Abstract
Avascular necrosis (avn) of the hip is a well-documented side effect of corticosteroid therapy, but it has also been described as a complication of radiation and chemotherapy. Many prostate cancer patients undergo treatment with all three of those therapeutic modalities, and yet reported cases of avn of the hip in prostate cancer patients are rare. Symptoms that might potentially alert physicians to this complication are nonspecific and may be attributed to cancer progression, in particular to progressive bone metastasis.
Here, we report on a 79-year-old man diagnosed with castration-resistant prostate cancer whose diagnosis of avn of the hip was confounded by his underlying malignancy. We discuss risk factors and diagnostic clues in this differential diagnosis of acute hip pain in patients with castration-resistant prostate cancer. Physicians might maintain a high index of suspicion for avn of the hip in prostate cancer patients presenting with new-onset hip pain. Surgical intervention may help to prevent the appearance of avn-associated pain and the negative impact of advanced avn on overall quality of life.
Keywords: Castration-resistant prostate cancer, avascular necrosis, corticosteroid, radiotherapy, chemotherapy, bisphosphonate
1. CASE DESCRIPTION
A 75-year-old man with a history of bilateral hip osteoarthritis, but otherwise healthy, was diagnosed with a T2b, Gleason score 4+3/10, prostate-specific antigen (psa) 6.7 μg/L adenocarcinoma of the prostate. He underwent external-beam radiation therapy (7600 cGy in 38 fractions) to achieve a psa nadir of 1.67 μg/L at 9 months. However, 2 years later, he was started on androgen deprivation therapy using leuprolide for asymptomatic psa progression and abdominopelvic lymphadenopathy. No concurrent definite evidence of bone metastases was found. Unfortunately, the response to leuprolide monotherapy (a psa decline to 1.16 μg/L from 43 μg/L within 8 months) was short-lived (<1 year), and both oral bicalutamide (50 mg daily) and a treatment attempt with oral ketoconazole (400 mg twice daily) combined with oral hydrocortisone (30 mg daily) had only a limited impact on further psa and disease progression.
Four years after his initial diagnosis, the patient presented with fatigue, an accelerated psa doubling time of 2.4 months, and widespread oligosymptomatic bone metastases. Plain radiography of the pelvis and hips revealed signs of coxarthritis, but no definite evidence of bone metastases in the area of the hip joints [Figure 1(a)]. The patient was started on an every-3-weeks docetaxel chemotherapy regimen (involving premedication with three 8-mg doses of oral dexamethasone, and twice-daily 5 mg oral prednisone continuously) accompanied by zoledronic acid administration. This treatment produced a prompt palliative benefit and a gradual psa decline from 366 μg/L to 135 μg/L. After 10 cycles of docetaxel, the patient opted for a treatment break and prednisone taper in the face of increasing fatigue after chemotherapy administration, and because of concerns about early cushingoid facies. Notably, imaging completed a couple of months earlier had not shown any signs of disease progression.
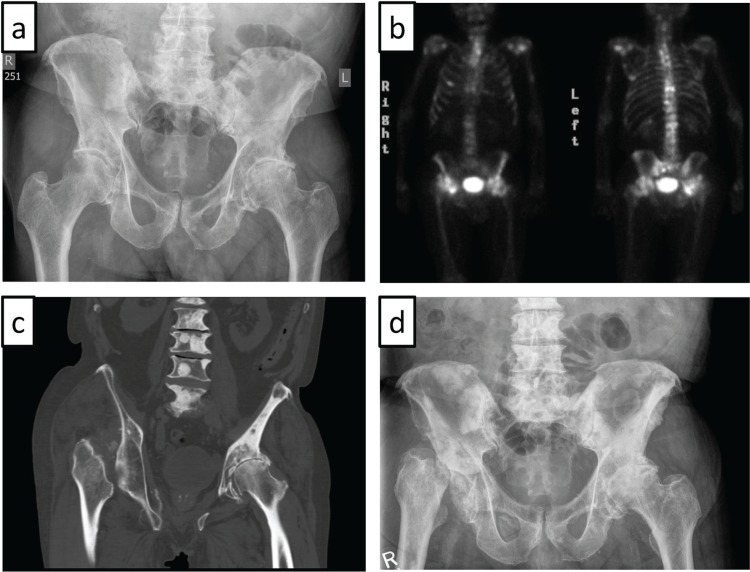
FIGURE 1.
(a) Plain radiography of the pelvis shows osteoarthritic changes of both hips, with joint-space narrowing particularly on the left side. (b) Technetium bone scan reveals multiple foci of increased activity compatible with bone metastases. Notably, the configuration of the right hip is unusual. (c) Near-complete resorption of the right femoral head, with superior displacement of the right femur as seen on computed tomography imaging. (d) Plain radiography of the pelvis reveals fragmentation of the right femoral head, with subluxation of the right femur, multifocal sclerotic metastases throughout the pelvis and lumbar spine, and severe superior migration osteoarthritis of the left hip.
Two months after the last dose of docetaxel, the patient returned with lower back and right hip pain radiating toward the knee, rapid psa increase (doubling time of 1.2 months), more intense bone scintigraphic foci in the spine, and worsening retroperitoneal lymphadenopathy. Otherwise, no significant changes in the appearance of diffuse, sclerotic osseous metastases of the pelvis and proximal femur bones were observed. Prednisone therapy was reinstated, and the patient underwent palliative radiation therapy (800 cGy in 1 fraction) to L5 and the right sacroiliac joint, pelvis, and proximal femur.
Unfortunately, the patient had to be admitted 2 weeks later with treatment-refractory right hip pain, right leg swelling, and difficulty ambulating. After spinal cord compression and right-sided deep venous thrombosis were excluded, the patient was successfully mobilized under morphine medication and discharged with a walker. He resumed most of his prior physical activities—albeit using the walker—without the need for dose-escalation of his pain medication (100 mg morphine sulfate daily), and he started further chemotherapy. Unexpectedly, bone scintigraphic restaging after an additional 6 cycles of docetaxel chemotherapy revealed a change in the configuration of the right hip [Figure 1(b)], which was also seen on computed tomography imaging and plain radiography, both of which showed near-complete resorption of the right femoral head and superior displacement of the right femur [Figure 1(c,d)]. Given the unequivocal clinical presentation of advanced avn of the femoral head, we abstained from obtaining magnetic resonance imaging. Considering his acceptable quality of life, the absence of relevant pain under morphine therapy, the limited life expectancy dictated by his prostate cancer, and the prospect of prolonged rehabilitation, our patient opted against hip replacement surgery.
2. DISCUSSION
Avascular necrosis of the hip is a fairly common complication of a wide range of medical conditions that may cause damage to the vascular supply of the femoral head. Posttraumatic osteonecrosis is the most common cause, and most nontraumatic cases (annual incidence of approximately 1 in 10,000) are associated with systemic steroid use and habitual alcohol consumption1. Less well-documented risk factors include radiation therapy and chemotherapy, among others. Plain radiography and bone scintigraphy successfully reveal the characteristic morphology changes of advanced avn, as evidenced in our patient. However, the signs of early avn seen on plain radiographs, such as sclerosis surrounding osteopenic areas and the “crescent” sign (that is, a lucent subchondral line), are often subtle; flattening of the femoral head is a sign of more advanced avn. The improved ability to discriminate fat from other tissues in the bone marrow makes magnetic resonance imaging the modality of choice for early avn detection2,3. In fact, subchondral “band-like” lesions showing low intensity on T1-weighted imaging are considered pathognomonic. Otherwise, advanced stages of avn can often be diagnosed with plain radiographs, as seen in our patient.
Advanced prostate cancer patients are often exposed to numerous risk factors for avn during treatment for their disease. However, although the risk constellation seen in our patient (that is, steroid use, radiation, and chemotherapy) applies to a large number of prostate cancer patients, reports of avn in prostate cancer patients—other than osteonecrosis of the jaw associated with bone-targeted agents4—are exceedingly rare5. In fact, to our knowledge, this report is only the third of nontraumatic femoral avn in patients with advanced prostate cancer.
The special circumstances in our patient may have contributed to the delayed and incidental avn diagnosis. First, the rapidly worsening pain after palliative radiation therapy was interpreted as radiation therapy–associated pain flare, which is experienced by approximately 40% of patients undergoing such treatment for bone metastases6. On the other hand, it is not uncommon for avn to be asymptomatic until sudden collapse of the cortical bone. The question of whether the pain increase seen in our patient was radiation-associated or a consequence of the beginning of femoral head collapse therefore remains open. Second, our patient remained on continuous opiate medication from the time of admission, which could have masked avn-related pain.
Steroid use is the most commonly accepted cause of nontraumatic avn. However, the pathophysiology of avn in general—and of steroid-induced avn in particular—remains poorly understood7. High daily steroid doses and, to a lesser degree, cumulative steroid dose correlate with the risk of developing avn. Furthermore, the nature of the underlying condition for which steroids are prescribed may modify the risk. The cumulative steroid dose in our patient was considered to put him at risk for avn.
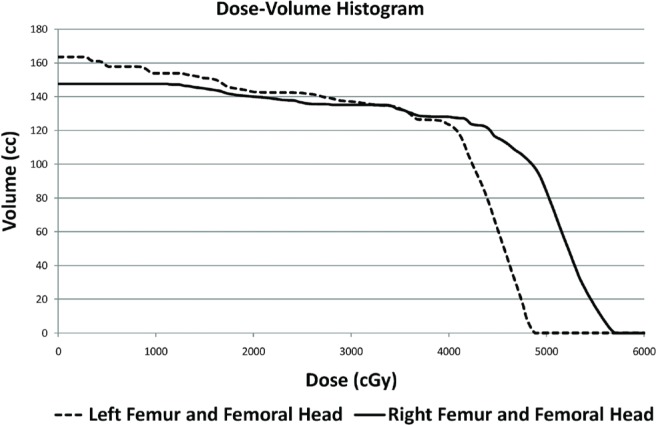
Avascular necrosis after irradiation is a well-documented phenomenon, and rare cases of radiation-associated hip avn have been reported8. However, the incidence of avn of the femoral head after radiation treatment is unknown. Mandibular osteoradionecrosis is seen in 3%–8% of head-and-neck cancer patients undergoing high-dose radiation therapy. The risk of avn is increased with radiation dosages greater than 6000 cGy. Furthermore, the rate of avn appears to vary with the type of radiation applied. Conventional external-beam radiation poses the highest risk (7.4%), followed by brachytherapy (5.3%), and intensity-modulated radiation therapy (5.2%)9. The dose–volume histogram for our patient indicates that the maximum cumulative point dose to the right femoral head was 5710 cGy, of which 4860 cGy was delivered in 39 fractions, and 850 cGy, in 1 fraction (Figure 2).
FIGURE 2.
A dose–volume histogram of both femoral heads and femur bones reveals a maximum cumulative point dose of 5710 cGy to the right femoral head (below the threshold of 6000 cGy usually associated with an increased risk of osteoradionecrosis).
The possible association between chemotherapy administration and avn may be confounded by concurrent steroid use, as is also typically the case in prostate cancer patients undergoing docetaxel chemotherapy. Interestingly, even though zoledronic acid administration is associated with a 1% risk of osteonecrosis of the jaw4, bisphosphonate-associated avn of other bones has presumably been described only sporadically10. Thus, it remains unclear whether the use of zoledronic acid may have contributed to the femoral avn seen in our patient. Interestingly, bisphosphonates are being actively studied for the treatment of early-stage avn11.
3. CONCLUSIONS
Considering the most common risk factors, most patients with advanced prostate cancer are expected to be at risk for avn. It is not clear at this moment whether the rather sporadic reporting of avn (other than osteonecrosis of the jaw) truly reflects a very low avn incidence, or whether its low occurrence in the literature can be attributed to publication bias or underdiagnosis of early-stage avn. Nonetheless, it is important to be wary that worsening hip pain in prostate cancer patients may not only be a symptom of progressive bone metastasis, radiation-associated pain flare, or osteoarthritis, but also of avn.
4. ACKNOWLEDGMENTS
The authors acknowledge the Ivan H. Smith Memorial Studentship to E. Chan and the Clinician–Scientist Award from Prostate Cancer Canada to U. Emmenegger for making this publication possible.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest relevant to the present work to declare.
6. REFERENCES
- 1.Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 2010;468:2715–24. doi: 10.1007/s11999-010-1292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Powell C, Chang C, Gershwin ME. Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin Rev Allergy Immunol. 2011;41:102–13. doi: 10.1007/s12016-010-8217-z. [DOI] [PubMed] [Google Scholar]
- 4.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald AG, Bissett JD. Avascular necrosis of the femoral head in patients with prostate cancer treated with cyproterone acetate and radiotherapy. Clin Oncol (R Coll Radiol) 2001;13:135–7. doi: 10.1053/clon.2001.9237. [DOI] [PubMed] [Google Scholar]
- 6.Hird A, Chow E, Zhang L, et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three Canadian cancer centers. Int J Radiat Oncol Biol Phys. 2009;75:193–7. doi: 10.1016/j.ijrobp.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Powell C, Chang C, Naguwa SM, Cheema G, Gershwin ME. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9:721–43. doi: 10.1016/j.autrev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deleeuw HW, Pottenger LA. Osteonecrosis of the acetabulum following radiation therapy. A report of two cases. J Bone Joint Surg Am. 1988;70:293–9. [PubMed] [Google Scholar]
- 9.Peterson DE, Doerr W, Hovan A, et al. Osteoradionecrosis in cancer patients: the evidence base for treatment-dependent frequency, current management strategies, and future studies. Support Care Cancer. 2010;18:1089–98. doi: 10.1007/s00520-010-0898-6. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Jain P, Kumar P, Parikh PM. Zoledronic acid induced osteonecrosis of tibia and femur. Indian J Cancer. 2009;46:249–50. doi: 10.4103/0019-509X.52967. [DOI] [PubMed] [Google Scholar]
- 11.Cardozo JB, Andrade DM, Santiago MB. The use of bisphosphonate in the treatment of avascular necrosis: a systematic review. Clin Rheumatol. 2008;27:685–8. doi: 10.1007/s10067-008-0861-9. [DOI] [PubMed] [Google Scholar]