,K+-ATPase has a turnover number of 8,000/min/mol and that for each ATP split, two K+ ions are pumped from the extracellular space into the muscle cells. The Na+,K+ pumps measured by [3H]ouabain binding to rat skeletal muscle are all functional and have been shown to become fully activated during intense electrical stimulation (120 Hz for 10 s; Everts and Clausen, 1994; Nielsen and Clausen, 1997). Assuming that this happens, the Na+,K+ pumps in rat EDL may reduce [K+]o in the extracellular space by 60 mM in 1 min (10.7/0.177). Thus, the Na+,K+ pumps have sufficient capacity to keep pace with the rapid excitation-induced rise in [K+]o, even in the high range detected in the present study. These results indicate that under physiological in vivo conditions, extracellular K+ in working muscles reaches a level requiring full utilization of the entire population of Na+,K+ pumps measured in the same muscles. The physiological importance of the Na+,K+ pumps is evident from the observation that when the pumps are blocked by ouabain, the rate of force decline during 20 s of continuous electrical stimulation is accelerated by 565% (Clausen, 2008a). During static work with isometric contractions sufficient to compress the blood vessels (Barcroft and Millen, 1939), the clearance of K+ via the circulation is abolished and depends on transport into the muscle cells via the Na+,K+ pumps and inward rectifier K+ channels. The ensuing rise in [K+]o caused by inadequate K+ clearance via circulation is likely to interfere with contractile performance, an extra risk of heavy static work.
In addition, the depolarizing effect of elevated [K+]o is counterbalanced by increased influx of Cl−, leading to partial repolarization and increased uptake of K+ via inward rectifier channels (Dutka et al., 2008). This is likely to happen already after 15 s of stimulation at 60 Hz, allowing continuation of excitation and contraction, albeit with declining force (Clausen, 2011). Finally, excitability is improved by the excitation-induced reduction in GCl (Pedersen et al., 2005), mediated by activation of protein kinase C (Pedersen et al., 2009). This was recently shown to increase contractile endurance (de Paoli et al., 2012). It should be recalled that the inhibitory effect of elevated [K+]o on contractile performance is likely to reflect depolarization. Depolarization and loss of contractility were also shown to be induced by carbacholine (Macdonald et al., 2005) or unspecific membrane leakage (Clausen and Gissel, 2005), effects which could both be restored by stimulating the Na+,K+ pumps with the β2-agonist salbutamol. A recent study demonstrated that the inhibitory effect of anoxia on rat EDL muscle contractility could be counteracted by stimulating the Na+,K+ pumps with β2-agonists or theophylline (Fredsted et al., 2012). Patients with lung diseases (e.g., asthma or chronic obstructive pulmonary disease) complain of leg fatigue and peripheral muscle dysfunction. Leg performance was shown to be improved by treatment with the β2-agonist salmeterol (Brouillard et al., 2008).
Conclusions and perspectives
The present experiments confirm and amplify the earlier demonstration of pronounced excitation-induced increase in extracellular [K+]o. (Clausen, 2008a, 2011). Even at a low frequency (5 Hz) stimulation in vitro induces a loss of cellular K+ sufficient to induce a rise in interstitial K+ that would severely interfere with excitability and cause fatigue. More importantly, even larger increases could be demonstrated in vivo, likely to require full utilization of the Na+,K+ pump capacity. This emphasizes the significance of the down-regulation of Na+,K+ pumps in skeletal muscles seen under a wide range of pathological conditions (Clausen, 1998).
The role of K+ in muscle fatigue has repeatedly been discussed, but often dismissed, first because the exercise-induced increase in plasma K+ seemed too modest, second because the decisive increase seemed to happen in the t-tubular lumen or in the interstitial water space of skeletal muscles, regions not readily accessible for accurate measurements, and third because simultaneous changes in local concentrations of other ions seemed to make it unlikely that K+ acts in isolation during fatigue (Cairns and Lindinger, 2008). The present observations indicating an excitation-induced pronounced increase in the extracellular concentration of K+ offer new and strong arguments for the idea that K+ is a sufficient cause of excitation-induced fatigue also at low frequencies and in vivo. It should be noted that this does not eliminate the important contributions of Ca2+ handling and myofibrilar proteins to muscle fatigue (Allen et al., 2008). The rapid effect of excitation on intracellular 36Cl uptake both in vitro and in vivo indicate that a substantial part of the excited muscle cells undergo sufficient depolarization to favor Cl− influx, further evidence that they lose excitability. Finally, the in vivo procedures developed here offer new possibilities to quantify excitation-induced transport of Na+, K+, and Cl− in muscles in the physiological environment.
Acknowledgments
I thank Tove Lindahl Andersen, Susanne Jespersen, Marianne Stürup-Johansen, and Vibeke Uhre for skilled technical assistance and Hanne Gissel for providing data for water content in rat EDL muscles.
This study was supported by grants from The Lundbeck Foundation (J. Nr. R31-A2536), The Karen Elise Jensen Foundation (J. Nr. 22401 8/04/05), and The Velux Foundation (J. Nr. 30118).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- CGRP
- calcitonin gene-related peptide
- ECV
- extracellular volume
- EDL
- extensor digitorum longus
- ICV
- intracellular volume
- KR buffer
- Krebs-Ringer bicarbonate buffer
- TCA
- trichloroacetic acid
References
- Allen D.G., Lamb G.D., Westerblad H. 2008. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88:287–332 10.1152/physrev.00015.2007 [DOI] [PubMed] [Google Scholar]
- Armstrong M.L., Dua A.K., Murrant C.L. 2007. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J. Physiol. 581:841–852 10.1113/jphysiol.2007.130013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcroft H., Millen J.L. 1939. The blood flow through muscle during sustained contraction. J. Physiol. 97:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouclin R., Charbonneau E., Renaud J.M. 1995. Na+ and K+ effect on contractility of frog sartorius muscle: implication for the mechanism of fatigue. Am. J. Physiol. 268:C1528–C1536 [DOI] [PubMed] [Google Scholar]
- Brouillard C., Pepin V., Milot J., Lacasse Y., Maltais F. 2008. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur. Respir. J. 31:579–584 10.1183/09031936.00119007 [DOI] [PubMed] [Google Scholar]
- Buchanan R., Nielsen O.B., Clausen T. 2002. Excitation- and β(2)-agonist-induced activation of the Na(+)-K(+) pump in rat soleus muscle. J. Physiol. 545:229–240 10.1113/jphysiol.2002.023325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.P., Lindinger M.I. 2008. Do multiple ionic interactions contribute to skeletal muscle fatigue? J. Physiol. 586:4039–4054 10.1113/jphysiol.2008.155424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.P., Hing W.A., Slack J.R., Mills R.G., Loiselle D.S. 1997. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am. J. Physiol. 273:C598–C611 [DOI] [PubMed] [Google Scholar]
- Cairns S.P., Ruzhynsky V., Renaud J.M. 2004. Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle. Am. J. Physiol. Cell Physiol. 287:C762–C770 10.1152/ajpcell.00589.2003 [DOI] [PubMed] [Google Scholar]
- Clausen T. 1998. Clinical and therapeutic significance of the Na+,K+ pump. Clin. Sci. 95:3–17 10.1042/CS19970254 [DOI] [PubMed] [Google Scholar]
- Clausen T. 2003. Na+-K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 83:1269–1324 [DOI] [PubMed] [Google Scholar]
- Clausen T. 2008a. Clearance of extracellular K+ during muscle contraction—roles of membrane transport and diffusion. J. Gen. Physiol. 131:473–481 10.1085/jgp.200809971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. 2008b. Role of Na+,K+-pumps and transmembrane Na+,K+-distribution in muscle function. The FEPS lecture–Bratislava 2007. Acta Physiol. (Oxf.). 192:339–349 10.1111/j.1748-1716.2007.01798.x [DOI] [PubMed] [Google Scholar]
- Clausen T. 2011. In isolated skeletal muscle, excitation may increase extracellular K+ 10-fold; how can contractility be maintained? Exp. Physiol. 96:356–368 [DOI] [PubMed] [Google Scholar]
- Clausen T., Gissel H. 2005. Role of Na,K pumps in restoring contractility following loss of cell membrane integrity in rat skeletal muscle. Acta Physiol. Scand. 183:263–271 10.1111/j.1365-201X.2004.01394.x [DOI] [PubMed] [Google Scholar]
- Clausen T., Everts M.E., Kjeldsen K. 1987. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J. Physiol. 388:163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Overgaard K., Nielsen O.B. 2004. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol. Scand. 180:209–216 10.1111/j.0001-6772.2003.01251.x [DOI] [PubMed] [Google Scholar]
- de Paoli F.V., Broch-Lips M., Pedersen T.H., Nielsen O.B. 2012. Relationship between membrane Cl- conductance and contractile endurance in isolated rat muscles. J. Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua A.K., Dua N., Murrant C.L. 2009. Skeletal muscle contraction-induced vasodilator complement production is dependent on stimulus and contraction frequency. Am. J. Physiol. Heart Circ. Physiol. 297:H433–H442 10.1152/ajpheart.00216.2009 [DOI] [PubMed] [Google Scholar]
- Dutka T.L., Murphy R.M., Stephenson D.G., Lamb G.D. 2008. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation-contraction coupling and fatigue. J. Physiol. 586:875–887 10.1113/jphysiol.2007.144667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts M.E., Clausen T. 1992. Activation of the Na-K pump by intracellular Na in rat slow- and fast-twitch muscle. Acta Physiol. Scand. 145:353–362 10.1111/j.1748-1716.1992.tb09375.x [DOI] [PubMed] [Google Scholar]
- Everts M.E., Clausen T. 1994. Excitation-induced activation of the Na(+)-K+ pump in rat skeletal muscle. Am. J. Physiol. 266:C925–C934 [DOI] [PubMed] [Google Scholar]
- Fenn W.O. 1940. The role of potassium in physiological processes. Physiol. Rev. 20:377–415 [Google Scholar]
- Fredsted A., Gissel H., Ørtenblad N., Clausen T. 2012. Effects of β2-agonists on force during and following anoxia in rat extensor digitorum longus muscle. J. Appl. Physiol. 112:2057–2067 10.1152/japplphysiol.01558.2011 [DOI] [PubMed] [Google Scholar]
- Gissel H., Clausen T. 1999. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am. J. Physiol. 276:R331–R339 [DOI] [PubMed] [Google Scholar]
- Gurnett C.A., Kahl S.D., Anderson R.D., Campbell K.P. 1995. Absence of the skeletal muscle sarcolemma chloride channel ClC-1 in myotonic mice. J. Biol. Chem. 270:9035–9038 10.1074/jbc.270.16.9035 [DOI] [PubMed] [Google Scholar]
- Hallén J., Gullestad L., Sejersted O.M. 1994. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without β-adrenoceptor blockade. J. Physiol. 477:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward B.S., Barchi R.L. 1980. Chloride efflux measurements in mammalian skeletal muscle. Muscle Nerve. 3:207–215 10.1002/mus.880030304 [DOI] [PubMed] [Google Scholar]
- Hník P.M., Holas M., Krekule I., Kŭriz N., Mejsnar J., Smiesko V., Ujec E., Vyskocil F. 1976. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 362:85–94 10.1007/BF00588685 [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., Horowicz P. 1959. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J. Physiol. 148:127–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer I. 1965. The potassium ion as a vasodilator during muscle exercise. Acta Physiol. Scand. 63:460–468 10.1111/j.1748-1716.1965.tb04089.x [DOI] [PubMed] [Google Scholar]
- Kotchen T.A., Galla J.H., Luke R.G. 1978. Contribution of chloride to the inhibition of plasma renin by sodium chloride in the rat. Kidney Int. 13:201–207 10.1038/ki.1978.30 [DOI] [PubMed] [Google Scholar]
- Krnjevic K., Miledi R. 1958. Failure of neuromuscular propagation in rats. J. Physiol. 140:440–461 [PMC free article] [PubMed] [Google Scholar]
- Law R.O., Phelps C.F. 1966. The size of the sucrose, raffinose, and inulin spaces in the gastrocnemius muscle of the rat. J. Physiol. 186:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger M.I., Heigenhauser G.J.F., Spriet L.L. 1987. Effects of intense swimming and tetanic electrical stimulation on skeletal muscle ions and metabolites. J. Appl. Physiol. 63:2331–2339 [DOI] [PubMed] [Google Scholar]
- Lo A., Fuglevand A.J., Secomb T.W. 2004. Theoretical simulation of K(+)-based mechanisms for regulation of capillary perfusion in skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 287:H833–H840 10.1152/ajpheart.00139.2004 [DOI] [PubMed] [Google Scholar]
- Macdonald W.A., Nielsen O.B., Clausen T. 2005. Na+-K+ pump stimulation restores carbacholine-induced loss of excitability and contractility in rat skeletal muscle. J. Physiol. 563:459–469 10.1113/jphysiol.2004.080390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen U.R., Fredsted A., Gissel H., Clausen T. 2004. Excitation-induced Ca2+ influx and muscle damage in the rat: loss of membrane integrity and impaired force recovery. J. Physiol. 559:271–285 10.1113/jphysiol.2004.067199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen U.R., Gissel H., Fredsted A., Clausen T. 2006. Excitation-induced cell damage and beta2-adrenoceptor agonist stimulated force recovery in rat skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R265–R272 10.1152/ajpregu.00392.2005 [DOI] [PubMed] [Google Scholar]
- Murphy K.T., Macdonald W.A., McKenna M.J., Clausen T. 2006. Ionic mechanisms of excitation-induced regulation of Na+-K+-ATPase mRNA expression in isolated rat EDL muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R1397–R1406 10.1152/ajpregu.00707.2005 [DOI] [PubMed] [Google Scholar]
- Murphy K.T., Nielsen O.B., Clausen T. 2008. Analysis of exercise-induced Na+-K+ exchange in rat skeletal muscle in vivo. Exp. Physiol. 93:1249–1262 10.1113/expphysiol.2008.042457 [DOI] [PubMed] [Google Scholar]
- Nielsen O.B., Clausen T. 1997. Regulation of Na(+)-K+ pump activity in contracting rat muscle. J. Physiol. 503:571–581 10.1111/j.1469-7793.1997.571bg.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.J., Mohr M., Klarskov C., Kristensen M., Krustrup P., Juel C., Bangsbo J. 2004. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J. Physiol. 554:857–870 10.1113/jphysiol.2003.050658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørgaard A., Kjeldsen K., Clausen T. 1981. Potassium depletion decreases the number of 3H-ouabain binding sites and the active Na-K transport in skeletal muscle. Nature. 293:739–741 10.1038/293739a0 [DOI] [PubMed] [Google Scholar]
- Overgaard K., Nielsen O.B., Clausen T. 1997. Effects of reduced electrochemical Na+ gradient on contractility in skeletal muscle: role of the Na+-K+ pump. Pflugers Arch. 434:457–465 10.1007/s004240050421 [DOI] [PubMed] [Google Scholar]
- Palade P.T., Barchi R.L. 1977. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J. Gen. Physiol. 69:325–342 10.1085/jgp.69.3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T.H., de Paoli F., Nielsen O.B. 2005. Increased excitability of acidified skeletal muscle: role of chloride conductance. J. Gen. Physiol. 125:237–246 10.1085/jgp.200409173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T.H., Macdonald W.A., de Paoli F.V., Gurung I.S., Nielsen O.B. 2009. Comparison of regulated passive membrane conductance in action potential–firing fast- and slow-twitch muscle. J. Gen. Physiol. 134:323–337 (published erratum appears in J. Gen. Physiol. 2009. 134:525) 10.1085/jgp.200910291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R.L., Simoncini L., Stühmer W. 1988. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 11:502–510 10.1002/mus.880110514 [DOI] [PubMed] [Google Scholar]
- Sacco P., McIntyre D.B., Jones D.A. 1994. Effects of length and stimulation frequency on fatigue of the human tibialis anterior muscle. J. Appl. Physiol. 77:1148–1154 [DOI] [PubMed] [Google Scholar]
- Sahlin K., Alvestrand A., Brandt R., Hultman E. 1978. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J. Appl. Physiol. 45:474–480 [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Inaishi Y., Kashihara Y., Kuno M. 1991. Release of calcitonin gene-related peptide from nerve terminals in rat skeletal muscle. J. Physiol. 434:257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejersted O.M., Sjøgaard G. 2000. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 80:1411–148111015618 [Google Scholar]
- Sever M.S., Erek E., Vanholder R., Kantarci G., Yavuz M., Turkmen A., Ergin H., Tulbek M.Y., Duranay M., Manga G., et al. ; Marmara Earthquake Study Group 2003. Serum potassium in the crush syndrome victims of the Marmara disaster. Clin. Nephrol. 59:326–333 [DOI] [PubMed] [Google Scholar]
- Sheff M.F., Zacks S.I. 1982. Interstitial space of mouse skeletal muscle. J. Physiol. 328:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjøgaard G. 1990. Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol. Scand. Suppl. 593:1–63 [PubMed] [Google Scholar]
- Thomassen M., Rose A.J., Jensen T.E., Maarbjerg S.J., Bune L., Leitges M., Richter E.A., Bangsbo J., Nordsborg N.B. 2011. Protein kinase Cα activity is important for contraction-induced FXYD1 phosphorylation in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301:R1808–R1814 10.1152/ajpregu.00066.2011 [DOI] [PubMed] [Google Scholar]