INTRODUCTION
As part of its System Performance initiative, the Canadian Partnership Against Cancer released Breast Cancer Control in Canada: A System Performance Special Focus Report in September 2012, presenting a broad range of system performance indicators that measure breast cancer control across the continuum. Among the indicators reported for the first time at the national level is the percentage of women with invasive breast cancer who were tested for tumour biomarkers. Data for that measure have just recently become available through the population-wide collection of collaborative stage in the provincial cancer registries.
RESULTS OF TUMOUR BIOMARKER TESTS GUIDE TREATMENT DECISIONS FOR WOMEN DIAGNOSED WITH BREAST CANCER
For women with breast cancer, knowledge about the presence of specific tumour markers can be useful in selecting appropriate adjuvant drug therapy.
The biomarkers most commonly used for predicting response to therapy are the estrogen (er) and progesterone (pr) receptors. In 2009, a task force convened by the U.S. National Comprehensive Cancer Network (nccn) recommended that all women with invasive primary breast cancer be tested for er and pr status1.
Another useful tumour marker in the management of breast cancer is the human epidermal growth factor receptor 2 (her2) protein. Another nccn task force recommended that all women with invasive breast cancer be tested for her22.
Immunohistochemistry is the standard method for biomarker testing in breast cancer.
ER, PR, AND HER2 TESTING
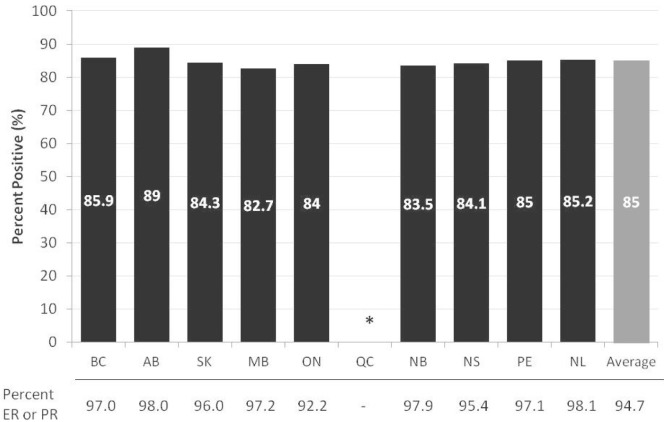
The data show that er or pr hormone receptor testing is done for most women with breast cancer. Data from 9 provinces on the use of er and pr hormone receptor testing in 2010 show that 94.7% of women newly diagnosed with invasive breast cancer had an er test, a pr test, or both, with little variation between those provinces (Figure 1). The proportion of patients with invasive breast cancer who were tested and determined to be er or pr positive (or both) ranged from 82.7% to 89.0% in the reporting provinces, with an overall average of 85.0% (Figure 1). That rate is consistent with studies from the United States showing that 75%–85% of breast cancers are expected to be er or pr positive (or both)3.
FIGURE 1.
Percentage of women newly diagnosed with invasive breast cancer in 2010 who tested as positive for the estrogen receptor (er) or the progesterone receptor (pr), or both, by province (includes women who were staged and who had an er or pr test completed). * Data for Quebec not available. Data source: Provincial cancer registries.
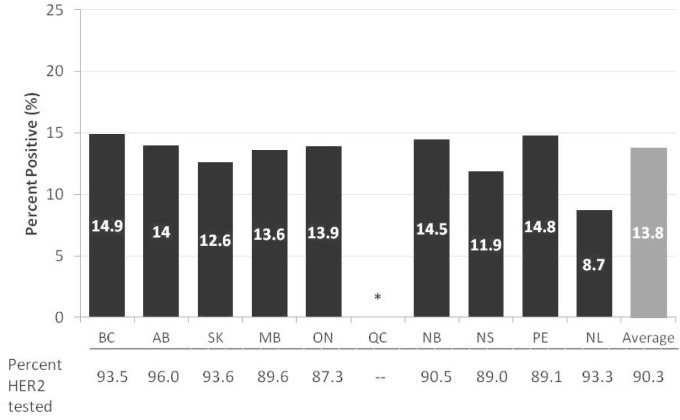
The proportion of women with invasive breast cancer who had a her2 test in 2010 ranged from 87.3% to 96.0% in the 9 provinces providing testing data. Of tests ordered in those provinces, an average of 13.8% found the tumour to be her2 positive (Figure 2), with test positivity ranging from 8.7% to 14.9% in the reporting provinces. To put those findings into perspective, the literature suggests that her2 positivity among women with invasive breast cancer can range from 15% to 25%2,3, although differences in definitions can limit the applicability of that comparison.
FIGURE 2.
Percentage of women newly diagnosed with invasive breast cancer in 2010 who tested as positive for the human epidermal growth factor receptor 2 (her2), by province (includes women who were staged and who had a her2 test completed). For Newfoundland and Labrador (NL), her2 testing was performed outside the province in a centralized lab. * Data for Quebec not available. Data source: Provincial cancer registries.
Results indicate that, in 2010, there was already a high uptake of testing and that er or pr positivity rates were in line with expectations, based on reports in the literature. Future analysis will further explore patterns, including examinations of stage at diagnosis and linkages to utilization rates for adjuvant therapies.
ABOUT BREAST CANCER CONTROL IN CANADA: A SYSTEM PERFORMANCE SPECIAL FOCUS REPORT
The Breast Cancer Control in Canada: A System Performance Special Focus Report was published by the Canadian Partnership Against Cancer and made possible through the dedicated efforts of the System Performance Steering Committee and Technical Working Group, each comprising representatives from all 10 provinces. An Editorial Panel of national cancer control experts oversaw production of the report.
To view Breast Cancer Control in Canada: A System Performance Special Focus Report, visit http://www.cancerview.ca/systemperformancereport.
Downloadable slides of figures in this communication and the report can be accessed at http://www.cancerview.ca/downloadableslides.
CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Allred DC, Carlson RW, Berry DA, et al. nccn Task Force Report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Canc Netw. 2009;7(suppl 6):S1–21. doi: 10.6004/jnccn.2009.0079. [DOI] [PubMed] [Google Scholar]
- 2.Carlson RW, Moench SJ, Hammond ME, et al. on behalf of the nccn her2 Testing in Breast Cancer Task Force her2 testing in breast cancer: nccn Task Force report and recommendations. J Natl Compr Canc Netw. 2006;4(suppl 3):S1–22. [PubMed] [Google Scholar]
- 3.American Society of Clinical Oncology (asco) Cancer.net > Cancer Types > Breast Cancer > Diagnosis [Web page] Alexandria, VA: ASCO; 2008. [Available at: http://www.cancer.net/patient/Cancer+Types/Breast+Cancer?sectionTitle=Diagnosis; cited May 2012] [Google Scholar]