Abstract
The new millennium has witnessed the emergence of minimally invasive, non-posterior based surgery of the lumbar spine, in particular via lateral based methodologies to discectomy and fusion. In contrast, and perhaps for a variety of reasons, anterior motion preservation (non-fusion) technologies are playing a comparatively lesser, though incompletely defined, role at present. Lateral based motion preservation technologies await definition of their eventual role in the armamentarium of minimally invasive surgical therapies of the lumbar spine. While injury to the major vascular structures remains the most serious and feared complication of the anterior approach, this occurrence has been nearly eliminated by the use of lateral based approaches for discectomy and fusion cephalad to L5-S1. Whether anterior or lateral based, non-posterior approaches to the lumbar spine share certain access related pitfalls and complications, including damage to the urologic and neurologic structures, as well as gastrointestinal and abdominal wall issues. This review will focus on the recognition, management and prevention of these anterior and lateral access related complications.
Keywords: Anterior spinal exposure, Lumbar spine, Complications
INTRODUCTION
Anterior spinal access is often required for the treatment of spinal deformity, bony and/or discogenic infection, trauma, tumor and degenerative disease. Advantages of this approach include performance of a thorough discectomy and release, capability to implant high profile interbody fusion and non-fusion devices, debridement and excision of necrotic tissue, removal of migrated/misplaced devices, and a favorable milieu for interbody fusion with rich blood supply and graft/device placement under compression. The most common associated complications include damage to the vascular, urologic and neurologic structures, as well as gastrointestinal and abdominal wall issues.
VASCULAR INJURY
Anterior exposure of the spine at the L4-L5 and L5-S1 levels requires mobilization of the left common iliac vessels, as they course obliquely across the anterior aspect of the L5 body, traversing variable portions of the L4-L5 and L5-S1 disc spaces in the process. The most dorsally located, the left common iliac vein is the most likely vascular structure to be injured during anterior lumbar spinal surgery. Apart from intraoperative hemorrhage and the challenge associated with vascular control and repair, thrombotic occlusion may occur in the postoperative period following seemingly uncomplicated iliac venorrhaphy, or simply as a result of prolonged retraction of the iliac vein or inferior vena cava. The ascending iliolumbar vein acts as an important dorsolateral tether to the left common iliac vein, therefore routine ligation and division will facilitate anterior exposure of the L4-L5 disc space[1]. Similarly, ligation and division of the L4 segmental vessels will release the aortic terminus and the terminal inferior vena cava (IVC), thus permitting retraction to the right side of the spine, further facilitating anterior exposure of the L4-L5 disc space. Previous osteomyelitis/discogenic infection, previous anterior spinal surgery, spondylolisthesis, osteophyte formation, transitional lumbosacral vertebra and anterior migration of interbody device have been identified as risk factors for injury to the major vascular structures during anterior spinal surgery[1]. With the sole exception of transitional anatomy, the identified conditions share the underlying pathogenesis of inflammation of the annular and pre-vertebral soft tissues, as well as of the periosteum, thereby limiting mobility of the overlying vascular structures. The vast majority of major vascular injuries to the great vessels of the abdomen occur during attempts at anterior exposure of L4-L5 and L5-S1.
The reported incidence of significant venous injury is in the 2%-4% range (Table 1). Arterial thrombosis occurs in less than 1% of cases, and is typically associated with fixed retraction of the large vessels, either via a table mounted mechanical system[2] or through Steinman pins placed directly into the vertebral body[3]. Although we do use a table mounted mechanical retractor system, the major vascular structures are manipulated only through the use of hand held retractors, with release of traction at regular intervals of no longer than fifteen minutes. In addition, attempts to mobilize heavily calcified vessels should be tempered, as loss of normal elasticity and recoil will predispose to plaque fracture and arterial thrombosis.
Table 1.
Reported incidence of major vascular injury during anterior spinal surgery n (%)
| Ref. | Year | n | Arterial injury | Venous injury |
| Fantini et al[1] | 2007 | 345 | 1 (0.3) | 9 (2.6) |
| Brau et al[2] | 2004 | 1315 | 6 (0.5) | 19 (1.4) |
| Kulkarni et al[3] | 2003 | 336 | 8 (2.4) | NA |
| Gumbs et al[4] | 2005 | 64 | 0 | 2 (3.1) |
| Fritzell et al[5] | 2003 | 72 | 0 | 2 (3.7) |
| Holt et al[6] | 2003 | 450 | 0 | 7 (1.6) |
| Kaiser et al[7] | 2002 | 98 | 0 | 3 (3.1) |
| Oskouian et al[8] | 2002 | 207 | 2 (0.9) | 7 (3.4) |
| Kuslich et al[9] | 1998 | 591 | 0 | 10 (1.7) |
NA: Not available.
The use of lateral based approaches for discectomy and fusion of the lumbar spine cephalad to L5-S1 has nearly eliminated the occurrence of great vessel injury. That said, it is not uncommon to encounter the aforementioned ascending iliolumbar vein during performance of a lateral based approach to the L4-L5 disc from the left. In this setting, ligation and division of the ascending iliolumbar vein in controlled fashion is the preferred approach.
Principles of venous repair
Initial maneuvers following recognition of injury to a major venous structure (e.g., iliac vein or vena cava) are of critical importance and may very well determine outcome. Aggressive use of suction and/or traction at the venotomy site, prior to gaining control, can cause further damage to the injured vessel and must be avoided. Trendelenburg’s position should be utilized. Control of hemorrhage should be obtained with compression proximal and distal to venotomy, typically through the use of Kitner peanut dissectors and/or sponge-sticks. Wylie renal vein retractors may also prove useful in this regard. No attempt to encircle the iliac vein or to apply vascular clamps should be made, as this will generally result in further venous disruption and increased bleeding. Once adequate visualization of the venotomy has been obtained, primary repair with 5-0 prolene suture on a cardiovascular needle may be carried out (Figure 1). Should a minimal access incision not permit formal suturing and tying, vascular clips may be placed at right angles to the long axis of the vessel in “railroad track” fashion (Figure 1C). Recent experience with endovascular repair of the left common iliac vein with a covered stent suggests that this will be a viable methodology as this technology becomes more widely available[10]. Topical hemostatic agents including Gelfoam® (Pfizer, New York, NY), Surgicel® Fibrillar™ and Surgiflo® (Ethicon, Somerville, NJ), and Tisseel (Baxter, Deerfield, IL) are important adjuncts to direct repair, and in many instances can be effective as the sole method of hemostasis.
Figure 1.
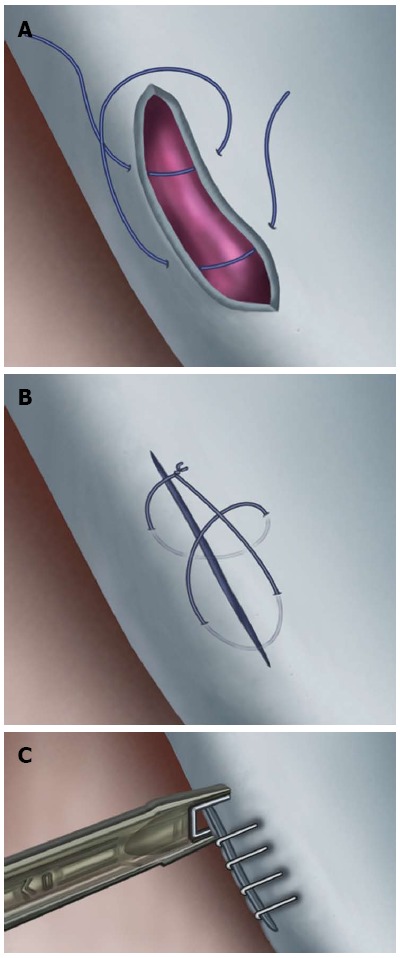
Lateral repair of iliac vein with 5-0 prolene suture placed in figure-of-eight fashion (A, B) and vascular clips placed in “railroad track” fashion (C).
Postoperative surveillance for iliac vein thrombosis
Successful repair of seemingly minor injuries of the iliac vein can result in thrombosis in the postoperative period. Remarkably, manifestations of leg swelling may not be readily apparent in the setting of bed rest and limited ambulation. Venous duplex scanning is notoriously unreliable in detecting thrombosis cephalad to the inguinal ligament. For this reason, iliac venous imaging by computed tomographic angiography (CTA) or magnetic resonance venography is performed routinely following iliac venous repair[1]. Detection of iliac vein thrombosis in the early postoperative period typically mandates placement of a vena caval filter, as anticoagulation is generally not an option.
Management of arterial injury
As noted above, arterial complications can be minimized by avoiding the use of fixed retraction systems on the large vessels, and by limiting the degree of arterial mobilization in the setting of heavy vessel calcification. Arterial hemorrhage can be managed with traditional lateral suture repair, applying vascular clamps above and below the arteriotomy if necessary. Arterial thrombosis can be a more difficult problem in the patient with atherosclerotic disease. Continuous pulse oximetry of the lower extremity ipsilateral to the site of arterial retraction, typically the left, is a useful monitor to employ routinely. Management by catheter thrombectomy and repair of the culprit lesion, sometimes requiring adjunct methods of endarterectomy or bypass, will be required. Consideration to leg fasciotomy should be given, depending upon the degree and duration of extremity ischemia.
UROLOGIC INJURY
Blood supply to the ureter is segmental in nature, and as such, no attempt to skeletonize the ureter should be made. Rather, the ureter should be rotated medially along with the visceral sac. Incidence of ureteral injury during primary retroperitoneal exposure is exceedingly low, though has been reported[4]. In contrast, the ureter is at significant risk of injury during revision anterior spinal surgery. This is especially true in the setting of removal of anterior instrumentation, as the ureter may be encased in scar tissue immediately overlying the instrumentation. In this instance, delayed images taken during pre-operative CTA will delineate the course of the ureters, and typically signal the need for ipsilateral ureteral stent placement. In addition, methylene blue is administered intravenously on a routine basis at the start of the revision anterior procedure.
Retrograde ejaculation
The sympathetic fibers of the hypogastric plexus are adherent to the posterior surface of the peritoneum at the level of L5-S1, thus further emphasizing the importance of en bloc mobilization of the visceral sac. Avoidance of electrical and/or thermal injury to the hypogastric plexus can be achieved by using a scalpel for the annulotomy and by using bipolar electrocautery sparingly and only as absolutely necessary. Modern series have reported low incidences of retrograde ejaculation. The ProDisc® (Synthes, West Chester, PA) lumbar total disc replacement (TDR) trial reported an incidence of 1.2% (1/82) in males undergoing TDR[11], while the Charité™ (DePuy, Raynham, MA) artificial disc trial reported an incidence of 4% (6/147) in males undergoing either TDR or anterior fusion[12]. A recent retrospective consecutive cohort study implicates the inflammatory reaction associated with recombinant human bone morphogenetic protein-2 (rhBMP-2) use as an adjunct to anterior lumbar interbody fusion (ALIF) at L5-S1 in generating an increased incidence of retrograde ejaculation[13]. A 6.3% incidence (15/239) of retrograde ejaculation was identified in male patients receiving rhBMP-2 as an adjunct to one (L5-S1) or two (L4-5/L5-S1) level ALIF, as compared to an incidence of 0.9% (2/233) absent rhBMP-2 use in the control arm. Noteworthy is that of 12 patients with retrograde ejaculation followed for at least 2 years postoperatively, six (50%) reported resolution.
GASTROINTESTINAL COMPLICATIONS
The most common gastrointestinal issue complicating the postoperative course of the patient undergoing anterior lumbar spinal surgery is ileus. Routine measures taken to reduce the incidence of ileus include preoperative mechanical bowel preparation, use of an orogastric tube intraoperatively, and avoidance of nitrous oxide as an anesthetic agent. Use of preoperative mechanical bowel preparation is especially worthwhile in the setting of a significant preoperative narcotic requirement, as gastrointestinal transit time may be dramatically prolonged. Methylnaltrexone bromide (Relistor®, Salix Pharmaceuticals, Raleigh, NC) injection is particularly useful in treating opiate induced constipation postoperatively. In cases of refractory ileus, as well as colonic pseudo-obstruction (Ogilvie’s syndrome), neostigmine administered intravenously is frequently effective, although a monitored setting is required as bradycardia is a recognized side effect of this parasympathomimetic agent[14].
ABDOMINAL WALL COMPLICATIONS
Sometimes referred to as abdominal asymmetry, a change in contour of the abdominal wall is a recognized outcome following oblique flank incisions during the course of aortic, renal and/or anterior spinal surgery. The resulting prominence or bulge is not a true hernia, as there is no accompanying fascial defect, and consequently no risk of incarceration exists. Generally thought to occur as a result of denervation of the oblique musculature of the flank, an increased incidence has been noted with incisions extending into the eleventh intercostal space[15], suggesting an important role for the eleventh intercostal nerve in preserving normal muscular function of the abdominal wall. Innervation of the oblique and rectus abdominis musculature is by the anterior divisions of intercostal nerves VII-XII, referred to as thoraco-abdominal nerves (Figure 2A). Coursing between the internal oblique and transversus abdominal muscles, the thoraco-abdominal nerves perforate the rectus sheath and terminate as anterior cutaneous branches of the abdomen. Further innervation of the internal oblique and transversus abdominal muscles is by the iliohypogastric (superior branch) and ilioinguinal nerves (inferior branch), arising together from the anterior rami at T12 and L1 (Figure 2B). Preservation of the thoraco-abdominal neurovascular bundle in the interval between the internal oblique and transversus abdominal muscles is felt to be an important element in maintaining integrity of muscular function of the abdominal wall during performance of lateral transpsoatic interbody fusion.
Figure 2.
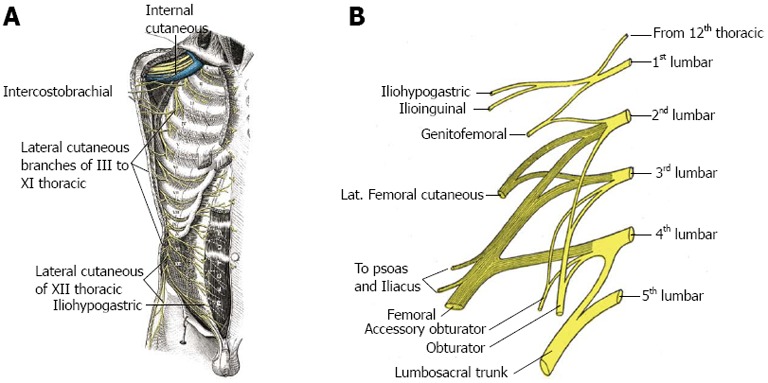
Anatomy of thoraco-abdominal nerves (A) and iliohypogastric and ilioinguinal nerves (B).
Footnotes
P- Reviewers Al-Eisa E, Nagashima H S- Editor Cheng JX L- Editor A E- Editor Zhang DN
References
- 1.Fantini GA, Pappou IP, Girardi FP, Sandhu HS, Cammisa FP. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976) 2007;32:2751–2758. doi: 10.1097/BRS.0b013e31815a996e. [DOI] [PubMed] [Google Scholar]
- 2.Brau SA, Delamarter RB, Schiffman ML, Williams LA, Watkins RG. Vascular injury during anterior lumbar surgery. Spine J. 2004;4:409–412. doi: 10.1016/j.spinee.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni SS, Lowery GL, Ross RE, Ravi Sankar K, Lykomitros V. Arterial complications following anterior lumbar interbody fusion: report of eight cases. Eur Spine J. 2003;12:48–54. doi: 10.1007/s00586-002-0460-4. [DOI] [PubMed] [Google Scholar]
- 4.Gumbs AA, Shah RV, Yue JJ, Sumpio B. The open anterior paramedian retroperitoneal approach for spine procedures. Arch Surg. 2005;140:339–343. doi: 10.1001/archsurg.140.4.339. [DOI] [PubMed] [Google Scholar]
- 5.Fritzell P, Hägg O, Nordwall A. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J. 2003;12:178–189. doi: 10.1007/s00586-002-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt RT, Majd ME, Vadhva M, Castro FP. The efficacy of anterior spine exposure by an orthopedic surgeon. J Spinal Disord Tech. 2003;16:477–486. doi: 10.1097/00024720-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser MG, Haid RW, Subach BR, Miller JS, Smith CD, Rodts GE. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51:97–103; discussion 103-5. doi: 10.1097/00006123-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Oskouian RJ, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96:1–5. doi: 10.3171/jns.2002.96.1.0001. [DOI] [PubMed] [Google Scholar]
- 9.Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976) 1998;23:1267–178; discussion 1279. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 10.Zahradnik V, Lubelski D, Abdullah KG, Kelso R, Mroz T, Kashyap VS. Vascular Injuries During Anterior Exposure of the Thoracolumbar Spine. Ann Vasc Surg. 2012:Epub ahead of print. doi: 10.1016/j.avsg.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Zigler J, Delamarter R, Spivak JM, Linovitz RJ, Danielson GO, Haider TT, Cammisa F, Zuchermann J, Balderston R, Kitchel S, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;32:1155–162; discussion 1163. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 12.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R, Regan JJ, Ohnmeiss DD. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976) 2005;30:1565–175; discussion 1565-175;. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 13.Comer GC, Smith MW, Hurwitz EL, Mitsunaga KA, Kessler R, Carragee EJ. Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a 10-year cohort controlled study. Spine J. 2012;12:881–890. doi: 10.1016/j.spinee.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 14.Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 15.Gardner GP, Josephs LG, Rosca M, Rich J, Woodson J, Menzoian JO. The retroperitoneal incision. An evaluation of postoperative flank 'bulge'. Arch Surg. 1994;129:753–756. doi: 10.1001/archsurg.1994.01420310085015. [DOI] [PubMed] [Google Scholar]


