Abstract
Background
A growing body of evidence is demonstrating that the nitrogen-containing bisphosphonate zoledronic acid (zol) improves clinical outcomes in various cancer settings, including multiple myeloma. Those findings provided the rationale for conducting an open-label randomized controlled phase iii trial to evaluate the effect of zol on overall survival (os) and progression-free survival (pfs) in patients with previously untreated high-risk multiple myeloma.
Methods
The trial randomly assigned 308 adult patients less than 65 years of age with previously untreated symptomatic multiple myeloma (1:1) to receive zol 4 mg intravenously once every 28 days for 24 months (n = 151) or no zol (n = 157). Before autologous stem-cell transplantation (asct), all patients received a high-dose noncytotoxic induction regimen of dexamethasone, all-trans-retinoic acid, and interferon alpha 2b.
Results
After a median follow-up of 69.8 months (range: 36.5–96 months), the 10-year pfs (66% vs. 52%, p < 0.001) and os (67% vs. 48%, p < 0.001) rates were significantly higher in treated patients than in control patients. Overall response (77% zol vs. 75% control), complete response (52% vs. 46%), and very good partial response (25% vs. 29%) rates were similar between the groups. Treatment was generally well tolerated, with no reports of renal impairment or osteonecrosis of the jaw.
Conclusions
In symptomatic previously untreated multiple myeloma patients, zol combined with high-dose therapy followed by asct improved os and pfs without appreciable toxicity. These findings provide additional evidence of the meaningful anticancer activity of zol in this patient population.
Keywords: High-dose therapy, multiple myeloma, stem-cell transplantation, zoledronic acid, bisphosphonates
1. INTRODUCTION
Multiple myeloma is a plasma-cell malignancy that can cause osteolytic bone lesions, excess immunoglobulin secretion, renal impairment, and myelosuppression1. Although treatment is rarely curative, great strides have been made in the management of multiple myeloma. Just since the early 2000s, newly introduced therapies such as bortezomib, lenalidomide, and thalidomide have revolutionized the treatment paradigm in multiple myeloma and likely contributed to improved survival2,3. Survival of patients diagnosed with multiple myeloma since then has risen significantly to 44.8 months, compared with historical reports of 29.9 months (p < 0.001)3.
High-dose induction therapy with subsequent autologous stem-cell transplantation (asct) is the current standard of care for previously untreated patients with symptomatic multiple myeloma4. Currently, the optimal induction therapy has not been established for patients who are potential candidates for transplantation. Although a variety of active regimens that combine dexamethasone, doxorubicin, bortezomib, thalidomide, and lenalidomide5 are available, no single best regimen has emerged.
Bisphosphonates such as zoledronic acid (zol), clodronate, and pamidronate inhibit osteoclast-mediated osteolysis and, by minimizing skeletal complications, provide significant benefit to patients with bone metastases from solid tumours or bone lesions from multiple myeloma6,7. Current clinical guidelines for the treatment of multiple myeloma recommend bisphosphonate therapy for all patients regardless of whether bone lesions have been detected, and in this setting, zol is the bisphosphonate of choice5,7–9. In addition to its bone-protective effects, zol has demonstrated clinically meaningful anticancer activity in multiple cancer settings, including that of multiple myeloma10–15. In particular, results from the large U.K. Medical Research Council (mrc) Myeloma ix trial demonstrated a significant overall survival (os) benefit for zol compared with the weaker oral bisphosphonate clodronate in patients with newly diagnosed multiple myeloma15. In our initial study, we showed that, compared with induction chemotherapy alone, adding zol to cyclophosphamide, vincristine, melphalan, and prednisone (our standard induction regimen at that time) significantly improved 5-year event-free survival (80% vs. 52%, p < 0.01) and 5-year os (80% vs. 46%, p < 0.01)10. Thus, it appears that zol could have antitumour effects in patients with multiple myeloma.
Recent observations might provide the rationale to explain those effects. Animal studies show that zol inhibits soft-tissue tumour growth, decreases tumour cell proliferation, and enhances immune surveillance; and in vitro studies suggest that zol inhibits tumour cell dissemination in bone marrow16,17. Given those promising results, we conducted an open-label randomized controlled phase iii trial to examine the effect of zol on progression-free survival (pfs) and os in the same patient population treated using our institution’s current standard of care, a high-dose induction regimen of dexamethasone, all-trans-retinoic acid, and interferon (dai) followed by asct. When the study was planned, the routine use of zol or other bisphosphonates was not included in the standard regimen in this setting.
2. METHODS
Our study (search for NCT01234129 at http://clinicaltrials.gov) was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent, and the study protocol was approved by the Ethical and Scientific Committee of the Mexican Institute of Social Security (HO 2002-83 R) before study initiation.
This single-centre study was conducted from June 1, 2002, to December 30, 2007, in the Oncology Hospital, National Medical Center, Instituto Mexicano del Seguro Social, a national tertiary reference centre in Mexico City, Mexico (population served: 32 million).
Adult patients at least 18 years but less than 65 years of age with untreated symptomatic multiple myeloma and measurable paraprotein in serum and urine, Eastern Cooperative Oncology Group performance status 0–2, and adequate renal (no end-stage renal failure and creatinine clearance > 30 mL/min), hematologic (platelet count > 50×109/L, neutrophil count > 0.75×109/L), and liver function were eligible for inclusion according to International Myeloma Working Group (imwg) guidance. Patients with nonsecretory paraprotein also were included (assessed for response and follow-up using the serum free light-chain assay, again according to imwg guidance18). Patients with amyloidosis, hiv infection, or uncontrolled diabetes were excluded. Participation in any other clinical trial was also an exclusion criterion.
As they presented clinically at our institution, patients were randomly assigned (in a 1:1 ratio by computer-generated randomization sequence placed in sealed envelopes) to receive zol 4 mg (or dose-adjusted based on creatinine clearance) intravenously once monthly for 24 months (zol arm) or no zol (control arm). After 24 months, zol was stopped, and no other bisphosphonate was allowed. No supplemental support with calcium or vitamin D was considered. All patients received six 1-month cycles of dai (dexamethasone 40 mg intravenously on days 1–4, all-trans-retinoic acid 45 mg/m2 orally once daily on days 5–14, and interferon alpha 2b 5.0×106 U subcutaneously once daily on days 5–14) as high-dose induction therapy. After 6 months, patients were restaged by bone marrow biopsy, and those who had achieved a complete response (cr) or a very good partial response (vgpr) were eligible for asct. Patients with a partial response or progressive disease after induction therapy were considered to have experienced treatment failure, and their participation in the study was discontinued, although they continued to receive treatment on an individual basis.
Before asct, patients received granulocyte colony–stimulating factor 10 μg/kg subcutaneously, daily for 2 days, starting on day 15, cycle 4, of induction therapy to mobilize stem cells, and stem-cell collection was first attempted on day 18 after cycle 4 of chemotherapy. The target yield of stem cells was more than 5×106 CD34+ cells per kilogram. If the first attempt at stem-cell collection was inadequate, patients underwent a second mobilization with cyclophosphamide 2 g/m2 intravenously and granulocyte colony–stimulating factor 10 μg/kg daily for 2 days after completion of high-dose induction therapy cycle 4. The high-dose conditioning regimen before infusion of stem cells was melphalan 200 mg/m2 intravenously. After asct, patients received interferon alpha 2b 5.0×106 U subcutaneously 3 times weekly for 1 year. A second asct was not allowed in the study. At relapse, patients were treated at the physician’s discretion. Most patients received thalidomide and dexamethasone or bortezomib and dexamethasone; in some cases, when clinical performance was poor, patients received melphalan and prednisone.
The primary endpoints were pfs and os at 10 years, and the secondary endpoints included overall response, rates of cr and vgpr, and safety.
When the study was planned, the current international criteria for evaluation of treatment response in this population had not been published. We therefore converted the response criteria defined in our protocol to be consistent with the terminology in the new imwg criteria19,20. Response was assessed after completion of high-dose induction therapy and 3 months after asct. Blood and 24-hour urine samples were obtained at baseline, after induction chemotherapy and asct, and at 3, 6, and 12 months after asct. During the follow-up period, blood and 24-hour urine samples were obtained every 6 months.
Targeted enrollment was approximately 320 patients to ensure that 310 would be available for analysis (150 patients in each arm), providing 80% power (2-sided α = 0.05) to detect a 10% difference in median pfs and os between the zol and control arms. At 3 and 6 years, the anticipated median pfs was 83% and 75% respectively, and the anticipated median os was 80% and 65% respectively. Comparisons of outcomes used the intention-to-treat population, which comprised all patients who were randomized. All patients who received the planned treatment were considered evaluable for efficacy and toxicity.
The 10-year pfs and os (primary endpoints) were estimated using the Kaplan–Meier method and compared between the groups using the chi-square test. Progression-free survival was defined as time from the start of high-dose induction therapy (or time from the start of next treatment) to time of progression, relapse, or death. Both groups underwent radiologic studies every 6 months to detect the presence of skeletal complications, because the primary purpose of zol is to prevent skeletal-related events (sres).
3. RESULTS
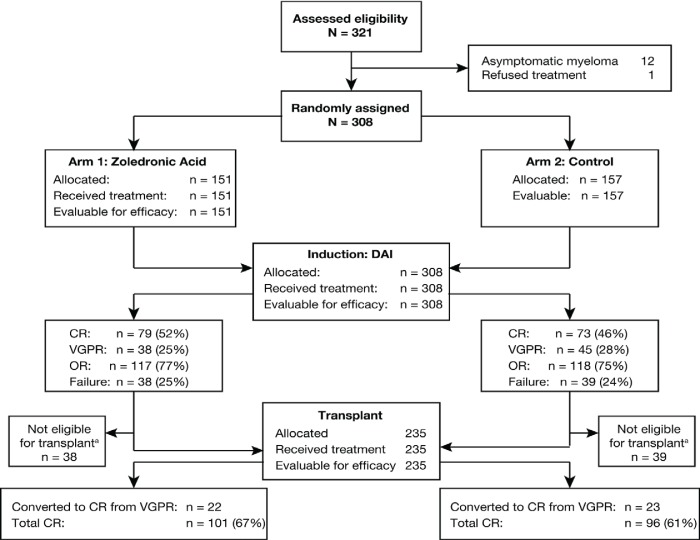
Of the 321 patients assessed for enrollment, 308 were randomly assigned to the zol (n = 151) or control (n = 157) arm (Figure 1). All randomized patients were included in the intention-to-treat and safety populations. Demographic and disease characteristics were well-balanced between the treatment groups at baseline (Table i)19. Overall, approximately half the patients were male, and all had bone lesions.
FIGURE 1.
Trial profile.aPatients who failed to achieve a complete response (cr) or very good partial response (vgpr) were not eligible for transplant. dai = dexamethasone, all-trans-retinoic acid, and interferon; or = overall response.
TABLE I.
Demographic and disease characteristics at baseline in the intention-to-treat population
| Characteristic | Patient group | |
|---|---|---|
| zol | Control | |
| Patients (n) | 151 | 157 |
| Age (years) | ||
| Median | 56.4 | 57.8 |
| Range | 29–65 | 33–65 |
| Sex [n (%)] | ||
| Male | 71 (47) | 85 (54) |
| Female | 80 (53) | 72 (46) |
| Risk [n (%)]a | ||
| High | 134 (89) | 138 (88) |
| Intermediate | 17 (11) | 19 (12) |
| Stage (Durie–Salmon) | ||
| iib | 11 (8) | 19 (12) |
| iiia | 2 (1) | 2 (1) |
| iiib | 138 (90) | 136 (86) |
| Myeloma type [n (%)] | ||
| Immunoglobulin G | 110 (73) | 108 (69) |
| Immunoglobulin A | 21 (14) | 30 (19) |
| Light chain | 10 (7) | 9 (6) |
| Nonsecretory | 10 (7) | 10 (6) |
| Anemia [n (%)] | 151 (100) | 157 (100) |
| Hypercalcemia [n (%)] | 38 (25) | 27 (17) |
| Renal failure [n (%)] | 17 (11) | 21 (13) |
| Bone lesions [n (%)] | 151 (100) | 157 (100) |
| β2-Microglobulinb [n (%)] | 151 (100) | 157 (100) |
| ecog performance status [n (%)] | ||
| 0–1 | 0 | 0 |
| 2 | 96 (64) | 99 (63) |
| >2 | 55 (36) | 58 (37) |
Based on the International Staging System19.
Exceeding twice the upper limit of normal.
zol = zoledronic acid; ecog = Eastern Cooperative Oncology Group.
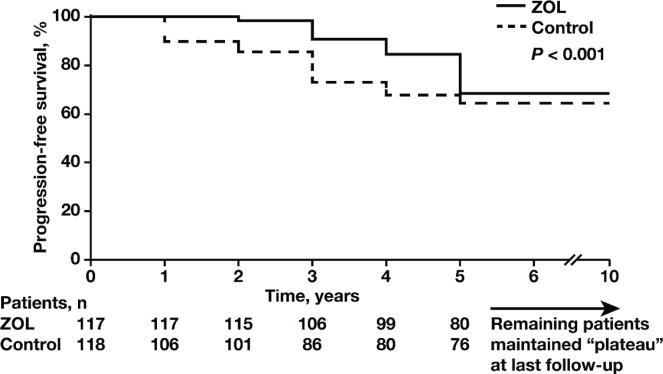
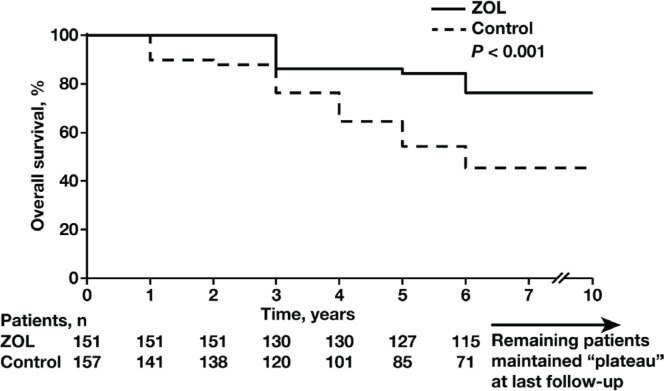
Median follow-up was 69.8 months (range: 36.5–96 months). All patients completed maintenance interferon treatment after asct. The median pfs was 60.4 months in the zol arm and 38.4 months in the control arm. The 10-year pfs was significantly higher (p < 0.001) in the zol arm [66%; 95% confidence interval (ci): 60% to 73%] than in the control arm (52%; 95% ci: 46% to 57%; Figure 2). Myeloma progression resulted in the deaths of 51 patients (33%; 95% ci: 28% to 38%) in the zol arm and 85 patients (52%; 95% ci: 47% to 56%) in the control arm. The 10-year os rate was significantly higher in the zol arm (67%; 95% ci: 60.1% to 72.0%) than in the control arm (48%; 95% ci: 43.9% to 55.4%; p < 0.001; Figure 3).
FIGURE 2.
Kaplan–Meier estimates of progression-free survival (pfs) at 10 years in the zoledronic acid (zol, n = 151) and control arms (n = 157). The 10-year pfs rate was significantly higher (p < 0.001) in the zol arm than in the control arm (66% vs. 52%; 95% confidence interval: 60% to 73% vs. 46% to 57%).
FIGURE 3.
Kaplan–Meier estimates of overall survival (os) at 10 years in the zoledronic acid (zol, n = 151) and control arms (n = 157). The 10-year os rate was significantly higher in the zol arm than in the control arm (67% vs. 48%; 95% confidence interval: 60.1% to 72.0% vs. 43.9% to 55.4%; p < 0.001).
Overall response, cr, and vgpr rates after high-dose induction therapy did not differ significantly between the treatment arms (Table ii). The overall response rate was 77% in the zol arm and 75% in the control arm, and the cr rate was 52% in the zol arm and 46% in the control arm. Very good partial responses were reported in 25% of patients in the zol arm compared with 29% in the control arm. A total of 235 patients—117 in the zol arm and 118 in the control arm—underwent asct. Stem-cell yields were greater than 1×106 CD34+ cells per kilogram in 97% of patients, with only 1 patient in the control arm requiring a second stem-cell mobilization. Very good partial responses achieved by 45 patients after high-dose induction therapy (22 in the zol arm, 23 in the control arm) were converted to crs after asct.
TABLE II.
Response to treatment in the intention-to-treat population
| Response | Responders [n (%)] | |
|---|---|---|
| zol | Control | |
| After high-dose therapy and before autologous stem cell transplantation (total) | 151 | 157 |
| Complete response | 79 (52) | 73 (46) |
| Very good partial response | 38 (25) | 45 (29) |
| Overall response | 117 (77) | 118 (75) |
| After autologous stem cell transplantation (total) | 117 | 118 |
| Very good partial response converted to complete response after autologous stem cell transplantation | 22 (15) | 23 (15) |
| Complete response (total)a | 101 (67) | 96 (61) |
Includes patients with a complete response before autologous stem cell transplantation and those with a very good partial response that converted to a complete response after autologous stem cell transplantation (p = 0.08).
zol = zoledronic acid.
Overall, treatment was well tolerated in both treatment arms, with most events being mild in severity. None of the patients discontinued treatment because of adverse events, including the patients who received interferon for 1 year. During high-dose induction therapy, only nonhematologic events were reported. Transient hyperglycemia developed in 37 patients (20 in the zol arm, 17 in the control arm) and was controlled with dietary changes and insulin in 16 patients. Mild dry skin experienced by 7 patients resolved with topical therapies. During asct, no marked differences in toxicity were observed between the groups (Table iii). Thrombocytopenia was the most frequently reported grade 3 or 4 event in both groups, occurring in 42% of patients in the zol arm and in 59% of patients in the control arm. Skeletal-related events were observed in 22 patients (14%) in the zol group and in 38 patients (24%) in the control group (p < 0.001). In all cases, local radiotherapy was sufficient for local control.
TABLE III.
Adverse events during autologous stem cell transplantation, by severity
| Event | Patient group [n (%)] | |||
|---|---|---|---|---|
| zol (n=117) | Control (n=118) | |||
| Grades 1–2 | Grades 3–4 | Grades 1–2 | Grades 3–4 | |
| Hematologic | 67 (57) | 50 (42) | 68 (57) | 67 (59) |
| Anemia | 27 (23) | 7 (6) | 23 (19) | 11 (9) |
| Neutropenia | 67 (57) | 16 (14) | 68 (58) | 18 (15) |
| Thrombocytopenia | 21 (18) | 50 (34) | 17 (14) | 59 (50) |
| Infection-related | 34 (29) | 21 (18) | 18 (15) | 24 (20) |
| Nonhematologic | 113 (96) | 10 (9) | 104 (88) | 6 (5) |
| Fatigue | 84 (72) | 7 (6) | 67 (57) | 4 (3) |
| Gastrointestinal | 29 (25) | 3 (3) | 37 (31) | 2 (2) |
zol = zoledronic acid.
Radiologic studies were performed in all patients every 6 months, including images of the jaw. Osteonecrosis of the jaw was not observed, and no specific measures were taken to avoid that adverse event. No patients experienced renal impairment, and no treatment-related deaths, secondary neoplasms, or acute leukemias were reported.
4. DISCUSSION
Bisphosphonates are an essential component of symptomatic multiple myeloma care for preventing sres and treating hypercalcemia5,7,21. A growing body of evidence is demonstrating that zol also improves non-skeletal outcomes in cancer patients with advanced bone disease10,13–15. Those benefits might be based primarily on two potential mechanisms:
a decrease in the rate of sres22–24, which can be life-limiting events25; and
indirect and direct anticancer effects26.
Indeed, in the mrc Myeloma ix trial (n = 1960), zol (compared with clodronate) significantly improved os in patients who initiated treatment for newly diagnosed multiple myeloma (50.0 months vs. 44.5 months respectively), with a 16% reduction in mortality (hazard ratio: 0.84; p = 0.0118)15. The os benefit with zol remained significant even after adjustment for the significant reduction in risk of sres, suggesting that the anti-myeloma activity of zol was independent of its effect on sres.
In the present randomized trial, at a median follow-up of more than 5 years, the addition of zol to our institute’s current standard of care (that is, high-dose dai followed by asct) in patients with previously untreated symptomatic multiple myeloma proved superior to the standard of care alone with respect to survival and disease progression. Notably, the objective response rate, vgpr, and cr were similar in both groups, suggesting that induction therapy with dai retained the clinical effectiveness shown in earlier studies. Patients who received zol demonstrated a significant improvement of 16% and 19% in 10-year pfs and os rates respectively, and those improvements may have been related to zol administration. Moreover, there were no statistically significant differences in baseline disease characteristics between the treatment arms that were likely to influence pfs or os, suggesting that the improved outcomes could be attributed to zol or the potential synergy between zol and dexamethasone27.
Consistent with those findings, our initial study in the same patient population demonstrated significantly improved disease outcomes in patients who received zol combined with chemotherapy (albeit using an older cyclophosphamide, vincristine, melphalan, and prednisone regimen that was our institute’s standard at the time) compared with chemotherapy alone10. Additionally, Sezer and colleagues28 reported a trend favouring zol with respect to sres and progression in patients with stage i multiple myeloma; however, that study was underpowered to demonstrate statistical significance. In contrast, zol had no significant anticancer benefits in patients with asymptomatic myeloma (n = 163)29. That discrepancy is likely a result of the low risk of progression in asymptomatic disease and reduced statistical power for disease-related endpoints. The current consensus is that, given the indolent nature of their condition, patients with asymptomatic (“smouldering”) myeloma should not be treated with anticancer agents or bisphosphonates30,31.
Recent data suggest that, in smouldering myelomas, cytogenetic changes could be an important prognostic factor and predictive factor for progression32. As a result, cytogenetics could potentially influence treatment decisions. However, our institution is a public facility with limited resources, and cytogenetic studies were not available.
In our study, treatment was well tolerated in both groups, and adverse events were consistent with the established safety profiles of the respective therapies33–35. While no reports of osteonecrosis of the jaw or renal toxicity emerged in our study, a low incidence of such events was observed in the mrc Myeloma ix trial15, suggesting that our younger patient population and the less cytotoxic dai regimen may have contributed to renal tolerability and absence of osteonecrosis of the jaw.
The reported incidence of osteonecrosis of the jaw among patients with advanced cancer receiving complex treatment regimens including bisphosphonates ranges from 0.6% to 2.4% across tumour types, with the highest incidence reported in multiple myeloma36,37. However, prevention strategies have emerged since the initial reports of osteonecrosis of the jaw in this setting38–40, and that knowledge could have improved oral health management in our patients, who did not receive preventive dental care. Notably, oral health recommendations were provided to investigators in the mrc Myeloma ix trial starting in June 2006, based on the recommendations of Weitzman and colleagues40, to reduce the risk of osteonecrosis of the jaw and to identify and manage suspected cases of that adverse event15.
Renal impairment is common in patients with multiple myeloma41, and intravenous bisphosphonates are associated with dose– and infusion-rate–dependent effects on renal function42. In the present study, adequate renal function was required in all patients at baseline to facilitate eligibility for subsequent asct, and renal safety was monitored according to the prescribing information for zol.
The present study has certain shortcomings. Although our trial was randomized and carried out using the standard treatment protocols at our institution, the dai regimen used as high-dose induction therapy might not be widely used in multiple myeloma patients, given the availability of newer therapies such as bortezomib, lenalidomide, and thalidomide. However, dai offers a favourable toxicity profile and has demonstrated efficacy comparable to that of other more aggressive and toxic regimens in this patient population, including adequate yields of CD34+ cells in patients underging asct33,34. In addition, as mentioned earlier, cytogenetics were not performed in our study. The present study was conducted at a single centre, an approach with inherent shortcomings. First, single-centre studies have potentially limited external validity because they are conducted in a discrete clinical setting that may not necessarily generalize to a broader population43. Additionally, a recent metaanalysis showed that single-centre trials may report larger treatment effects than multicentre trials44. Nevertheless, single-centre studies remain an essential part of clinical research as “hypothesis-generating investigations”43. Finally, our study did not systematically collect information pertaining to specific salvage therapies, a shortcoming that might have influenced the os (but not the pfs) results.
In our study, zol was the bisphosphonate of choice for evaluation because, compared with pamidronate, it has higher antiresorptive activity and a more convenient infusion schedule. Denosumab, an approved inhibitor of receptor activator of nuclear factor κB ligand, is an alternative to bisphosphonates in patients with bone metastases from solid tumours, but it is not indicated for patients with bone lesions from multiple myeloma45. Until recently, there was no consensus as to which bisphosphonate is superior at improving disease outcomes. However, emerging prospective clinical data now favour zol over other approved bisphosphonates for this purpose9,15,46. In the mrc Myeloma ix trial, zol was superior to clodronate in newly diagnosed multiple myeloma patients for several endpoints15,46. In a mixed-treatment analysis of randomized controlled clinical trials in patients with multiple myeloma, zol generally outperformed other bisphosphonates—including clodronate and pamidronate— with respect to os and pfs and also prevention of sres9. Those data, especially the outcomes from the Myeloma ix trial15, suggest that the improved pfs and os with zol therapy are not secondary to reductions in sres. Additional large head-to-head studies are warranted to further evaluate whether zol is the optimal bisphosphonate for improving disease outcomes in multiple myeloma patients.
5. CONCLUSIONS
In this randomized controlled study of patients with previously untreated symptomatic multiple myeloma, the addition of zol to our institute’s current standard of care significantly improved disease progression and survival outcomes. Those data add to the growing body of evidence supporting the anticancer benefits of zol in patients starting a broad range of anticancer treatment regimens for symptomatic multiple myeloma9,10,12,15.
6. ACKNOWLEDGMENTS
This study was supported entirely with resources from the Mexican Institute of Social Security. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation, but the study was not sponsored by Novartis or by any other pharmaceutical company. We thank Marithea Goberville phd, ProEd Communications, Inc., for medical editorial assistance with this manuscript.
7. CONFLICT OF INTEREST DISCLOSURES
Aside from support from Novartis Pharmaceuticals Corporation for medical editorial assistance, all authors declare that they have no financial conflicts of interest.
8. REFERENCES
- 1.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9:278–88. doi: 10.3816/CLM.2009.n.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica. 2009;94:270–5. doi: 10.3324/haematol.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harousseau JL, Attal M, Avet–Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–46. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (nccn) Multiple Myeloma NCCN Clinical Practice Guidelines in Oncology Ver 1.2012. Fort Washington, PA: NCCN; 2012. [Google Scholar]
- 6.Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 7.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303–17. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 8.Bird JM, Owen RG, D’Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 9.Mhaskar R, Redzepovic J, Wheatley K, et al. Comparative effectiveness of bisphosphonates in multiple myeloma [abstract 3028]. Presented at the 52nd ASH Annual Meeting and Exposition; Orlando, FL. December 4–7, 2010; [Available online at: http://ash.confex.com/ash/2010/webprogram/Paper27802.html; cited September 15, 2011] [Google Scholar]
- 10.Avilés A, Nambo MJ, Neri N, Castañeda C, Cleto S, Huerta–Guzmán J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol. 2007;24:227–30. doi: 10.1007/BF02698044. [DOI] [PubMed] [Google Scholar]
- 11.Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the zo-fast Study. Ann Oncol. 2010;21:2188–94. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 12.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [Erratum in: N Engl J Med 2009;360:2379] [DOI] [PubMed] [Google Scholar]
- 13.Zaghloul MS, Boutrus R, El-Hossieny H, Kader YA, El-Attar I, Nazmy M. A prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer. Int J Clin Oncol. 2010;15:382–9. doi: 10.1007/s10147-010-0074-5. [DOI] [PubMed] [Google Scholar]
- 14.Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705–9. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]
- 15.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (mrc Myeloma ix): a randomised controlled trial. Lancet. 2010;376:1989–99. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modi ND, Lentzsch S. Bisphosphonates as antimyeloma drugs. Leukemia. 2012;26:589–94. doi: 10.1038/leu.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JR, Guenther A. The backbone of progress—preclinical studies and innovations with zoledronic acid. Crit Rev Oncol Hematol. 2011;77(suppl 1):S3–12. doi: 10.1016/S1040-8428(11)70003-8. [DOI] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [Errata in: Leukemia 2006;20:2220 and 2007;21:1134] [DOI] [PubMed] [Google Scholar]
- 19.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durie BG. Role of new treatment approaches in defining treatment goals in multiple myeloma—the ultimate goal is extended survival. Cancer Treat Rev. 2010;36(suppl 2):S18–23. doi: 10.1016/S0305-7372(10)70008-6. [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Yee GC, Somerfield MR, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–72. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 22.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–21. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 23.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–44. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Gleason DM, Murray R, et al. on behalf of the Zoledronic Acid Prostate Cancer Study Group Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–82. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 25.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–7. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 26.Guenther A, Gordon S, Tiemann M, et al. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer. 2010;126:239–46. doi: 10.1002/ijc.24758. [DOI] [PubMed] [Google Scholar]
- 27.Tassone P, Forciniti S, Galea E, et al. Growth inhibition and synergistic induction of apoptosis by zoledronate and dexamethasone in human myeloma cell lines. Leukemia. 2000;14:841–4. doi: 10.1038/sj.leu.2401770. [DOI] [PubMed] [Google Scholar]
- 28.Sezer O, Jakob C, Aldaoud A, et al. Zoledronic acid therapy versus control in patients with multiple myeloma in stage i (Durie and Salmon): results of a phase iii study of the dsmm and osho [abstract 0361] Haematologica. 2010;95(suppl 2):145. [Google Scholar]
- 29.Musto P, Petrucci MT, Bringhen S, et al. on behalf of gimema (Italian Group for Adult Hematologic Diseases)/Multiple Myeloma Working Party and the Italian Myeloma Network A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–95. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca R, Bergsagel PL, Drach J, et al. on behalf of the International Myeloma Working Group International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–21. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders J, Crawford B, Gibson J, Joy Ho P, Iland H, Joshua D. Is there a case for the early use of bisphosphonates in smouldering myeloma and mgus? (Bisphosphonates in smm and mgus) Int J Lab Hematol. 2007;29:395–7. doi: 10.1111/j.1365-2257.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 32.Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (mgus) and smoldering multiple myeloma (smm): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573–81. doi: 10.1182/blood-2011-01-270140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avilés A, Nambo MJ, Neri N, et al. Biological modifiers as cytoreductive therapy before stem cell transplant in previously untreated patients with multiple myeloma. Ann Oncol. 2005;16:219–21. doi: 10.1093/annonc/mdi048. [DOI] [PubMed] [Google Scholar]
- 34.Avilés A, Neri N, Nambo MJ, et al. Novel therapy in multiple myeloma. Invest New Drugs. 2005;23:411–15. doi: 10.1007/s10637-005-2900-6. [DOI] [PubMed] [Google Scholar]
- 35.Berenson JR. Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist. 2005;10:52–62. doi: 10.1634/theoncologist.10-1-52. [DOI] [PubMed] [Google Scholar]
- 36.Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13:911–20. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 37.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/jbmr.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (onj) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117–20. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 39.Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20:137–45. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 40.Weitzman R, Sauter N, Eriksen EF, et al. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients—May 2006. Crit Rev Oncol Hematol. 2007;62:148–52. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Smith MR. Zoledronic acid to prevent skeletal complications in cancer: corroborating the evidence. Cancer Treat Rev. 2005;31(suppl 3):19–25. doi: 10.1016/j.ctrv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Lipton A. The safety of zoledronic acid. Expert Opin Drug Saf. 2007;6:305–13. doi: 10.1517/14740338.6.3.305. [DOI] [PubMed] [Google Scholar]
- 43.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;37:3114–19. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 44.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39–51. doi: 10.7326/0003-4819-155-1-201107050-00006. [DOI] [PubMed] [Google Scholar]
- 45.Xgeva (denosumab) injection [package insert] Thousand Oaks, CA: Amgen; 2010. [Available online at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125320s007lbl.pdf; cited September 19, 2011]. [Google Scholar]
- 46.Morgan GJ, Child JA, Gregory WM, et al. on behalf of National Cancer Research Institute Haematological Oncology Clinical Studies Group Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (mrc Myeloma ix): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743–52. doi: 10.1016/S1470-2045(11)70157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]