Abstract
The purpose of this review is to examine the validity of positive claims regarding the direct anterior approach (DAA) with a fracture table for total hip arthroplasty. Recent literature regarding the DAA was searched and specific claims investigated including improved early outcomes, speed of recovery, component placement, dislocation rates, and complication rates. Recent literature is positive regarding the effects of total hip arthroplasty with the anterior approach. While the data is not definitive at present, patients receiving the anterior approach for total hip arthroplasty tend to recover more quickly and have improved early outcomes. Component placement with the anterior approach is more often in the “safe zone” than with other approaches. Dislocation rates tend to be less than 1% with the anterior approach. Complication rates vary widely in the published literature. A possible explanation is that the variance is due to surgeon and institutional experience with the anterior approach procedure. Concerns remain regarding the “learning curve” for both surgeons and institutions. In conclusion, it is not a matter of should this approach be used, but how should it be implemented.
Keywords: Total hip arthroplasty, Anterior approach, Hip, Arthritis, Joint replacement
INTRODUCTION
The direct anterior approach (DAA) for total hip arthroplasty was first described by Judet[1] in 1947, and recently popularized in North America by Matta et al[2,3]. It is attractive to patients and surgeons because of its muscle sparing approach, which allows for a faster recovery[4,5], less pain after surgery[6], and post-operative hip precautions are not necessary[7]. It can be performed on a standard operating table or with the use of a specialized orthopedic table that facilitates femoral exposure. The patient is positioned supine, which allows for accurate assessment of leg lengths intra-operatively. Fluoroscopy and computer navigation can also be utilized to provide real time information about component position during surgery.
The surgeon’s level of experience with the approach does directly correlate with complication rates until reaching a plateau after the first 40-100 cases[8-10]. The low rate of dislocation consistently reported using the DAA for total hip arthroplasty[2,11] is a testament to the accuracy of component placement, as well as the preservation of important soft tissue structures that confer hip stability.
Advantages and disadvantages of the approach
The DAA is applicable for both primary[5] and revision total hip arthroplasty[12]. Bilateral hip replacement can easily be performed without re-positioning the patient. Achieving adequate femoral exposure is the most technically challenging aspect of the DAA for surgeons new to the technique.
There are some anatomic features of the native hip and pelvis that make the DAA more difficult, and all surgeons who desire to utilize this approach for total hip arthroplasty should be mindful of these morphologies. A wide or horizontal iliac wing can limit access to the femoral canal for broaching and placement of the femoral component. Acetabular protrusio brings the femoral canal closer to the center of the pelvis, which can obstruct access to the femur. A high neck shaft angle with decreased offset positions the femoral canal deeper in the thigh. Obese muscular males can limit the space available to place the components, and it takes considerable knowledge of how to position retractors as well as the leg in three-dimensional space to achieve enough exposure to do this accurately. A straight impactor that attaches to the acetabular component often impinges against the large muscular thigh distally, which can lead to more vertical and anteverted placement of the cup. An offset inserter is helpful in this situation. These are all technical aspects of the procedure that surgeons early in the learning curve are advised to consider in their patient selection process. Patients with a previous acetabular fracture associated with posterior heterotopic ossification, which requires excision, and when extensive exposure of the posterior acetabulum/column is necessary to address large posterior acetabular defects are relative contraindications[3].
Mast et al[12] described their operative experience in 51 patients with an average follow-up of 4.5 years using the DAA for revision total hip arthroplasty with an orthopedic table. When performing isolated acetabular liner exchange, cup revision or conversion of hip resurfacing to THA, the authors were able to perform these surgeries without proximal or distal extension of the standard approach.
CONTRAINDICATIONS
Mast et al[12] identify three scenarios that highlight the limitations of the anterior approach in revision surgery: (1) revision of long, extensively porous-coated femoral stems; (2) managing severe proximal bone loss or osteolysis; and (3) revision of a femoral stem with significant retroversion. It is worth pointing out that each of these three limitations involves the femoral exposure. Complications in this case series included loosening of the acetabular component (4%), heterotopic bone formation (2%), limb-length inequality (2%), trochanteric fracture (2%), with a reported complication rate of 9.8%. Interestingly, they reported no dislocations after revision surgery with a mean follow-up of 4.5 years.
SURGICAL TECHNIQUE
The surgeon can use a regular operating table or an orthopedic table designed to facilitate femoral exposure. The surgical technique described is with the use of an orthopedic table. The operative team consists of the surgeon, a single scrubbed assistant that stands on the opposite side of the table, a scrub nurse, a circulating nurse and the anesthesiologist. The patient is positioned supine on the operating table between a perineal post, which affords the benefit of being able to expeditiously utilize intra-operative fluoroscopy or computer navigation to assess leg lengths and ensure optimum placement of the components before leaving the operating room. Both feet are placed in boots that lock into a mobile spar that allows the leg to be positioned and rotated in any direction during the procedure. The original Judet orthopedic table has been modified to include a bracket that parallels the operative leg and supports a femoral hook, which holds the femur in an elevated position when broaching the canal. The operative extremity is draped from the iliac crest to the knee (Figure 1).
Figure 1.
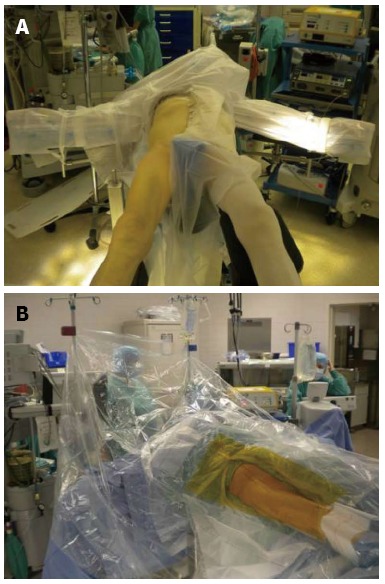
Patient positioned supine on a specialized orthopedic table (A) with the operative leg prepped and draped (B).
ACETABULAR EXPOSURE
The proximal aspect of the incision is marked 2-3 cm posterior and 1-2 cm distal from the anterior superior iliac spine, and extends distally in line with and over the tensor fascia lata muscle belly. The incision is placed laterally to the interval between the tensor fascia muscle and the sartorius to minimize the risk of lateral femoral cutaneous nerve injury. By developing the interval within the tensor fascia, the lateral femoral cutaneous nerve remains medial to the sartorial fascia and is avoided in the superficial dissection.
The skin and subcutaneous tissues are dissected down to the translucent fascia over the tensor, where two or three perforating blood vessels are encountered. The fascia is then incised in line with the muscle just anterior to these perforating vessels. An Alice clamp is attached to the medial aspect of the fascial incision and provides counter traction as the surgeon uses his finger to bluntly sweep the tensor muscle off the sartorial fascia.
A blunt cobra retractor is placed over the superior lateral aspect of the femoral neck which enhances the interval exposure between the tensor muscle and gluteus medius laterally and the sartorial and rectus fascia medially. The lateral femoral circumflex vessels are found within this interval encased in a layer of fat in the middle of the wound. They are carefully dissected and cauterized, or tied off and transected, as bleeding from these vessels can be profuse and difficult to control if they retract. A Cobb elevator is then used to mobilize the indirect head of the rectus off the capsule at the base of the neck followed by placement of a second cobra retractor along the inferior medial portion of the femoral neck. A double bent homan retractor is then slid perpendicular to the inguinal ligament directly above the capsule and along the acetabulum (Figure 2).
Figure 2.
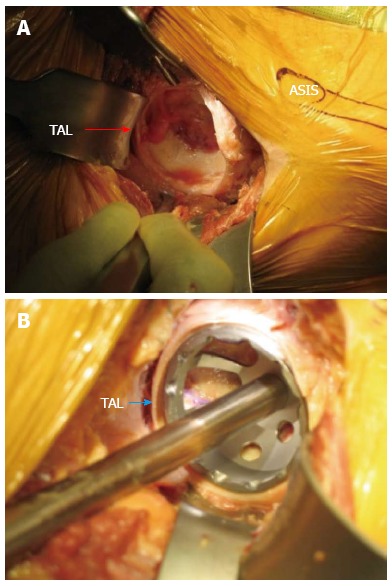
Acetabular exposure. A: Acetabular exposure with the direct anterior approach; B: Acetabular exposure using the direct anterior approach with trial component in place. The transverse acetabular ligament (TAL) is clearly identified by arrow. ASIS: Anterior superior iliac spine.
The hip capsule is then incised as an inverted “T” parallel to the lateral aspect of the intertrochanteric line along the lateral portion of the femoral neck and extended medially along the inferior portion of the femoral neck. The capsule is tagged with non-absorbable suture and repaired at the conclusion of the case. This capsular closure provides an additional layer of soft tissue to theoretically minimize the risk of deep infection. Alternatively the capsule may also be excised.
A hip skid is then slid between the femoral head and the acetabulum to break up any adhesions to facilitate an atraumatic dislocation of the femoral head. The femoral neck cut is made in one of three ways: (1) with the hip reduced; (2) a “napkin ring” segment of bone is created and removed by making two parallel neck cuts which leaves a smaller segment of the femoral head; or (3) after dislocating the femoral head. After dislocation fixed bony landmarks such as the superior aspect of the femoral head or the lesser trochanter are used to determine the desired level of femoral neck resection. A corkscrew with a removable handle is placed in the femoral head prior to making the femoral neck cut. The corkscrew will allow the surgeon to remove the femoral head in a controlled fashion without damaging the tensor muscle with the residual sharp spike of bone created by the distal end of the osteotomy. These steps allow expeditious removal of the femoral head after the neck cut. The femur is then externally rotated approximately 20 to 45 degrees, with slight adduction and flexion of the leg which enhances the exposure of the acetabulum for reaming and placement of the cup. Fluoroscopy and computer navigation can be used at this point to assess the placement of the socket, and adjustments made to component orientation if necessary (Figure 3).
Figure 3.
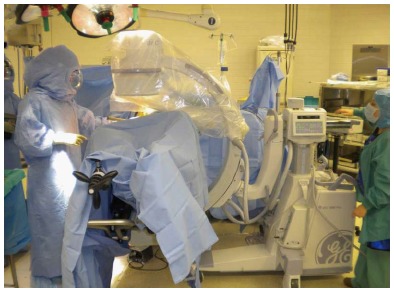
The supine position of the patient on the operating table facilitates the use fluoroscopy during surgery to assess component position and alignment.
FEMORAL EXPOSURE
After the acetabular component is seated, any traction on the operative leg is released and the femur is rotated back to a neutral position. If a femoral hook is used to assist with femoral exposure, it should be placed just distal and posterior to the vastus ridge. It should slide in easily and without resistance superficial to the vastus lateralis. The leg is then externally rotated so the calcar is facing directly anterior and the greater trochanter posterior. The operative leg is then positioned so the hip is extended 25-30 degrees by bringing the foot to the floor, and then maximally adducted. The surgeon laterally displaces the proximal femur and manually lifts the femoral hook to elevate the femur and uses a foot pedal to bring the motorized bracket arm up to dock the hook. If the surgeon attempts to elevate the femur by only using the motorized bracket arm with the hook in place, the femur can easily fracture.
The key to obtaining femoral exposure is performing sequential capsular and soft tissue releases along the medial aspect of the greater trochanter and femoral neck under tension (Figure 4). This ultimately allows the greater trochanter to clear the posterior wall of the acetabulum. With the operative leg hyper-extended and adducted, a long curved homan retractor is placed behind the greater trochanter to sufficiently tension these soft tissue attachments. This allows the surgeon to see and feel the femur move with each structure that is released. The goal is to release the minimum amount of soft tissue attachments to translate the femur laterally and elevate it up and out of the wound. The capsule is the first structure taken down with electrocautery, followed by the piriformis, the gemelli and the obturator internus until sufficient exposure is achieved. Preserving the obturator externus is important for maintaining hip stability and should not be released unless necessary as this effects the most direct medial pull of the femur to the pelvis.
Figure 4.
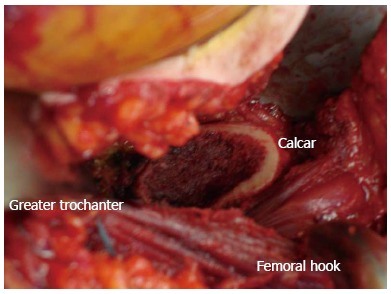
Exposure following femoral neck osteotomy with the direct anterior approach using a specialized orthopedic table.
Offset broach handles and occasional use of flexible reamers facilitate preparation of the femur and placement of the final femoral component. Fluoroscopy and computer navigation are again optional (Figure 3). They allow the surgeon additional information to intra-operatively assess the center of rotation, offset, leg lengths, femoral stem alignment, and fit within the canal.
The hip is reduced and can be checked for stability and component impingement. If the approach is performed with the utilization of a special table, lowering the foot to the floor and then adducting and externally rotating the operative leg can check anterior stability. Simply unhooking the boot from the mobile spar allows the surgeon to assess posterior stability and impingement.
The wound is copiously irrigated, the capsule re-approximated with heavy non-absorbable braided suture, and a deep drain drain is placed. The fascia of the tensor fascia lata muscle is closed with a running suture, and the subcutaneous and subcuticular layer closed with interrupted and a running 3-0 monocryl suture.
POST-OPERATIVE CARE
Patients are mobilized the day of surgery and post-operative hip precautions are not necessary. Post-operative pain and narcotic use is often significantly less compared to other surgical approaches for total hip arthroplasty[6]. Patients are more frequently discharged to home instead of extended care facilities, thus further decreasing the time of exposure to harmful pathogens[13]. The length of time in the hospital after total hip arthroplasty is significantly less in some European countries with the DAA[6,13].
If the patient has a pendulous abdomen that rests on the incision, precautionary steps are taken to minimize prolonged moisture on the incision. The senior author (Moskal JT) applies an abdominal binder at the conclusion of the case for patients with a pendulous abdomen to keep the pannus from resting on the incision until it has healed. Keeping the inguinal crease clean, dry and the incision covered with a sterile bandage also minimizes the risk of post-operative infection.
In a large prospective series, the average time to discontinuing the use of a cane or walker was 21 d, with 80% of patients discontinuing ambulatory assist devices by 7.6 d[11]. Gait analysis studies show quicker recovery of motor function for the DAA compared to other surgical exposures[14]. The patient returns for follow-up at 2 wk, 6 wk, 1 year, and then every 2 years for routine clinical and radiographic surveillance.
OUTCOMES
The literature, in general, makes numerous positive claims regarding the DAA with a fracture table for total hip arthroplasty including quicker recovery and return to unassisted ambulation, and reduced soft tissue damage, surgery time, pain, and risk of dislocation with early elimination of hip precautions[4,5,8,11,15,16].
In 2004, Sculco[15] wrote an early review of less extensive THA surgery. Sculco[15] stated in his review of minimally invasive total hip arthroplasty, “The rationale for performing hip arthroplasty through a less extensive exposure is to reduce hospital stay, speedy recovery, decrease surgical trauma. Certainly patients are happier with a smaller incision, and recovery is faster.” As less invasive THA continues to evolve, it is important to consider patient satisfaction and the speed at which they recover is a critical factor in their satisfaction and their return to normal activities of daily living.
The benefits of the anterior approach are mostly accrued from “muscle preservation” rather than the more traditional “muscle splitting” approaches[2,4,5,9,17-19]. Various authors have contributed to the literature focused on the mini-incision anterior approach, numerous aspects of this surgical technique are discussed: early outcomes and speed of recovery[4,5,9,14,17,20-22], component placement[2,4], dislocation rates[2,11,18,22-24], complication rates[2,4,9,11,21-23], and the impact of surgeon experience with this technique[2,4,9,11,22-25].
Early outcomes and speed of recovery
There are many ways to measure the early outcomes of THA and the speed of recovery from the surgery, such as time to full weight bearing, incidence of limping, biochemical muscle recovery, gait variables, range of motion, and traditional clinical measures[4,5,9,14,17,20-22].
In 2004, Siguier et al[22] reported that all patients were able to full weight bear within two days postoperatively and that most patients were able to discontinue walking aids within 8 d to 3 wk of surgery. There were no cases of limping secondary to gluteus medius insufficiency because the buttock muscles and greater trochanter were not affected by the surgical approach.
In an early investigational study, Pilot et al[17] were concerned with specific indicators of muscle recovery following anterior approach THA. They found no significant difference in inflammation as measured by interleukin-6 levels, in muscle damage as measured by heart type fatty acid binding protein, or in hemoglobin levels when comparing the mini-incision anterior approach with the standard posterolateral approach for THA (10 subjects in each group). Although they speculate that the term minimally invasive surgery is “at least doubtful in terms of being less traumatic” that there were no significant negative outcomes in terms of muscle recovery with minimally invasive surgery using the anterior approach.
In a very recent study, Bergin et al[19] reported the extent of muscle damage from the limited incision anterior approach (n = 29) as compared to the standard incision posterior approach (n = 28). The biochemical markers of inflammation, serum creatine kinase, C-reactive protein, interleukin-6, interleukin-1 beat, and tumor necrosis factor-alpha, were in general lower in the anterior approach group from post-surgery through post-operative day 2. The rise in creatine kinase was 5.5 times greater in the posterior approach group than in the anterior approach group post-surgery (P < 0.05) and nearly twice as high over the measurement period (P < 0.05). Serum creatine kinase levels indicated that the anterior approach causes significantly less muscle damage than the posterior approach[19].
Roth et al[21] looked at the early outcomes for 195 THA using the anterior approach in the supine position and found early restoration of full weight bearing and range of motion.
In a kinematic study comparing the DAA and the traditional anterolateral approach, Mayr et al[14] found that both gait and total range of motion were better with the DAA. Gait was improved in more categories than with the traditional anterolateral approach, including: significant improvement in cadence, stride time, stride length, walking speed, hip flexion at foot contact, maximum hip flexion in swing.
Nakata et al[4] compared the DAA and the mini-posterior approach in one of the few articles reporting on two different minimally invasive procedures. They found more rapid recovery of hip function and gait ability with the DAA. In the same year, Seng et al[9] also reported an earlier recovery and return to activities of daily living with the anterior approach.
In a more recent study, Klausmeier et al[20] compared the anterior approach with the anterolateral approach and a control group that did not have THA, their focus was the short term recovery of hip strength and motion. Hip abductor strength was lower in both of the THA groups when compared with the control group preoperatively, at six weeks, and at 16 wk. At 6 wk, the late stance peak abductor moment was not significantly different between the anterior approach and the control group; this measure was significantly lower for the anterolateral group. While the authors found no difference between the two approaches with regards to speed of recovery, or isometric strength and dynamic gait measures at six and sixteen weeks, the anterior approach was associated with improved gait velocity and peak flexor moment at 6 wk[20].
Most studies do not evaluate the differences in standard clinical measures such as Harris Hip Scores, SF-36, WOMAC, and VAS energy, daily activities, or overall quality, however Restrepo et al[5] did in 2010. In a study comparing the single-incision-modified Smith-Peterson anterior approach and the direct lateral approach, the outcomes using validated measures were found to be were significantly better for the anterior approach group at 6 wk, 6 mo, and 12 mo[5].
Other studies that reported on these factors consistently found that the anterior approach provided for faster recovery and improved early outcomes when employing the anterior approach[4,5,9,14,17,21,22].
Component placement
Component placement is an important factor in the success of THA, two sources reported on this outcome using the anterior approach[2,4]. Matta et al[2] had “safe zone” placement rates for the acetabular component; 96.07% (440 of 458 THA) in safe zone abduction angle and 93.01% (426 of 458 THA) in safe zone anteversion angle. Nakata et al[4] stated that significantly more acetabular components were placed in “safe zones” with DAA (98 of 99 THA, 98.99%) as compared to the mini-posterior approach (87 of 96 THA, 90.63%) (P = 0.008).
Dislocation rates
Dislocation rates are a common and useful metric when discussing THA, 8 studies discuss the dislocation rate using the anterior approach[2,4,9,11,21-23,25]. Of the 5801 THA reported in these studies, there were 55 dislocations (0.95%). Dislocation risk tends to be less than 1.0%, excepting for the rate reported by Sariali et al[18] (1.53%).
Complication rates
Various complications were reported: total complications, nerve related complications, and fractures (dislocation reported above)[2,4,9,11,21-23,25]. The overall complication rates ranged from 2.03% to 15.79%[22,24]. The two highest rates of overall complications, 15.79% and 15.63%, were in studies focused on complication rates with anterior THA using fracture tables[23,24]. The overall complication rate from aggregated data was 7.74% (320 of 4136 THA)[2,4,9,11,22,24].
The rate of nerve related complications was reported to range from 0.00% to 14.81%[21,25]. Most rates of nerve related complicates were less than 2%; the rate reported by Bhargava et al[25] was clearly much higher than the others, possibly due to this study being focused on nerve related complications[2,4,11,19,21,22,24].
The rate of fracture complications ranged from 0.10% to 7.29%[22,24]. Most complication rates were less than 3%; the rate reported by Woolson et al[24] came from a study of complications in a community hospital and may have been influenced by the setting and surgeon experience[2,4,11,19,21-23].
Impact of surgeon experience with this technique
The level of experience that an orthopaedic surgeon has with any new technique clearly impacts the successful execution of that technique; various authors have reiterated this with regards to the DAA using a fracture table[4,9,11,23-25]. Jewett et al[23] and Woolson et al[24] found disturbingly high rates of complications with this technique when performed by surgeons still in the “learning curve.” When Woolson et al[24] examined outcomes associated with the early experience of four community surgeons; the series was only of the early cases. Jewett et al[23] examined the complication rates for the first 800 cases performed using this technique and found that after the first 400 cases, intraoperative complications such as fracture no longer occurred. Bhargava et al[25] noted that the incidence of nerve impairment decreases as surgeon experience increases.
Two studies attempted to quantify the “learning curve” for the DAA using a fracture table[9,11]. Bhandari et al[11] found a clear decline in complications after the first 100 cases were performed by creating subgroups for analysis, one group contained surgeons with less than 100 cases and the other group contained surgeons with over 100 cases. Surgeons who had performed less than 100 cases had complication rates double that of more experienced surgeons[11]. Seng et al[9] sought to define the learning curve for joint arthroplasty surgeons in high volume practices. After six months and 57 cases, over 50% of DAA THA were performed comfortably and surgical time and intraoperative blood loss decreased[9].
CONCLUSION
What are the benefits of the anterior approach? In contrast to muscle-splitting approaches such as the direct lateral approach, the anterolateral approach, or the posterior approaches, the anterior approach is a muscle-sparing procedure thus no muscles are cut or detached. Muscle-splitting approaches require the cutting and detachment of soft tissues. This in turn disturbs the natural dynamic stabilization of the hip and makes it impossible for the hip to function normally until those structures have healed. Therefore, with muscle-splitting approaches, patients require at least six weeks of muscle healing plus additional time and rehabilitation effort to recover lost muscle strength. In short, patients must recover from both the surgical approach and the hip arthroplasty. Additionally, restrictions are required regarding patient movement and weightbearing to allow the soft tissues adequate time to heal.
In contrast to muscle-splitting approaches, with a muscle-sparing procedure, such as the DAA, no muscles are cut or detached. The patient must recover/heal from the surgical procedure only, not the approach. Recovery requires no additional time for healing of the muscle sleeve or its attachment, thus patients recover more quickly and may rehabilitate without restrictions. Experience suggests that patients benefit from a quicker recovery and elimination of postoperative restrictions, particularly younger and/or more active patients who need to return to work or return to other activities without restriction.
As more studies regarding the anterior approach for total hip arthroplasty are published, it becomes clearer that this approach does present distinct benefits for patient focused outcomes. However, there are concerns when incorporating new techniques into surgical practice; these often create a “learning curve” and unforeseen technical complications.
In conclusion, the DAA is a muscle-sparing approach with a quicker rehabilitation because the recovery is faster since the patients need only to recover from the procedure and not the approach. The question is not whether the orthopaedic community will embrace this technique but rather how should it be introduced into routine practice.
Footnotes
P- Reviewer Massoud EIE S- Editor Cheng JX L- Editor A E- Editor Zhang DN
References
- 1.Judet J, Judet R. The use of an artificial femoral head for arthroplasty of the hip joint. J Bone Joint Surg Br. 1950;32-B:166–173. doi: 10.1302/0301-620X.32B2.166. [DOI] [PubMed] [Google Scholar]
- 2.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115–124. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 3.Yerasimides J, Matta JM. Primary total hip arthroplasty with a minimally invasive anterior approach. Seminars in Arthroplasty. 2005;16:1860–1890. [Google Scholar]
- 4.Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24:698–704. doi: 10.1016/j.arth.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25:671–9.e1. doi: 10.1016/j.arth.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Goebel S, Steinert AF, Schillinger J, Eulert J, Broscheit J, Rudert M, Nöth U. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop. 2012;36:491–498. doi: 10.1007/s00264-011-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Restrepo C, Mortazavi SM, Brothers J, Parvizi J, Rothman RH. Hip dislocation: are hip precautions necessary in anterior approaches? Clin Orthop Relat Res. 2011;469:417–422. doi: 10.1007/s11999-010-1668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berend KR, Lombardi AV, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 6:107–120. doi: 10.2106/JBJS.I.00525. [DOI] [PubMed] [Google Scholar]
- 9.Seng BE, Berend KR, Ajluni AF, Lombardi AV. Anterior-supine minimally invasive total hip arthroplasty: defining the learning curve. Orthop Clin North Am. 2009;40:343–350. doi: 10.1016/j.ocl.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31 [PubMed] [Google Scholar]
- 11.Bhandari M, Matta JM, Dodgin D, Clark C, Kregor P, Bradley G, Little L. Outcomes following the single-incision anterior approach to total hip arthroplasty: a multicenter observational study. Orthop Clin North Am. 2009;40:329–342. doi: 10.1016/j.ocl.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Mast NH, Laude F. Revision total hip arthroplasty performed through the Hueter interval. J Bone Joint Surg Am. 2011;93 Suppl 2:143–148. doi: 10.2106/JBJS.J.01736. [DOI] [PubMed] [Google Scholar]
- 13.Alecci V, Valente M, Crucil M, Minerva M, Pellegrino CM, Sabbadini DD. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach: perioperative findings. J Orthop Traumatol. 2011;12:123–129. doi: 10.1007/s10195-011-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayr E, Nogler M, Benedetti MG, Kessler O, Reinthaler A, Krismer M, Leardini A. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech (Bristol, Avon) 2009;24:812–818. doi: 10.1016/j.clinbiomech.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Sculco TP. Minimally invasive total hip arthroplasty: in the affirmative. J Arthroplasty. 2004;19:78–80. doi: 10.1016/j.arth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Woolson ST. In the absence of evidence--why bother? A literature review of minimally invasive total hip replacement surgery. Instr Course Lect. 2006;55:189–193. [PubMed] [Google Scholar]
- 17.Pilot P, Kerens B, Draijer WF, Kort NP, ten Kate J, Buurman WA, Kuipers H. Is minimally invasive surgery less invasive in total hip replacement? A pilot study. Injury. 2006;37 Suppl 5:S17–S23. doi: 10.1016/S0020-1383(07)70007-4. [DOI] [PubMed] [Google Scholar]
- 18.Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23:266–272. doi: 10.1016/j.arth.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Bergin PF, Doppelt JD, Kephart CJ, Benke MT, Graeter JH, Holmes AS, Haleem-Smith H, Tuan RS, Unger AS. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93:1392–1398. doi: 10.2106/JBJS.J.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klausmeier V, Lugade V, Jewett BA, Collis DK, Chou LS. Is there faster recovery with an anterior or anterolateral THA? A pilot study. Clin Orthop Relat Res. 2010;468:533–541. doi: 10.1007/s11999-009-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth A, Venbrocks RA. Total hip replacement through a minimally invasive, anterolateral approach with the patient supine. Oper Orthop Traumatol. 2007;19:442–457. doi: 10.1007/s00064-007-1019-2. [DOI] [PubMed] [Google Scholar]
- 22.Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res. 2004;(426):164–173. doi: 10.1097/01.blo.0000136651.21191.9f. [DOI] [PubMed] [Google Scholar]
- 23.Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469:503–507. doi: 10.1007/s11999-010-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplasty. 2009;24:999–1005. doi: 10.1016/j.arth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Bhargava T, Goytia RN, Jones LC, Hungerford MW. Lateral femoral cutaneous nerve impairment after direct anterior approach for total hip arthroplasty. Orthopedics. 2010;33:472. doi: 10.3928/01477447-20100526-05. [DOI] [PubMed] [Google Scholar]


