Abstract
Interferon beta and glatiramer acetate have been mainstays of treatment in relapsingremitting multiple sclerosis for two decades. Remarkable advances in our understanding of immune function and dysfunction as well as increasingly sophisticated clinical trial design have stemmed from efforts to better understand these drugs. In this chapter, we review the history of their development and elaborate on known and theorized mechanisms of action. We describe the pivotal clinical trials that have led to their widespread use. We evaluate the clinical use of the drugs including tolerability, side effects, and efficacy measures. Finally, we look to the future of interferon beta and glatiramer acetate in the context of an ever growing armamentarium of treatments for relapsing remitting multiple sclerosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0163-4) contains supplementary material, which is available to authorized users.
Keywords: Interferon beta, Glatiramer acetate, Multiple sclerosis therapeutics, Review, History
Interferon-Beta
Introduction
The development of disease modifying agents (DMA) for multiple sclerosis (MS) stands among the great achievements in medicine. Remarkable advances in our understanding of immune function and dysfunction as well as increasingly sophisticated clinical trial design have stemmed from efforts to better understand these drugs. In this chapter, we describe the development of the first DMAs for MS, the interferon betas (IFNB) and glatiramer acetate (GA). We examine historical aspects of development, their mechanisms of action, the important clinical trials leading to their approval, and address questions about the future use these agents.
History
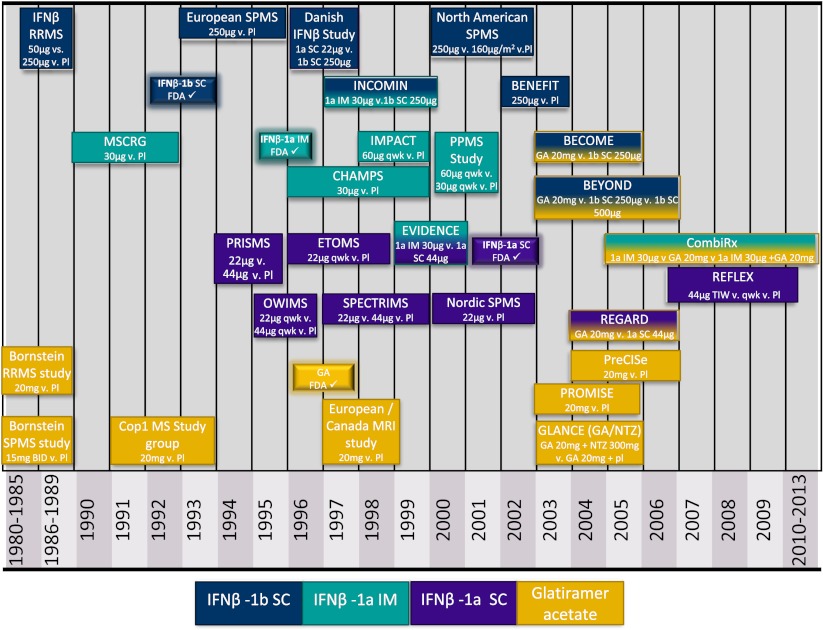
The FDA approval of interferon beta-1b in 1993 marked a new era in MS care (Fig. 1), but the work leading to this momentous accomplishment stretched back as far as four decades. Isaac and Lindenmann first described interferons (IFN) in 1957 [1]. After much work over the next decades implicating viral infections and immune dysregulation as underlying mechanisms of disease in MS, it was theorized that IFNs might be of benefit to patients with MS. In a rare leap to direct human experimentation, a number of studies with IFNs were undertaken [2]. IFN gamma, a type II IFN, had been observed to be deficient in leukocytes in patients with MS, though it was also known to stimulate the immune system at multiple levels. Panitch et al. [3] performed a historical open-label, pilot study in which 18 RRMS patients received IFN gamma at various doses. Seven participants throughout the dose range experienced relapses, representing an increase in relapse rate from their pre study rate.
Fig. 1.
Timeline of IFN and GA trials. All dosing frequencies are as per current FDA approvals except where indicated. Representations of lengths of studies are approximate. Dates were derived from trial publications and listings on ClinicalTrials.gov. Pl = placebo, SC = subcutaneous, IM = Intramuscular, qwk = once weekly, BID = twice daily, FDA ✓ = US food and drug administration initial approval
Based on these disappointing results, work shifted to the type I IFNs, IFN alpha and IFN beta, known inhibitors of IFN gamma [4]. Early work with IFN alpha suggested a reduction in rate of exacerbations [5], but in larger, better designed trials, IFN alpha showed no benefit over placebo [6, 7]. Furthermore, it was poorly tolerated with increased flu-like symptoms and transient worsening in neurological symptoms on administration [6, 7]. Work with IFN beta had begun as early as 1979 when Ververken et al. had treated three progressive MS subjects with intramuscular IFNB for just two weeks with no effect on progression [7]. Other early work included administration of IFNB intrathecally, which decreased relapses, but did not change disease progression [8]. Based on these results, larger randomized, placebo controlled trials were initiated. With the completion of The IFNB Multiple Sclerosis Study Group trial, IFNB-1b emerged as the first proven, effective IFN in the treatment MS. This and other trials demonstrating efficacy of IFN formulations will be described in the following sections. First, we look more closely at IFNBs complex mechanisms of action and consider the implications in clinical practice.
Mechanisms of Action
In contrast to some other disease modifying agents to be discussed in following chapters, which have known specific targeted biological effects, IFNB has a myriad of effects throughout the immune system with perhaps hundreds of genes affected and multiple immune cell types regulated.
IFNB belongs to the type I interferon family along with subtypes of IFN alpha, epsilon, kappa, and omega. This cytokine family is widely expressed and has immunomodulatory and anti-infectious properties [9]. IFNB is produced in multiple cell types, though classically thought of as a fibroblast product. Induction of IFNB can occur by various paths, but ultimately by activation of common signaling molecules.
TNF receptor-associated factors (e.g. TRAF3) and IFN regulatory factors (IRF3, 7) join with nuclear factor-kappa-beta to stimulate the IFNB (and alpha) promoters within the nucleus [10]. Once produced, IFNB acts through the IFN alpha/beta receptor (IFNAR) found on many cell types. The heterodimeric subunits, IFNAR1 and 2, are associated with Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2) which, once activated, phosphorylase the signal transducer and activators of transcription (STAT) family. The JAK/STAT activation cascade leads to activation of nuclear IFN-stimulated response elements (ISREs) to promote numerous gene activation and suppression [11]. Additionally, the Type I IFNs are known to act through JAK/STAT-independent pathways as well [9]. Much work continues to be done on the mechanism of action of IFNB and there is emerging evidence of differing effects of different IFNB formulations [12], though here we present common mechanisms.
The actions of IFNB antagonize many of the pathological processes thought to underlie the pathogenesis of MS. IFNB increases the production of anti-inflammatory cytokines including IL-4 and IL-10, which promote a Th2 response [13]. In part, this may be accomplished by affecting certain cell populations including increasing production of the IL-10-producing CD56bright Natural Killer cells. IFNB decreases the production of proinflammatory cytokines including IL-12, IL-17, IL-23, and osteopontin [12, 13]. In MS patients, IFNB may decrease IFN gamma and TNF alpha [14]. IFNB may decrease the migration of pro-inflammatory leukocytes into the CNS by affecting both cell adhesion molecules of the endothelial surface and on the activated T-cell surface including VLA-4, LFA-1, and MMP-9 among others. There may be a differential effect favoring Th2 migration while blocking Th1 migration [15]. IFNB induces apoptosis of activated T-cells through downregulation of anti-apoptotic proteins and upregulation of surface Fas and CTLA4 expression [16]. IFNB modulates Treg function and increases expansion of naïve Treg populations [16].
While the many actions of IFNB obscures a complete understanding of its biological effects in MS, this same complexity may be its greatest strength. The web of autoimmunity in individual patients may be driven by diverse elements. Though the current trend in MS therapeutics is toward monoclonal antibodies and other drugs with highly targeted mechanisms, there may be value in a more multifaceted and durable treatment effect. Despite its broad mechanisms of actions, there remain responders and non-responders to IFNB [17]. Work continues to identify biological markers that can be measured prior to initiation of treatment to guide clinicians in choosing a disease modifying therapy.
Clinical Trials
Relapsing Remitting MS
Over the last 20 + years of IFNB research in MS, there have been many noteworthy scientific studies, though a comprehensive review of each is outside the scope of this chapter. Here, we review the critical trials that have shaped our understanding of IFNB use. When reviewing such trials it is important to remember that study populations vary, definitions of outcome measures differ, and different statistical methods are employed. These variations between trials make comparisons difficult.
The IFNB Multiple Sclerosis Study Group trial, whose results were published as a pooled analysis in 1993, began enrolling in June 1988 [18]. This multicenter, double-blind, placebo-controlled trial examined the efficacy and safety of subcutaneous (SC) IFN beta-1b every other day (QOD) at two doses (1.6 million international units – later measured as 50 μg and 8 MIUs – later 250 μg) versus placebo. Though one of the earliest trials of its kind, outcome measures are familiar today reflecting its major impact on research in MS over the next 25 years. Primary measures included annual exacerbation rate (now more commonly called the annualized relapse rate (ARR)) and proportion of exacerbation free patients with secondary measures including time to first exacerbation, duration and severity, change in the Expanded Disability Status Scale (EDSS), and MRI outcomes of disease burden and activity. The pivotal two-year data demonstrated ARR of 1.27, 1.17, and 0.84 in the placebo, 50 μg, and 250 μg arms, respectively (p = 0.0001 placebo vs 250 μg), a 34 % reduction. To measure the accrual of disability due to MS disease, the authors used EDSS scores at two time points separated by 90 days to demonstrate a “confirmed end point”, now commonly referred to as confirmed/sustained disability progression. This trial did not demonstrate a significant difference in this outcome. After two years, participants were given the option of continuing within the trial in a double-blind fashion. 5 year data was published in 1995 [19]. The difference in ARR between the groups in the 3-5 year data did not reach statistical significance and differences in disability progression could not be demonstrated. IFN beta-1b SC 250 μg QOD was approved for use in RRMS in 1993 as the first disease modifying therapy for MS heralding an important advancement in MS care.
Beginning in November 1990, the randomized, double-blind, placebo-controlled trial began testing the next IFNB formulation, IFN beta-1a intramuscular (IM) 30 μg [20]. The MS Collaborative Research Group (MSCRG) study used ARR and a longer time frame for EDSS sustained disability of six months. Using 2 year data of 172 participants, the trial demonstrated a reduction in ARR from 0.90 in the placebo arm to 0.61 on the treatment arm, a 32 % reduction. However, in early 1993, the trial was terminated prematurely concurrent to the FDA approval of IFN beta-1b with the stated reason for termination being an unusually low participant dropout rate allowing for analysis of the primary endpoint, time to sustained disability, earlier than expected. Due to this, 129 of the 301 participants were followed for less than two years and analysis of the ARR for all patients including those followed for the shorter period revealed an ARR reduction of 18 %. Importantly, this study was able to demonstrate a significant difference between groups in the proportion of patients reaching a six month sustained disability of EDSS increase by at least one point (34.9 % placebo vs 21.9 % IFN beta-1a, p = 0.02). In part, this led to the FDA approval of IFNB-1a IM in 1996.
In May 1994, the Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in MS Study Group (PRISMS) [21], began enrolling in this randomized, placebo controlled study of two doses of IFN beta -1a SC three times weekly (TIW) at doses of 22 μg and 44 μg. Two year data was available for 95 % of participants and showed a reduction in the number of relapses by 27 % for the 22 μg arm and 33 % for the 44 μg arm. Other efficacy measures also favored the treatment groups. The PRISMS group published a separate analysis of MRI data in 1999. The results of the 2 years data demonstrated significant effects of study drug on median increase in burden of disease, number of T2 active lesions, and percentage of scans showing T2 activity [22].
In March 1995, the Once Weekly Interferon for MS Study Group (OWIMS) began enrolling for a trial of IFN beta-1a SC 22 μg and 44 μg given just once weekly [23]. As an exploratory trial, the primary endpoint was a MRI measure, combined unique active lesions (CUA) at 24 weeks. CUA was defined as new T2/proton density lesions or T1-gadolinium enhanced lesions. CUA continues to be an important MRI measure today. The one-year data for this trial demonstrated a 53 % reduction in CUA for the 44 μg vs placebo groups. A non-significant reduction in relapse rate of 19 % was observed for the 44 μg vs placebo groups. IFN beta-1a SC TIW was FDA approved in 2002.
Clinically Isolated Syndrome
With trials showing the efficacy of IFNB in RRMS, interest shifted to evaluating its use earlier in the disease course. Clinically isolated syndrome (CIS) refers to the development of a neurological attack most likely due to demyelinating disease and is suggestive of the future development of MS. To assess the efficacy of IFNB in CIS, a number of studies were undertaken.
In April 1996, the Controlled High-Risk Subjects Avonex MS Prevention Study (CHAMPS) group began enrolling across North America [24]. Participants experienced a unifocal attack of demyelinating disease no more than 27 days before randomization and had characteristic MRI brain findings. Participants were randomized to IFN beta-1a IM 30 μg weekly or placebo. The primary endpoint was the development of clinically definite MS (CDMS) defined as a new neurological event or progressive neurological worsening. The cumulative probability of conversion to CDMS over 3 years was significantly lower in the IFNB arm compared with placebo in an unadjusted analysis, 35 % vs 50 % (rate ratio 0.56, p = 0.002). MRI findings favored the treatment arm in T2 lesion volume, number of T2 lesions, number of gadolinium-enhancing lesions. Because participants were no longer followed after conversion to CDMS, long-term data on early initiation of treatment could not be determined.
In August 1995, the Early Treatment of MS Study Group (ETOMS) began enrolling across 14 European countries [25]. Participants had experienced a first clinical attack of demyelinating disease within the prior 3 months, which was unifocal or multifocal, and had characteristic MRI findings. Participants were randomized to IFN beta-1a 22 μg SC weekly vs placebo for 2 years. The primary outcome measure was conversion to CDMS, defined by an occurrence of a second attack, which occurred in 34 % of the IFN beta-1a group and 45 % of the placebo group (odds ratio after logistic regression for baseline characteristics was 0.61, p = 0.045). Participants had high levels of MRI disease activity pointing to a study population at high risk additional disease activity, but there was a significant decrease in number of new T2 lesions in the active treatment arm. There was a non-significant reduction in ARR of 23 % in the IFNB arm.
In February 2002, the Betaferon in Newly Emerging MS for Initial Treatment (BENEFIT) trial began enrolling participants with first neurological events suggestive of MS (CIS) and characteristic MRI lesions within 60 days of onset [26]. Participants were randomized to IFN beta-1b 250 μg SC QOD or placebo in a 5:3 ratio. The primary endpoint was conversion to CDMS, defined as a second clinical attack or a 3 month sustained progression on EDSS of at least 1.5 points reaching a total EDSS of at least 2.5. After 2 years, 28 % of the treatment group and 45 % of the placebo group converted to CDMS with a hazard ratio of 0.50 (p < 0.0001) after a proportional hazards regression. The absolute risk reduction was 17 %. The number needed to treat (NNT) in order to prevent one case of CDMS was 5.9. Secondary endpoints of MRI efficacy variables were also significantly reduced.
In a more recent trial of IFNB efficacy in CIS, the Rebif Flexible dosing in early MS (REFLEX) study evaluated the use of IFN beta-1a SC 44 μg given three times weekly vs once weekly vs placebo in participants with a first neurological event within 60 days of onset and with characteristic MRI lesions [27]. The trial was conducted with the newer, serum-free formulation of the drug available in Europe. The primary endpoint was time to diagnosis of MS as diagnosed by McDonald 2005 criteria [28]. After two years, the three times weekly group compared with placebo had a 51 % relative reduction in conversion (p < 0.0001) and the once weekly group compared with placebo had a 31 % relative reduction (p = 0.008). Secondary MRI outcomes including CUA also showed significant reductions, greater in the three times weekly arm.
Taken together, the results of the CIS trials demonstrate a benefit for use of IFNB earlier in the MS disease course. However, in the 2 and 3 year data, the absolute clinical benefits are modest [29]. Inclusion criteria selected for patients with active clinical and MRI disease. Subgroup analyses demonstrated more pronounced effects in those with more disease activity [30]. Regression to the mean, an issue that continues to plague more recent MS trials, posits that variables that are extreme on the first measurement will tend to be close to the average on the second measurement. In one small study of 24 clinically active MS patients, as much as 40 % of the reduction in relapse rate may have been due to regression to the mean [31]. In the follow up study of 3 year data from the BENEFIT trial and 5 year data from the CHAMPS trials (CHAMPIONS) [32], early treatment reduced the risk for conversion to CDMS and confirmed EDSS worsening. While suggestive, it is difficult to draw firm conclusions about the long-term benefits of early treatment from these studies.
Progressive Forms
With IFNB’s beneficial effects on relapses and MRI activity becoming increasingly established, interest in its use for progressive forms of MS grew.
The European Study Group on IFNB-1b in Secondary Progressive MS (SPMS) undertook a trial published in 1998 [33]. This randomized, placebo-controlled, double masked trial tested IFNB-1b 250 μg SC QOD in 358 participants who had had 6 months of neurological deterioration independent of relapses and who had a history of RRMS. Baseline EDSS was 3 to 6.5 and the study required a history of 2 relapses or an EDSS increase of 1 point in the last 2 years. The primary outcome was time to 3 month confirmed disability defined as a sustained increased in EDSS of 1 point (or 0.5 points if baseline EDSS was 6 to 6.5). EDSS scores collected during a relapse were excluded unless they were sustained 90 days after the relapse. A number of other clinical outcomes were also assessed as were MRI variables. After three years, 38.9 % of IFNB-1b group and 49.8 % of the placebo group met the primary outcome, a significant difference which persisted in an intention-to-treat analysis. A significantly positive effect was also seen in secondary EDSS-based efficacy measures.
Treatment effect was similar irrespective of baseline EDSS or superimposed relapses per the analysis. ARR was reduced from 0.64 in the placebo group to 0.44 in the IFNB-1b group, a 31.3 % relative reduction. Significant reductions in MRI T2 lesion volume and new lesions were also observed. Notably, this was one of the first trials to employ time to confirmed disability as an outcome [34, 35]. Because the EDSS is poorly responsive to changes in the 6-6.5 range, this group also employed a different definition of EDSS change in that range of just 0.5 points, a trend that persists in MS research. Following the European Study group results, IFNB-1b was approved for the treatment of SPMS in Europe and Canada. To further study the effect of IFNB-1b on progressive disease, the North American Study Group on IFNB-1b in SPMS study was conducted and published in 2004 [36]. Participants had CDMS for at least 2 years, a history of at least one relapse followed by progressive deterioration sustained for at least 6 months, EDSS 3-6.5 with an increase of at least 1 point in the 2 years prior to screening (or 0.5 points with baseline EDSS 6.5). There was no requirement to have had relapses in a timeframe preceding enrollment. 939 participants were randomized to receive IFNB-1b SC 250 μg vs 160 μg/m2 (to assess whether body mass affected response) vs placebo. The primary outcome measure was days to increased EDSS of at least 1 point (0.5 points for EDSS 6-6.5) confirmed at 2 consecutive assessments at least 6 months from the onset of progression. A number of secondary clinical and MRI outcomes were included. At an interim analysis, the data safety monitoring board recommended early termination of the trial based on the finding that the primary outcome was not significantly different between groups, i.e. there was no difference in time to confirmed disability progression. There was a significant difference in ARR for the 250μg arm (43 % reduction) and for the pooled IFNB-1b groups (36 % reduction) but not the 160μg/m2 alone. MRI outcomes significantly favored the IFNB-1b groups. This study further demonstrated the dissociation of treatment effect between relapse-related outcomes and disease progression outcomes, underscoring their theorized pathophysiological differences. In comparing the two trials, the different definitions of time to confirmed progression (i.e. the European study using only 3 months and the North American study using 6 months) may have accounted for the differences in the outcomes of the trials. The shorter time frame may capture more relapse-related EDSS changes.
The Secondary Progressive Efficacy Clinical Trial of Recombinant Interferonbeta-1a in MS (SPECTRIMS) study group enrolled 618 participants to study the effect of IFNB-1a SC three times weekly at doses of 22 μg vs 44 μg vs placebo over 3 years [37]. The primary outcome, time to 3 month confirmed disability progression defined as an increase in at least 1 EDSS point (or 0.5 if baseline EDSS ≥ 5.5), was not significantly different between groups. As in other studies, relapse-related outcomes were significantly affected in the treatment arms. In a post-hoc analysis of treatment-by-sex interaction, there was an unexpected finding that women had a significant delay in progression in both treatment arms, which was also observed in MRI outcome making variability in EDSS assessments an unlikely explanation. After extensive review of the data, no explanation could be found for this difference.
The International MS Secondary Progressive Avonex Controlled Trial (IMPACT) studied the effect of IFNB-1a IM 60 μg (double the current RRMS treatment dose) vs placebo in a total of 436 participants over 2 years [38]. A unique aspect of this SPMS trial was the use of the MS Functional Composite (MSFC) as the primary outcome, a composite of the timed 25-foot walk (lower extremity function), the 9-hole peg test (upper extremity function), and the paced auditory serial addition test (PASAT3, cognitive function). There was a 40.4 % reduction in MSFC worsening in the treatment group compared to placebo, a significant result, primarily driven by reduced worsening in the 9-hole peg test and PASAT3. However, there was no difference in 3 month time to EDSS worsening, though the MSFC had been shown to correlate with EDSS [39]. Given that the EDSS is heavily weighted toward measurement of lower extremity function, one potential explanation for this finding may be that IFNB-1a was more effective at preserving upper extremity and cognitive function, which were not individually observed in prior studies. Despite the positive primary outcome of the study, IFNb-1a IM did not receive regulatory approval primarily based on the non-significant effect on EDSS.
The Nordic SPMS study group examined the use of IFNB-1a SC 22 μg once weekly vs placebo for three years in a total of 371 participants [40]. The primary outcome of 6 month time to confirmed disability was not significantly different between groups nor was differences in annualized relapse rate.
An exploratory study of IFN in PPMS examined doses of IFNB-1a IM 30 μg vs 60 μg vs placebo over 2 years in 50 participants total [41]. The primary outcome of 3 month sustained disability progression was not significantly different among the groups. One concern in designing progressive trials that continues to plague current research is how best to capture a cohort that are actively progressing. If a large proportion of participants have no worsening in measurable disability, a trial may be unable to detect a difference between treatment and placebo groups despite efficacy of the medication. In this study, 48 % of participants demonstrated progression throughout the trial suggesting that the negative results were not due to lack of progression.
Comparator Trials
With a growing number of choices of MS disease modifying therapies, a number of studies were undertaking in an attempt to demonstrate superiority of one product. Many of the studies, however, had methodological issues complicating interpretation of results. Three large studies compared different forms of IFNB. The Evidence of Interferon Dose-Response European American Comparative Efficacy (EVIDENCE) study group compared IFNB-1a SC 44 μg TIW with IFNB-1a IM 30 μg weekly in 677 RRMS participants [42]. While assessors of clinical and MRI parameters were blinded to treatment arm, participants and treating neurologists were not blinded. The primary endpoint was the proportion of participants relapse free at 24 weeks, which showed a significant difference of 74.9 % relapse free in the IFNB-1a 44 μg TIW group compared with 63.3 % in the IFNB-1a 30 μg weekly group, an odds ratio of 1.9 to stay relapse free. Similarly, MRI active lesions were fewer in the IFNB-1a 44 μg TIW group. In a 48 week extension the overall comparison remained significant, though it was driven by the changes in the first 24 weeks. In an additional 32 week switch study, participants in the IFNB-1a 30 μg arm were given the option to switch to IFNB-1a 44 μg TIW arm [43]. 73 % did switch and 91 % of the IFNB-1a 44 μg TIW remained on their original therapy. For those who switched, the ARR significantly decreased from 0.64 to 0.32 and there was a decrease in MRI active lesions. A number of methodological limitations are inherent to switch studies such as this. First, clinical assessors were aware that participants had switched to the more frequent IFNB. Second, both assessors and participant were aware of the results of the EVIDENCE study while in the switch study. This may have biased them in favor of the more frequent IFN, though the MRI results tracked with the clinical results, which supports a true difference. Third, those who opted to switch had a higher pretransition relapse rate and were less likely to be relapse free. They may be more likely to see a benefit from a switch, which would bias the results in favor of the switch. Overall, these factors make interpreting the results difficult.
The Independent Comparison of Interferon (INCOMIN) Trial Study Group randomized 188 RRMS participants to IFNB-1b SC 250 μg QOD or IFNB-1a IM 30 μg weekly [44]. After 2 years, the primary outcome of proportion of participants free from relapses and free from new MRI lesions favored IFNB-1b. A major criticism of this study was lack of blinding of assessors or participants.
The Danish IFNB study was an open-label, observational study in which patients were offered IFNB-1a SC 22 μg weekly or IFNB-1b SC 250 μg QOD, but participants who wished not to be randomized were placed on IFNB-1b [45]. In a 24 month followup, there was no difference in ARR and time to first relapse. Open-label design, lack of a placebo arm, and participants’ choice of randomization versus IFNB-1b all make the results difficult to interpret.
Three large trials compared IFNB formulations to glatiramer acetate. The Rebif vs Glatiramer Acetate in Relapsing MS Disease (REGARD) study randomized 764 RRMS participants naive to either study drug to either IFNB-1a SC 44 μg TIW or GA SC 20 mg daily and followed for 2 years [46]. There was no significant difference in time to first relapse or MRI parameters except that the IFNB group had significantly fewer enhancing lesions. The Betaseron vs Copaxone in MS with Triple-Dose Gadolinium and 3-tesla MRI Endpoints (BECOME) study randomized 75 RRMS or CIS participants to IFNB-1b SC 250 μg QOD vs GA SC 20 mg daily for up to two years [47]. This study used an optimized MRI protocol with the primary outcome being the number of combined active lesions. No difference was observed between the groups in number of lesions or clinical relapses. The Betaferon Efficacy Yielding Outcomes of a New Dose (BEYOND) trial randomized RRMS participants in a 2:2:1 fashion to IFNB-1b SC 250 μg QOD vs 500 μg vs GA SC 20 mg daily for three years [48]. Participants and treating physicians were unblinded to treatment assignment, but evaluating physicians remained blinded. There was no difference in the primary outcome of relapse risk, changes in EDSS, or MRI outcomes. Taken together, there does not appear to be a significant difference in the efficacy of high-dose IFNB and GA.
Combination Trials
Combining individual therapies has been successful for treating many other conditions. The underlying logic of this approach is to utilize more than one agent to exploit the therapeutic effects of each, while limiting potential (especially doserelated) toxic effects. The existence of multiple therapies, each with only moderate effect, yet working through different immunologic mechanisms, raises the question of whether combinations of these therapies might be safe and provide synergistic or additive benefit. From a trial design perspective, combination trials may have large patient bases from which to draw participants leading to robust designs, but present design difficulties including selecting candidate combinations from a large number of potential combinations, having difficult dose-finding processes, requiring multiple trial arms leading to large enrollment needs, and securing drug industry support for already approved drugs whose reputation may suffer from a negative combination trial [49].
In vitro study of the effects of combining IFN (in this case IFNB-1b) and GA suggested an additive effect of the combination [50]. However, studies in experimental allergic encephalomyelitis, an animal model of MS, with IFN alpha (oral and parenteral) and GA suggested that the combination was less effective than either therapy alone, raising a potential safety concern [51]. Other in vitro data suggests that there is no interference with the mechanisms of action of these agents [52].
The CombiRx study compared the efficacy of combining IFNB-1a IM weekly and GA daily compared to each agent individually in a randomized, double-blind study of RRMS [53]. The primary outcome was reduction in risk of ARR. The results were that the combination was not superior to GA alone in ARR or measures of disability reduction. Both the combination and GA alone were superior to IFN alone, although all treatment arms performed very well. The combination group was superior to either single agent in MRI measures of disease activity. The combination was also superior to the single agents in measure of disease activity free status (defined as no relapses, no progression of disability, no gadolinium enhancing lesions and no new or enlarging T2 lesions).
Two combination studies have utilized IFN and high dose steroids. The MECOMBIN trial randomized RRMS subjects to receive methylprednisolone 500 mg daily by mouth for three days every month or placebo in addition to IFNB-1a IM 30 mg weekly [54]. The primary outcome was time to onset of disability progression, which did not differ between the groups. The steroid/IFN group had a lower relapse rate than the placebo group. The NORMINS study enrolled subjects on IFNB-1a SC TIW who had at least 1 relapse in the previous year to receive oral methylprednisolone 200 mg or placebo daily for 5 days every four weeks for 96 weeks [55].The primary outcome was mean yearly relapse rate which was significantly better in the steroid group. The study results are confounded by a high drop-out rate and a loss of power due to slow recruitment.
The Avonex Combination Trial (ACT) examined use of IFNB-1a IM weekly combined with: 1) placebo, 2) oral methotrexate (MTX) 20 mg weekly, 3) bimonthly IV methylprednisolone (IVMP) 1000 mg daily for three days or, 4) both. The 313 participants had been treated with IFNB-1a IM weekly for at least 6 months and had had either a clinical relapse or MRI enhancing lesion at least 6 months after initiation of IFNB-1a IM weekly. The primary endpoint of new or enlarged T2 lesions comparing baseline and 12 month MRI scan was not met for any group. Non-significant trends in secondary outcomes variably favored either MTX or IVMP [56].
Long Term Follow Up
One of the greatest challenges in the field of MS research remains the elucidation of long term benefits of disease modifying treatments. Because MS is a lifelong disease, a major criticism of short term efficacy trials is that they may not reflect the long term effects of the medications on the disease process. Here, we will describe only the longest running of these follow up trials as an example. To address the question of long term survival in MS patients, the 16 year and 21 year Long Term Follow-up investigators evaluated a cohort of participants from the pivotal IFNB Multiple Sclerosis Study Group trial, which evaluated the use of IFNB-1b SC 50 mcg, 250 mcg QOD vs placebo [57, 58]. In the 21 year long term follow up study, the investigators were able to identify 366 of the original 372 participants. The cohort’s baseline trial characteristics remained balanced at the 21 year evaluation. Using all-cause mortality as the primary outcome, the study found that those randomized to either IFNB-1b arm had a significant reduction in mortality with a hazards ratio of 0.53-0.54, which was independent of baseline demographics, clinical, and MRI characteristics and held true in a sensitivity analysis. This study is remarkable for its near complete ascertainment of the original cohort, which decreases concern for informative censoring.
Clinical Use
Tolerability/Adherence
In a systematic review of both RCTs and observational studies, Giovannoni et al. evaluated the tolerability and adherence of IFNB [59]. Concerning tolerability, they found that the incidence of flu-like symptoms (typically fever, chills, headache, myalgias, arthralgias, fatigue) was present in 32 % of patients treated with IFNB-1b, 40 % with IFNB-1a SC, and 57 % with IFNB-1a IM. Injection site reactions (typically redness, bruising, pain and rarely secondary infections, skin necrosis, and atrophy) were found to be most common in IFNB-1a SC with an incidence of 65 % followed by IFNB-1b SC of 33 % and IFNB-1a IM of 22 %. However, there was large inter-study variability/range in part owing to differences in definitions and reporting. They could not find evidence of a change in incidence with extended use owning to the small numbers of studies with extended treatment periods, however, it is generally held that flu-like symptoms may lessen after 3-6 months of treatment. Depression was also evaluated and was experienced in 11 % of IFNB-1a IM, 7 % in IFNB-1a SC and less than 2 % in IFN-1b SC. However, given its co-morbidity in MS, it is difficult to conclude causation from IFNB use alone.
Concerning adherence, the authors reported an overall rate of discontinuation as well as a rate of discontinuation from studies lasting longer than 24 months, termed “long-term”. For IFNB-1b SC, the overall rate of discontinuation was 23 % and with a long-term rate of 34 %. For IFNB-1a IM, rates were 20 % and 30 % respectively, and for IFNB-1a SC rates were 17 % and 22 % respectively. Reasons for discontinuation of therapy were also evaluated. Adverse events accounted for discontinuations in 64 % of IFNB-1a SC, 44 % of IFNB-1b SC, and 23 % of IFNB-1a IM. Perceived lack of efficacy accounted for discontinuation in 28 % of IFN-1a IM, 26 % of IFNB-1b SC, and 11 % of IFNB-1a SC. Finally, patient decision accounted for 11-19 % of discontinuation and ‘lost to follow-up’ was less than 5 % for all IFNB’s.
Laboratory Abnormalities
Asymptomatic abnormalities in hematological and hepatic studies are common with IFNB use. They tend to be mild, transient, often occur in the first 6 months, are dose related, and usually do not present cause for discontinuation [60]. Leukopenia, usually lymphopenia, is the most common hematological abnormality, but any cell line may be affected. The IFNB dose may be temporarily reduced and reassessed with reintroduction of the drug. Rare hematological toxicity including severe leukopenia, autoimmune hemolytic anemia, and aplastic anemia have been reported. Abnormalities in hepatic transaminases are also common and should be closely monitored if they rise above 3 times the upper limit of normal. Reduction in dose, frequent monitoring, elimination of other hepatotoxic drugs, and reintroduction of the full dose may be sufficient, though persistently elevated measures may require discontinuation and evaluation for hepatic disease. Rarely serious liver damage has been reported.
Determinants of Efficacy
With IFNB being the most commonly prescribed DMA for MS and new agents becoming available, there is great interest in predetermining efficacy in individual patients. Currently, the most commonly used markers for efficacy are MRI and measurement of IFNB neutralizing antibodies (NAbs).
MRI
MRI active lesions have been validated at the individual patient level as a surrogate marker for relapses for patients participating in two large trials for IFNB-1a [61]. Furthermore, in RRMS patients treated with IFNB-1a, those with three or greater active lesions had more disease progression on EDSS and MSFC and more brain atrophy than those with less than three [62]. This and other studies have indicated that MRI active lesions found within the first 6 - 24 months after starting an IFNB predict poor prognosis, but one cannot discern whether this is an inadequate response to the therapy or a marker of disease severity [63].
NAbs
NAbs represent a humoral immune response to the presence of IFNB and have been shown to reduce its efficacy [63]. Rates of NAb positivity vary and are dependent on the IFN administered as well as method of NAb detection: For IFNB-1a IM, reported rates range from 1 - 22 % with an average of 5.3 %, for IFNB-1a SC 11-35 % with average 23.6 %, and IFNB-1b SC 22-41 % with average 35 % [64]. There is an expansive and, at times, charged body of literature concerning the proper measurement and interpretation of NAbs in IFNB treatment, the details of which are beyond the scope of this review. One consensus opinion from an international panel recommended measuring NAbs after 1-2 years of IFNB treatment and interpreting the results based on the clinical status of the patient and the NAb titer [65]. For patients doing poorly, defined as multiple relapses or a relapse plus extensive MRI activity, they recommended a switch to a non-IFNB drug regardless of NAb titers. For patients with intermediate disease activity, defined as one relapse or no/limited MRI activity, recommendations were based on NAb titers. For those with high titer, a remeasurement should be performed at 3-6 months and a switch should be made if the NAbs are persistently elevated. For those with negative titers, one could consider continuing IFNB and repeat NAbs in 12 months. Finally, for those doing well, defined as having no relapses and no/limited MRI activity, the group recommended that if high titers were found, a repeat in 3-6 months should be performed. If this repeat again showed high titers, a switch to non-IFNB drug may warranted. This final point differs most notable from our common practice, in which patients doing well do not have NAbs measured owing to the fact that we are unlikely to switch to a non-IFNB based on the presence NAb titers alone. The panel recommended to avoid switching from one IFNB medication to another in the presence of Nabs given the cross-reactivity of NAbs between products.
Future
While IFNB is now a well-established therapy for MS, there remains many questions and areas of uncertainty. Here, we briefly discuss some of these active areas of research.
Biomarkers
It is thought that disease response to IFNB varies greatly at the patient level ranging from highly efficacious to ineffective. Prospective identification of responders through biomarker measurement is a major goal in MS research, though such markers continue to be elusive. The difficulties in this area are illustrated by recent work on IL-17 F, a cytokine highly expressed by Th17 cells. Based on initial work in EAE in rats, which indicated the presence of Th17 cells corresponded to worsened disease scores [66], Axtell et al. retrospectively analyzed 26 patients with RRMS on IFNB and found that elevated pretreatment levels of IL-17 F were present in patients who were poor responders to the IFNBs. In an attempt to replicate these results, Bushnell et al. [67] used 118 samples from an IFNB-1a IM dose comparison study and reanalyzed the samples from Axtell et al. [66], using very similar methods and a comprehensive assessment for quality. Unfortunately, no association with IL-17 F levels and clinical response was found. One possible explanation for the discrepant results included selection bias introduced in the smaller Axtell cohort, which was not formed under a clinical trial protocol.
Other difficulties hampering progress in biomarker research include: lack of a unified definition of poor treatment response, elucidation of common measurable biological pathways in the setting of both a heterogeneous disease pathophysiology and heterogeneous drug effect, and the differentiation of biomarkers that predicts poor drug response from biomarkers that predict disease severity.
Follow-On-Biologics and New Formulations
Follow-on-biologics (FOBs) are medicinal products meant to serve as biological, pharmaceutical, and therapeutic equivalents to available biological agents [68]. High treatment costs in MS have driven economic interest in the development of FOBs, though there are a number of challenges to overcome including: 1. Replication of a complex biological agent to reproduce primary to quaternary structure, 2. Ensuring an agent undergoes similar biological reactivity within the body such as posttranslational modification, and 3. Developing appropriate regulatory frameworks for approval, i.e. requiring only demonstration of biological and pharmaceutical equivalence versus the need to conduct full clinical trials to demonstrate equivalence. Even with each of these challenges addressed, it remains uncertain if physicians and patients will be willing to accept an FOB as a viable alternative to branded DMA.
Clinical Use
Despite IFNBs long standing place as a first-line therapy for MS, the field continues to lack consensus on a number of issues including timing of use in the disease process including use in SPMS with relapses, timing of switch from IFNB in the setting of disease activity, and optimal dosing regimens. Studies already presented here have lent some guidance to these questions, but uncertainty remains. Active research in IFNB continues even as the field of available treatments grows with the hope that light will be shed on these unanswered questions.
Glatiramer Acetate
History
The development of GA stands as a great success in translational research and its rich history cannot be given full justice here, but we describe it briefly to introduce a framework for this section. Since the 1930’s with work by Rivers and others, experimental autoimmune encephalitis (EAE), a T-cell mediated acute demyelinating disease of laboratory animals, had been developed by immunization with CNS homogenates in complete Freund’s Adjuvant [69]. Later in the 1960s, EAE had been induced by myelin basic protein (MBP). Arnon and colleagues explored the production of synthetic molecules mimicking MBP with the goal of exploring more closely the induction of EAE, though in the late 1960s methods for creating sequenced polypeptides did not yet exist. Instead, they synthesized random copolymers of amino acids with high lysine content, but found that none induced EAE even when conjugated to sphingolipid moieties. These findings led to suppression studies in which EAE was induced in guinea pigs with MBP followed by introduction of the synthetic copolymers. In what Dr. Arnon described as an “overwhelming” result, the copolymers, most notably copolymer 1 (Cop 1), strongly suppressed EAE [69]. Cop 1 was shown to crossreact with MBP at both T- and B-cell levels, a potential key to its mechanism of action, as described later, and was shown to be specific for EAE suppression across multiple species including primates. In toxicological studies, they could not induce any adverse effects even with doses 8000-fold higher than expected for treatment. With this, human trials were initiated as will be described in the following sections.
Mechanisms of Action
More than 40 years after the discovery of Cop 1, now known as glatiramer acetate (GA), we still lack a full understanding of its biological effects. GA is a standardized combination of four amino acids, namely L-alanine, L-glutamic acid, Llycine, and L tyrosine in molar ratios of 4.2, 1.4, 3.4, and 1.0, respectively, which are randomly polymerized to an average molecular weight of 4700 to 13,000 dalton [70]. There are several proposed mechanisms to explain GA’s beneficial effects. It appears GA binds to the major histocompatibility complex (MHC) class II of MBPrecognizing antigen presenting cells (APCs). This GA-MHC complex is recognized by GA-reactive T cells, which exist de novo in the periphery. In doing so, GA may both block the autoreactive nature of MBP recognition and induce these GA-reactive T cells to shift from a primarily pro-inflammatory Th1 state to an anti-inflammatory Th2 state [70, 71]. While GA does not cross the blood brain barrier, these GA-reactive Th2 cells migrate into the CNS to exert their effects, though not all of these T-cells are cross-reactive with MBP suggesting GA may also act to induce Th2 shift independent of MBP autoreactivity [72]. The D-stereoisomer of GA also effectively binds MHC class II on APCs, but does not suppress EAE suggesting there may be other mechanisms of action [73]. GA treatment may restore the function of regulatory T cells (Treg) thereby increasing suppression of autoreactive lymphocytes [74]. GA’s effect on the adaptive immune system is not limited to T cell interactions, however. GA stimulates B-cells’ production of anti-GA antibodies, but unlike the NAbs of IFNB, there is no evidence that the anti-GA antibodies reduce GA’s effectiveness [75, 76]. GA may also decrease B-cell-mediated activation of autoreactive T cells and promote B-cell-stimulated Treg function. GA may also induce beneficial shifts in the function of monocytes and the antigen-presenting dendritic cells [77]. In addition to its many anti-inflammatory properties, GA may also have neuroprotective effects as well as stimulate neurogenesis. GA increases the CNS secretion of brain-derived neurotrophic factor (BDNF), which supports the survival of both neuronal and glial cells [78]. Additionally, other neurotrophic factors, NT-3 and NT-4, appear increased in GA treatment and may have similar effects [79]. In mouse models of EAE, the presence of GA stimulated cell proliferation, migration, and differentiation into damaged areas. GA may act on oligodendrocyte precursor cells to stimulate myelin repair, though it is difficult to separate the anti-inflammatory effects and neuroprotective effects since they occur in tandem [77, 80]. As with the IFNBs previously described, the complexity of mechanisms that GA exerts may underlie its durable and long lasting beneficial effects. It remains to be seen whether the highly tailored mechanisms of action of the newer MS medications, though efficacious in decreasing inflammatory lesion development, will also provide the depth of potential neuroprotective effects.
Clinical Trials
Early Studies
The first use of GA in humans was undertaken by Abramsky et al. in 1977 [81]. It was administered to only 4 severely disabled patients over 3-6 months. While there was no consistent clinical improvement, the drug was welltolerated, opening the door for larger trials. In 1982, Bornstein et al. [82] conducted a small open-label, uncontrolled trial with 16 participants, 4 with RRMS and 12 with chronic forms of MS. GA was administered IM initially five times per week and tapered down thereafter to once weekly over a 6 month period. Some participants were reported to have neurological improvements, which declined with the reduced dose so the dosage was again increased. 8 participants were treated for more than two years. GA was well tolerated and the impression of neurological improvement further supported a large, more rigorously designed trial.
In 1987, Bornstein et al. [83] published the first randomized, double-blind, placebo-controlled trial of GA in 50 participants who had RRMS, at least 2 exacerbation in the prior 2 years, and with a Kurtzke Disability Status Scale (precursor to the EDSS) of 6 or less. Participants were randomized to either GA SC 20 mg daily or placebo after matching for sex, exacerbation rate, and baseline disability. 24 pairs were created with 2 unmatched participants split between the two groups. The primary outcome was proportion of exacerbation-free participants.
During the two year trial period, there was an average of 2.7 exacerbations in the placebo group and 0.6 in the GA group, a significant difference. Other measures of exacerbations were also significantly different. Progression of disability was evaluated, defined as a one point increase in Kurtzke score maintained at 3 months. The placebo group met the progression definition sooner than the GA group in a survival analysis with a borderline significance of p = 0.05.
Relapsing Remitting MS
Beginning in October 1991, the Copolymer 1 Multiple Sclerosis Study group conducted a 2 year, double-blind, placebo-controlled trial with 251 participants who had an EDSS of 0-5 and a history of at least 2 relapses in the prior 2 years, including 1 in the last year [84]. Participants were well matched and withdrawals from each group were similar with 15 % withdrawing from the GA group and 13.5 % from placebo. The primary outcome of the study was mean number of relapses. The GA group experienced significantly fewer relapses with a mean of 1.19 relapses over 2 years (ARR 0.59) while the placebo group experienced 1.68 (ARR 0.84), a 29 % reduction in relapse rate.
Though participants with higher EDSS scores had more relapses, the treatment effect was more pronounced in those with lower EDSS scores of 0-2. The GA group had 24.8 % of participants demonstrate EDSS improvements with 54.4 % stable and 20.8 % worsening while the placebo group saw 15.2 % improve, 56 % remain stable, and 28.8 % worsen (p = 0.037). 3 month sustained disability progression, defined as an increase of at least one EDSS step, showed no significant difference between the groups. Based on this data, GA was approved by the FDA in December 1996. In 1998, the extension of the same trial was published [85]. During the original 2 year core study, it was decided to continue all participants within their arms until the last had reached the 2 year point. 215 participants remained and were eligible for the extension. 203 entered and 194 completed the 11 month extension period. Of the 12 participants who were eligible but did not enter the extension period, the primary reason for not doing so was the desire to receive IFNB-1b, which had gained approval toward the end of the glatiramer core study. This fact underscores an increasingly difficult factor in MS trial research, namely the availability of other efficacious DMAs making the design of placebo-controlled trials in the modern era challenging. Demographics and number of participants continuing or dropping out did not significantly differ between the groups suggesting this type of bias did not affect the outcome of this study. The primary outcome of the extension period remained mean relapse rate and when that data was added to the core study, the ARR for GA and placebo were 0.58 and 0.81, respectively, a 32 % reduction. At the completion of the randomized extension study, participants were offered continuation in an open-label long term follow up study, which will be described later.
The preceding trials did not include MRI outcomes, however the pivotal GA trial had some participants undergo MRIs, which were analyzed in separate work. Ge et al. [86] conducted MRIs in 27 of their participants at a single site, 14 in the GA arm, 13 in the placebo arm. Their analysis adjusted for baseline characteristics. Though they could not show a significant difference in clinical outcomes in this small group, they did find significantly increased brain volume loss and number of enhancing lesions in the placebo group compared to GA.
In addition to this small study, other MRI studies were also performed. Mancardi et al. [87] studied 10 participants with RRMS using monthly MRIs over 10-14 months and compared the findings to pretreatment MRIs from the preceding 9-27 months. The mean number of enhancing lesions per scan was 2.20 in the pretreatment phase and 0.92 during GA treatment, but this did not reach significance (p = 0.10). When comparing number of enhancing lesions per scan per participant, however, there was a significant reduction (p = 0.003). The authors recognized limitations of this design including lack of a control group and the potential of regression to the mean. Highly active disease patients may have been more likely to enroll in the study then subsequently spontaneously improve, which would enhance the apparent effect of the study drug.
Comi et al. [88] performed a randomized, placebo-controlled, double-blind MRI study of 239 RRMS participants over 9 months with monthly MRIs performed. Inclusion criteria were similar to the pivotal trial except this study required only 1 relapse in the previous 2 years, but also required at least 1 enhancing lesion on the baseline scan. This likely selected for a group with highly active MRIs at baseline. The primary outcome, total number of enhancing lesions, revealed a significant decrease in the GA arm (25.96) compared to placebo (36.80), a 29 % reduction. The number of enhancing lesions per patient per month was 2.9 for GA and 4.1 for placebo (p = 0.005). Interestingly, the statistical significance emerged at about 6 months suggesting that there may be a delay in time to effectiveness from initiation. Secondary outcomes of cumulative median enhancing lesion volume and new T2 lesions showed a divergence between the groups at about 4 months from randomization and significance at 6 months. For clinical measures, ARR for the GA arm was 0.81 and for the placebo arm 1.21, a 33 % reduction. Again, relapse rates were similar in the first 6 months then diverged thereafter.
Clinically Isolated Syndrome
The “early glatiramer acetate treatment in delaying conversion to clinically definite multiple sclerosis in subjects Presenting with a Clinically Isolated Syndrome” (PreCISe) trial began enrollment in January 2004 [89]. This randomized, double-blind, placebo-controlled study included participants who had experienced one unifocal neurological event in the prior 90 days and had positive brain MRIs (at least two T2 lesions of 6 mm diameter and present on at least two 3 mm slices). The primary endpoint was time to conversion to CDMS due to a second relapse. A relapse was defined as new or reappearing neurological abnormalities lasting at least 48 hours accompanied by an increase of 0.5 EDSS steps or an increase within the individual function system (FS) scores of one grade for any two FS or two grades for any one FS. Note the more rigorous definition here utilizing a 48 hour requirement rather than the usual clinical definition of 24 hours. 481 participants were enrolled, 243 were randomized to GA and 238 to placebo. The planned length of study was 3 years, but during an interim analysis after a mean GA exposure of 2.32 years, a significantly beneficial effect of GA was seen. The data monitoring committee unanimously recommended the placebo group be stopped and be offered open-label GA treatment. In an intention to treat analysis, there was a significant reduction of 45 % in risk of conversion to CDMS for the GA group. The number needed to treat to prevent one conversion to CDMS was 5.49. As seen in the RRMS trial, the Kaplan-Meier curves diverge between 3-6 months. Other clinical secondary endpoints showed similar results. MRI analysis showed significant reductions in new T2, enhancing, and T1 hypointense lesions.
Progressive Forms
In 1991, Bornstein et al. [90] published a 2 year study of GA in chronic progressive MS. Participants were aged 20-60, had EDSS scores of 2-6.5 and had evidence of a chronic-progressive course for at least 18 months with no more than 2 exacerbations in the previous 24 months. 169 participants were enrolled and entered into a pre-trial observation period of 6-15 months in which each participant was required to show signs of progression before entering into the randomized treatment phase. Qualifying changes included: increase in one FS by 2 grades, increase in 2 FS by 1 grade, worsening of 1 grade in the ambulatory index, or increase by 1 EDSS step. 63 participants were eliminated during the observation period (31 did not have sufficient progression, 10 chose alternative treatments, 8 progressed above EDSS of 6.5, 2 had exacerbations, and 12 were excluded for other reasons). Therefore, 24 % of otherwise qualified participants were excluded either because they lacked sufficient progression or progressed/relapsed beyond the inclusion criteria, which demonstrates the difficulties of capturing sufficiently progressing patients while still enrolling enough to meet power calculations. The primary outcome was time to 3 month sustained disability of 1 EDSS step if baseline was 5 or greater or 1.5 EDSS steps if baseline was less than 5. 106 participants began the trial, 86 completed the trial with 10 in each arm withdrawing early. Of those who withdrew, 3 were adjudicated to meet criteria for confirmed progression prior to the code break. Therefore, 23 (17.6 %) in the GA group and 14 (25.5 %) in the placebo group met the primary endpoint, not statistically significant by a number of methods.
To study the effects of GA on PPMS, the 3 year PROMISE trial was initiated [91]. This randomized, double-blind, placebo controlled study enrolled participants ages 30 to 65 with baseline EDSS scores of 3-6.5 inclusive. The diagnosis of PPMS was determined at each site, but required at least 6 months of progressive neurological symptoms including evidence for myelopathy (pyramidal FS score of at least 2) and exclusion of cervical spondylitic myelopathy, preferably by MRI. Lumbar puncture was required, but participants were not required to demonstrate abnormalities of IgG synthesis. Cases lacking abnormalities were reviewed by an eligibility committee to ensure consistency with the diagnosis of PPMS. Each of these inclusion criteria underscore difficulties in designing PPMS trials. Since PPMS patients are older at onset and diagnosis, the upper age limit must be higher, but this introduces more age-related disease, which may confound the PPMS diagnosis including cervical spondylosis, B12 deficiency, thyroid dysfunction, and other causes of myelopathy. Similarly, the upper limit of EDSS scores must also be higher to capture more PPMS patients, but as previously discussed, the EDSS is insensitive to neurological change other than changes in gait above an EDSS of 6. Despite these hurdles, the PROMISE trial was able to enroll 943 participants, randomizing 2:1 with 627 in the GA arm and 316 in the placebo arm. The primary outcome was 3 month confirmed disease progression (defined as a change of 1 EDSS point if baseline was 3-5, called stratum I, or change of 0.5 if baseline was 5.5-6.5, called stratum II). Two interim analyses were planned during the trial. The first occurred after 622 participants had completed 12 months of study and found no treatment effect and a surprisingly low rate of progression with only 16.1 % progressing in stratum I (expected 50 %) and 19.3 % in stratum II (expected 20 %). This again highlights the challenge of recruitment of an actively progressing group to allow for observation of a treatment effect. While more rigorous inclusion criteria might have captured such a group, it likely would have limited the pool of potential enrollees in an already uncommon disease. In the second interim analysis after 757 participants had completed 2 or more years on study, there remained no significant treatment effect, HR 0.87 (p = 0.1753). The data safety monitoring board recommended early termination of the study. MRI analysis revealed the mean change from baseline enhancing lesions was significantly reduced in the GA group, but lost significance in the 2nd and 3rd years. The GA group also had smaller increases in T2 lesion volume, but only in the 2nd year. In a post hoc analysis, it was found that males had a significantly delayed time to disability progression in the GA group with HR 0.71, p = 0.0193. In follow-up analysis, no clear explanation for this gender difference could be proven other than to note that males progressed faster in the trial, which may have allowed a treatment effect to become apparent [92]. It remains to be seen whether a true treatment effect in slowing progression could have been demonstrated had the whole group progressed more rapidly.
Comparator Trials
As described in previous sections, GA was compared to IFNB-1a SC (REGARD) and IFNB-1b SC (BECOME and BEYOND) [46–48] for the treatment of RRMS. Though each study under individual scrutiny had potential reasons for failing to demonstrate GA superiority, it has become accepted practice to consider GA and highdose interferons to have similar clinical efficacy.
Combination Trials
Given the modest benefits of individual DMAs, there has been longstanding interest in combining treatments to generate more robust effects on MS disease activity [93]. The unique mechanisms of action of GA has made it an interesting candidate for combination therapy. Because of the apparent delay in efficacy of GA, one model of combination therapy is that of induction with a powerful immunosuppressant followed by GA as a maintenance therapy. This has been most extensively studied with the use of mitoxantrone (MTX) [94]. While pilot studies of highly active disease patients have shown a robust decrease in inflammatory disease activity with induction using MTX followed by GA, these studies suffer from their observational design and the potentially strong effect of regression to the mean [16]. It remains to be seen whether exposing patients to the potential long term side effects of MTX in the setting of combination therapy is warranted. Another model for combination therapy with GA has been to combine it with other approved immunomodulators. As previously described, combination with IFNB-1a IM in the CombiRx study failed to demonstrate benefit in ARR compared to GA alone [53]. In the phase 2 randomized, double-blind, placebo-controlled 24-week Glatiramer Acetate and Natalizumab Combination Evaluation (GLANCE) study, natalizumab (NTZ) was added to RRMS patients with EDSS 0-5 who had been on GA for at least 12 months and had at least one relapse in that time [95]. All 110 participants remained on GA and were either randomized to receive NTZ (n = 55) or placebo (n = 55) in addition. The primary endpoint was the rate of development of new active lesions on brain MRI, which was significantly lower in the combination group than GA alone (p = 0.031), which became apparent after 4-8 weeks of treatment. The cumulative number of enhancing lesions was also lower in the combination group (0.6 ± 1.8) than the GA group (2.3 ± 5.3, p = 0.020). There was a 62 % reduction in new/newly enlarging T2 lesions (p = 0.029). Without a NTZ only arm, it cannot be determined if the MRI benefits of the combination arm derive from NTZ alone or a combined effect from NTZ + GA. ARR was not different between the groups and EDSS scores remained stable. Given the concern for progressive multifocal leukoencephalopathy in combination therapy with IFNB, participants were not continued into a planned extension phase and no additional combination trials of NTZ and GA have occurred.
Long Term Follow Up
After the conclusion of the initial pivotal trial and randomized extension phase, participants were offered the chance to remain in an open-label long term follow-up study, the results of which have been published at 6, 8, 10, and 15 years [96–99]. Concerning the most recent report of 15 year data, 232 participants were originally entered into the long term open label study (19 from the original placebo arm chose not to join and are excluded from the ongoing modified intention to treat analysis (mITT)). Of those 232 in the mITT cohort, 100 remained in the “ongoing cohort”, having been treated solely with GA for an average of 13. 6 years. The 131 who did not remain in the study constituted the “withdrawn cohort” and their data were calculated from last observation carried forward. Using a propensity score analysis, there appeared to be no differences in the baseline characteristics or original randomization arm between these two groups suggesting that neither of these factors affected the outcome of this 15 year analysis, a major potential source of bias. Results of the analysis included: ARR before and after GA initiation were: 1.18 ± 0.82 to 0.43 ± 0.58 for the entire mITT cohort, 1.12 ± 0.82 to 0.25 ± 0.34 for ongoing cohort, and 1.23 ± 0.83 to 0.56 ± 0.68 for the withdrawn cohort. EDSS scores were stable or improved for 54 % of the mITT, 57 % of the ongoing cohort, and 52 % for the withdrawn cohort. Progression to SPMS occurred in 25 % of the mITT, 35 % of the ongoing cohort, and 17 % of the withdrawn cohort. One may note that the withdrawn cohort appears to have performed comparably to the ongoing cohort, but it is important to remember that this data comes from the last observation carried forward whereas the ongoing cohort was observed throughout the 15 year period. In analyzing reason for withdrawal, 55/132 (42 %) withdrew due to “perception of disease worsening”, “desire to switch or combine therapies”, or “difficulty, inability, or unwillingness to adhere to study protocol”. This suggests that at least some of the withdrawals may have been due to clinical worsening, data not reflected in the last observation carried forward design. The remaining were either lost to follow up (14/132), had an adverse event (23/132) or became pregnant (8/132). It is difficult to draw firm conclusions from this data due to lack of a placebo comparator arm and the potential for selection bias favoring retention of participants doing well on GA, but it represents some of the best data on carefully conducted, prospective, long term observational use of a single agent. The GA treated group appears to maintain a low ARR, exhibit slow progression on EDSS, and tolerate the treatment without new safety concerns.
Clinical Use
GA is generally well tolerated. In the aforementioned systematic review of RCTs and observational studies, Giovannoni et al. [59] found injection site reactions to have occurred in 56-78 % of trial participants. This included injection site bruising, erythema, pain, pruritus, induration and rarely skin necrosis. In the initial phase three trial, 90 % of GA and 59 % of placebo participants experienced mild injection site reactions. It was suspected that the high number of injection site reactions in the placebo group may have been due in the inclusion of mannitol in both groups’ injection preparations. One unique injection-related tolerability issue with GA is the occurrence of immediate post-injection systemic reactions (IPISRs). IPISRs occur immediately to a few minutes after injecting. Patients experience flushing, chest tightness, dyspnea, palpitations, and anxiety. The duration is about 30 seconds to 30 minutes, resolving spontaneously [84]. In the initial phase three trial, IPISRs were observed in 19 participants (15.2 %) compared with 4 (3.2 %) of placebo. The number of episodes per participant ranged from 1 to 7 during the two year trial for the GA groups. These IPISRs have not been found to be harmful, but can be very concerning to patients if they are not informed of the reaction before GA is initiated. Flu-like symptoms were rare with a mean incidence of less than 5 %. Depression was experienced by less than 2 % [59]. Allergic and anaphylactic reactions are rare but have been reported [100, 101].
Concerning adherence, Giovannoni et al. [59] reported an overall rate of discontinuation for GA was 36 % with adverse events being the most common reported reason for discontinuation. Only 6 % discontinued for perceived lack of efficacy.
Future
GA has been a mainstay of MS treatment for over 15 years and continues to be frequently used. Research continues into its mechanism of action and its implications for understanding MS disease pathogenesis. The future of the treatment itself is somewhat unclear. An oral formulation failed to demonstrate efficacy on clinical and MRI parameters in a large phase 3 study [102]. As the branded patent is set to expire, competition with biosimilars may emerge. Glatiramoid compounds are under study and may add to the competition [103]. The field of effective treatments for RRMS continues to grow. Despite these challenges, the excellent record of safety, tolerability, and efficacy will likely guarantee the use of GA well into the future.
Electronic supplementary material
(PDF 509 kb)
Acknowledgments
CAM would like to acknowledge and thank the National MS Society for supporting his fellowship at the CGD Center for MS, Mount Sinai School of Medicine, New York, NY.
Conflicts of Interest
CAM: Consulting agreements: Biogen Idec
FDL: Sources of Funding for Research: Acorda Therapeutics, Inc.; Biogen Idec; Novartis Pharmaceuticals Corp; Teva Neuroscience, Inc.; Genzyme; Sanofi; Celgene; NIH; NMSS Consulting Agreements/Advisory Boards/DSMB: Bayer HealthCare Pharmaceuticals; Biogen Idec; EMD Serono, Inc.; Novartis; Pfizer; Teva Neuroscience; Actelion; Sanofi-Aventis; Acorda; Questcor; Roche, Genentech; Celgene; Johnson & Johnson; Revalesio; Coronado Bioscience, Genzyme, MedImmune; Bristol-Myers Squibb
Co-Chief Editor: Multiple Sclerosis and Related Diseases
Financial Interest: Cognition Pharmaceuticals, Inc.
Full conflict of interest disclosures is available in the electronic supplementary materials for this article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Derwenskus J, Lublin FD. Use of interferon-beta in the treatment of multiple sclerosis. Adv Neurol. 2006;98:257–271. [PubMed] [Google Scholar]
- 2.McFarlin DE. Multiple sclerosis (second of two parts) N Engl J Med. 1982;307:1246–1251. doi: 10.1056/NEJM198211113072005. [DOI] [PubMed] [Google Scholar]
- 3.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;18:893–895. doi: 10.1016/S0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 4.Lublin F. History of modern multiple sclerosis therapy. J Neurol. 2005;252(Suppl 3):iii3–iii9. doi: 10.1007/s00415-005-2010-6. [DOI] [PubMed] [Google Scholar]
- 5.Knobler RL, Panitch HS, Braheny SL, et al. Systemic alpha-interferon therapy of multiple sclerosis. Neurology. 1984;34:1273–1279. doi: 10.1212/WNL.34.10.1273. [DOI] [PubMed] [Google Scholar]
- 6.Camenga DL, Johnson KP, Alter M, et al. Systemic recombinant alpha-2 interferon therapy in relapsing multiple sclerosis. Arch Neurol. 1986;43:1239–1246. doi: 10.1001/archneur.1986.00520120023011. [DOI] [PubMed] [Google Scholar]
- 7.AUSTIMS Research Group Interferon-alpha and transfer factor in the treatment of multiple sclerosis: a double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 1989;52:566–574. doi: 10.1136/jnnp.52.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs L, Salazar AM, Herndon R, et al. Multicentre double-blind study of effect of intrathecally administered natural human fibroblast interferon on exacerbations of multiple sclerosis. Lancet. 1986;2:1411–1413. doi: 10.1016/S0140-6736(86)92730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer SL, Lin R, Moore PA, Hiscott J, Pitha PM. Regulation of type I interferon gene expression by interferon regulatory factor-3. J Biol Chem. 1998;273:2714–2720. doi: 10.1074/jbc.273.5.2714. [DOI] [PubMed] [Google Scholar]
- 11.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 12.Sega S, Wraber B, Mesec A, Horvat A, Ihan A. IFN-beta1a and IFN-beta1b have different patterns of influence on cytokines. Clin Neurol Neurosurg. 2004;106:255–258. doi: 10.1016/j.clineuro.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Mirandola SR, Hallal DE, Farias AS, et al. Interferon-beta modifies the peripheral blood cell cytokine secretion in patients with multiple sclerosis. Int Immunopharmacol. 2009;9:824–830. doi: 10.1016/j.intimp.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Prat A, Biernacki K, Antel JP. Th1 and Th2 lymphocyte migration across the human BBB is specifically regulated by interferon beta and copolymer-1. J Autoimmun. 2005;24:119–124. doi: 10.1016/j.jaut.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Graber JJ, McGraw CA, Kimbrough D, Dhib-Jalbut S. Overlapping and distinct mechanisms of action of multiple sclerosis therapies. Clin Neurol Neurosurg. 2010;112:583–591. doi: 10.1016/j.clineuro.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Chiu AW, Richert N, Ehrmantraut M, et al. Heterogeneity in response to interferon beta in patients with multiple sclerosis: a 3-year monthly imaging study. Arch Neurol. 2009;66:39–43. doi: 10.1001/archneur.66.1.noc80047. [DOI] [PubMed] [Google Scholar]
- 18.Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebocontrolled trial. UBC MS/MRI study group and the IFNB multiple sclerosis study group. Neurology. 1993;43:662–667. doi: 10.1212/WNL.43.4.662. [DOI] [PubMed] [Google Scholar]
- 19.The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277–1285. doi: 10.1212/WNL.45.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 21.PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group Randomised double-blind placebocontrolled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. doi: 10.1016/S0140-6736(98)03334-0. [DOI] [PubMed] [Google Scholar]
- 22.Li DK, Paty DW. Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsingremitting multiple sclerosis. Prevention of relapses and disability by interferon-beta1a subcutaneously in multiple sclerosis. Ann Neurol. 1999;46:197–206. doi: 10.1002/1531-8249(199908)46:2<197::AID-ANA9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.The Once Weekly Interferon for MS Study Group Evidence of interferon beta-1a dose response in relapsing-remitting MS: the OWIMS study. Neurology. 1999;53:679–686. doi: 10.1212/WNL.53.4.679. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS study group. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- 25.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357:1576–1582. doi: 10.1016/S0140-6736(00)04725-5. [DOI] [PubMed] [Google Scholar]
- 26.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 27.Comi G, De Stefano N, Freedman MS, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol. 2012;11:33–41. doi: 10.1016/S1474-4422(11)70262-9. [DOI] [PubMed] [Google Scholar]
- 28.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the “McDonald criteria”. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittock SJ. Interferon beta in multiple sclerosis: how much BENEFIT? Lancet. 2007;370:363–364. doi: 10.1016/S0140-6736(07)61170-2. [DOI] [PubMed] [Google Scholar]
- 30.Beck RW, Chandler DL, Cole SR, et al. Interferon beta-1a for early multiple sclerosis: CHAMPS trial subgroup analyses. Ann Neurol. 2002;51:481–490. doi: 10.1002/ana.10148. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Yelamos S, Martinez-Yelamos A, Martin Ozaeta G, et al. Regression to the mean in multiple sclerosis. Mult Scler. 2006;12:826–829. doi: 10.1177/1352458506070820. [DOI] [PubMed] [Google Scholar]
- 32.Kinkel RP, Kollman C, O'Connor P, et al. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology. 2006;66:678–684. doi: 10.1212/01.wnl.0000200778.65597.ae. [DOI] [PubMed] [Google Scholar]
- 33.European Study Group on interferon beta-1b in secondary progressive MS Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–1497. doi: 10.1016/S0140-6736(98)10039-9. [DOI] [PubMed] [Google Scholar]
- 34.Ebers GC, Heigenhauser L, Daumer M, Lederer C, Noseworthy JH. Disability as an outcome in MS clinical trials. Neurology. 2008;71:624–631. doi: 10.1212/01.wnl.0000313034.46883.16. [DOI] [PubMed] [Google Scholar]
- 35.Healy B, Chitnis T, Engler D. Improving power to detect disease progression in multiple sclerosis through alternative analysis strategies. J Neurol. 2011;258:1812–1819. doi: 10.1007/s00415-011-6021-1. [DOI] [PubMed] [Google Scholar]
- 36.Panitch H, Miller A, Paty D, Weinshenker B, North American Study Group on Interferon beta-1b in Secondary Progressive MS Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788–1795. doi: 10.1212/01.WNL.0000146958.77317.3E. [DOI] [PubMed] [Google Scholar]
- 37.Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group Randomized controlled trial of interferon- beta-1a in secondary progressive MS: clinical results. Neurology. 2001;56:1496–1504. doi: 10.1212/WNL.56.11.1496. [DOI] [PubMed] [Google Scholar]
- 38.Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679–687. doi: 10.1212/WNL.59.5.679. [DOI] [PubMed] [Google Scholar]
- 39.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122:871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 40.Andersen O, Elovaara I, Farkkila M, et al. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:706–710. doi: 10.1136/jnnp.2003.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leary SM, Miller DH, Stevenson VL, et al. Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology. 2003;60:44–51. doi: 10.1212/WNL.60.1.44. [DOI] [PubMed] [Google Scholar]
- 42.Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE trial. Neurology. 2002;59:1496–1506. doi: 10.1212/01.WNL.0000034080.43681.DA. [DOI] [PubMed] [Google Scholar]
- 43.Schwid SR, Thorpe J, Sharief M, et al. Enhanced benefit of increasing interferon beta-1a dose and frequency in relapsing multiple sclerosis: the EVIDENCE study. Arch Neurol. 2005;62:785–792. doi: 10.1001/archneur.62.5.785. [DOI] [PubMed] [Google Scholar]
- 44.Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/S0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- 45.Koch-Henriksen N, Sorensen PS, Christensen T, et al. A randomized study of two interferon-beta treatments in relapsing-remitting multiple sclerosis. Neurology. 2006;66:1056–1060. doi: 10.1212/01.wnl.0000204018.52311.ec. [DOI] [PubMed] [Google Scholar]
- 46.Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7:903–914. doi: 10.1016/S1474-4422(08)70200-X. [DOI] [PubMed] [Google Scholar]
- 47.Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72:1976–1983. doi: 10.1212/01.wnl.0000345970.73354.17. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8:889–897. doi: 10.1016/S1474-4422(09)70226-1. [DOI] [PubMed] [Google Scholar]
- 49.Conway D, Cohen JA. Combination therapy in multiple sclerosis. Lancet Neurol. 2010;9:299–308. doi: 10.1016/S1474-4422(10)70007-7. [DOI] [PubMed] [Google Scholar]
- 50.Milo R, Panitch H. Additive effects of copolymer-1 and interferon beta-1b on the immune response to myelin basic protein. J Neuroimmunol. 1995;61:185–193. doi: 10.1016/0165-5728(95)00085-G. [DOI] [PubMed] [Google Scholar]
- 51.Brod SA, Lindsey JW, Wolinsky JS. Combination therapy with glatiramer acetate (copolymer-1) and a type I interferon (IFN-alpha) does not improve experimental autoimmune encephalomyelitis. Ann Neurol. 2000;47:127–131. doi: 10.1002/1531-8249(200001)47:1<127::AID-ANA22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Chen M, Gran B, Costello K, et al. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Mult Scler. 2001;7:209–219. doi: 10.1177/135245850100700401. [DOI] [PubMed] [Google Scholar]
- 53.Lublin F, Cofield S, Cutter G, et al. The CombiRx trial: a multi-center, doubleblind, randomized study comparing the combined use of interferon beta-1a and glatiramer acetate to either agent alone in participants with relapsing-remitting multiple sclerosis - clinical outcomes. Neurology. 2012;78:PL02.003. doi: 10.1212/WNL.78.1_MeetingAbstracts.PL02.003. [DOI] [Google Scholar]
- 54.Ravnborg M, Sørensen PS, Andersson M, et al. Methylprednisolone in combination with interferon beta-1a for relapsing-remitting multiple sclerosis (MECOMBIN study): a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:672–680. doi: 10.1016/S1474-4422(10)70132-0. [DOI] [PubMed] [Google Scholar]
- 55.Sorensen PS, Mellgren SI, Svenningsson A, et al. NORdic trial of oral methylprednisolone as add-on therapy to interferon beta-1a for treatment of relapsingremitting multiple sclerosis (NORMIMS study): a randomised, placebo-controlled trial. Lancet Neurol. 2009;8:519–529. doi: 10.1016/S1474-4422(09)70085-7. [DOI] [PubMed] [Google Scholar]
- 56.Cohen JA, Imrey PB, Calabresi PA, et al. Results of the avonex combination trial (ACT) in relapsing-remitting MS. Neurology. 2009;72:535–541. doi: 10.1212/01.wnl.0000341934.12142.74. [DOI] [PubMed] [Google Scholar]
- 57.Ebers GC, Traboulsee A, Li D, et al. Analysis of clinical outcomes according to original treatment groups 16 years after the pivotal IFNB-1b trial. J Neurol Neurosurg Psychiatry. 2010;81:907–912. doi: 10.1136/jnnp.2009.204123. [DOI] [PubMed] [Google Scholar]
- 58.Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNbeta-1b trial. Neurology. 2012;78:1315–1322. doi: 10.1212/WNL.0b013e3182535cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012;18:932–946. doi: 10.1177/1352458511433302. [DOI] [PubMed] [Google Scholar]
- 60.Kremenchutzky M, Morrow S, Rush C. The safety and efficacy of IFN-beta products for the treatment of multiple sclerosis. Expert Opin Drug Saf. 2007;6:279–288. doi: 10.1517/14740338.6.3.279. [DOI] [PubMed] [Google Scholar]
- 61.Sormani MP, Stubinski B, Cornelisse P, et al. Magnetic resonance active lesions as individual-level surrogate for relapses in multiple sclerosis. Mult Scler. 2011;17:541–549. doi: 10.1177/1352458510391837. [DOI] [PubMed] [Google Scholar]
- 62.Rudick RA, Lee JC, Simon J, Ransohoff RM, Fisher E. Defining interferon beta response status in multiple sclerosis patients. Ann Neurol. 2004;56:548–555. doi: 10.1002/ana.20224. [DOI] [PubMed] [Google Scholar]
- 63.Killestein J, Polman CH. Determinants of interferon beta efficacy in patients with multiple sclerosis. Nat Rev Neurol. 2011;7:221–228. doi: 10.1038/nrneurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 64.Hegen H, Schleiser M, Gneiss C, et al. Persistency of neutralizing antibodies depends on titer and interferon-beta preparation. Mult Scler. 2012;18:610–615. doi: 10.1177/1352458511426738. [DOI] [PubMed] [Google Scholar]
- 65.Polman CH, Bertolotto A, Deisenhammer F, et al. Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol. 2010;9:740–750. doi: 10.1016/S1474-4422(10)70103-4. [DOI] [PubMed] [Google Scholar]
- 66.Axtell RC, de Jong BA, Boniface K, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bushnell SE, Zhao Z, Stebbins CC, et al. Serum IL-17F does not predict poor response to IM IFNbeta-1a in relapsing-remitting MS. Neurology. 2012;79:531–537. doi: 10.1212/WNL.0b013e318259e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reingold SC, Steiner JP, Polman CH, et al. The challenge of follow-on biologics for treatment of multiple sclerosis. Neurology. 2009;73:552–559. doi: 10.1212/WNL.0b013e3181b2a6ce. [DOI] [PubMed] [Google Scholar]
- 69.Arnon R. The development of Cop 1 (copaxone), an innovative drug for the treatment of multiple sclerosis: personal reflections. Immunol Lett. 1996;50:1–15. doi: 10.1016/0165-2478(96)02506-0. [DOI] [PubMed] [Google Scholar]
- 70.Carter NJ, Keating GM. Glatiramer acetate: a review of its use in relapsingremitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs. 2010;70:1545–1577. doi: 10.2165/11204560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Blanchette F, Neuhaus O. Glatiramer acetate: evidence for a dual mechanism of action. J Neurol. 2008;255:26–36. doi: 10.1007/s00415-008-1005-5. [DOI] [PubMed] [Google Scholar]
- 72.Hestvik AL, Skorstad G, Price DA, Vartdal F, Holmoy T. Multiple sclerosis: glatiramer acetate induces anti-inflammatory T cells in the cerebrospinal fluid. Mult Scler. 2008;14:749–758. doi: 10.1177/1352458508089411. [DOI] [PubMed] [Google Scholar]
- 73.Webb C, Teitelbaum D, Herz A, Arnon R, Sela M. Molecular requirements involved in suppression of EAE by synthetic basic copolymers of amino acids. Immunochemistry. 1976;13:333–337. doi: 10.1016/0019-2791(76)90344-X. [DOI] [PubMed] [Google Scholar]
- 74.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4 + CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teitelbaum D, Brenner T, Abramsky O, et al. Antibodies to glatiramer acetate do not interfere with its biological functions and therapeutic efficacy. Mult Scler. 2003;9:592–599. doi: 10.1191/1352458503ms963oa. [DOI] [PubMed] [Google Scholar]
- 76.Brenner T, Arnon R, Sela M, et al. Humoral and cellular immune responses to copolymer 1 in multiple sclerosis patients treated with copaxone. J Neuroimmunol. 2001;115:152–160. doi: 10.1016/S0165-5728(01)00250-8. [DOI] [PubMed] [Google Scholar]
- 77.Lalive PH, Neuhaus O, Benkhoucha, et al. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs. 2011;25:401–414. doi: 10.2165/11588120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarchielli P, Zaffaroni M, Floridi A, et al. Production of brain-derived neurotrophic factor by mononuclear cells of patients with multiple sclerosis treated with glatiramer acetate, interferon-beta 1a, and high doses of immunoglobulins. Mult Scler. 2007;13:313–331. doi: 10.1177/1352458506070146. [DOI] [PubMed] [Google Scholar]
- 79.Aharoni R, Eilam R, Domev H, et al. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skihar V, Silva C, Chojnacki A, et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc Natl Acad Sci U S A. 2009;106:17992–17997. doi: 10.1073/pnas.0909607106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abramsky O, Teitelbaum D, Arnon R. Effect of a synthetic polypeptide (COP 1) on patients with multiple sclerosis and with acute disseminated encephalomeylitis. Preliminary report. J Neurol Sci. 1977;31:433–438. doi: 10.1016/0022-510X(77)90220-9. [DOI] [PubMed] [Google Scholar]
- 82.Bornstein MB, Miller AI, Teitelbaum D, Arnon R, Sela M. Multiple sclerosis: trial of a synthetic polypeptide. Ann Neurol. 1982;11:317–319. doi: 10.1002/ana.410110314. [DOI] [PubMed] [Google Scholar]
- 83.Bornstein MB, Miller A, Slagle S, et al. A pilot trial of Cop 1 in exacerbatingremitting multiple sclerosis. N Engl J Med. 1987;317:408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- 84.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The copolymer 1 multiple sclerosis study group. Neurology. 1995;45:1268–1276. doi: 10.1212/WNL.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 85.Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 multiple sclerosis study group. Neurology. 1998;50:701–708. doi: 10.1212/WNL.50.3.701. [DOI] [PubMed] [Google Scholar]
- 86.Ge Y, Grossman RI, Udupa JK, et al. Glatiramer acetate (copaxone) treatment in relapsing-remitting MS: quantitative MR assessment. Neurology. 2000;54:813–817. doi: 10.1212/WNL.54.4.813. [DOI] [PubMed] [Google Scholar]
- 87.Mancardi GL, Sardanelli F, Parodi RC, et al. Effect of copolymer-1 on serial gadolinium-enhanced MRI in relapsing remitting multiple sclerosis. Neurology. 1998;50:1127–1133. doi: 10.1212/WNL.50.4.1127. [DOI] [PubMed] [Google Scholar]
- 88.Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian glatiramer acetate study group. Ann Neurol. 2001;49:290–297. doi: 10.1002/ana.64. [DOI] [PubMed] [Google Scholar]
- 89.Comi G. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. The Lancet (British edition) 2009;374:1503–1511. doi: 10.1016/S0140-6736(09)61259-9. [DOI] [PubMed] [Google Scholar]
- 90.Bornstein MB, Miller A, Slagle S, et al. A placebo-controlled, double-blind, randomized, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology. 1991;41:533–539. doi: 10.1212/WNL.41.4.533. [DOI] [PubMed] [Google Scholar]
- 91.Wolinsky JS, Narayana PA, O'Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61:14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- 92.Wolinsky JS, Shochat T, Weiss S, Ladkani D, PROMiSe Trial Study Group Glatiramer acetate treatment in PPMS: why males appear to respond favorably. J Neurol Sci. 2009;286:92–98. doi: 10.1016/j.jns.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 93.Tullman MJ, Lublin FD. Combination therapy in multiple sclerosis. Curr Neurol Neurosci Rep. 2005;5:245–248. doi: 10.1007/s11910-005-0053-9. [DOI] [PubMed] [Google Scholar]
- 94.Rieckmann P. Concepts of induction and escalation therapy in multiple sclerosis. J Neurol Sci. 2009;277:S42–S45. doi: 10.1016/S0022-510X(09)70012-7. [DOI] [PubMed] [Google Scholar]
- 95.Goodman AD, Rossman H, Bar-Or A, et al. GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study. Neurology. 2009;72:806–812. doi: 10.1212/01.wnl.0000343880.13764.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 multiple sclerosis study group. Mult Scler. 2000;6:255–266. doi: 10.1177/135245850000600407. [DOI] [PubMed] [Google Scholar]
- 97.Johnson KP, Ford CC, Lisak RP, Wolinsky JS. Neurologic consequence of delaying glatiramer acetate therapy for multiple sclerosis: 8-year data. Acta Neurol Scand. 2005;111:42–47. doi: 10.1111/j.1600-0404.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 98.Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309–320. doi: 10.1191/135248506ms1318oa. [DOI] [PubMed] [Google Scholar]
- 99.Ford C, Goodman AD, Johnson K, et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler. 2010;16:342–350. doi: 10.1177/1352458509358088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481–1482. doi: 10.1212/01.WNL.0000158675.01711.58. [DOI] [PubMed] [Google Scholar]
- 101.Baumgartner A, Stich O, Rauer S. Anaphylactic reaction after injection of glatiramer acetate (copaxone(R)) in patients with relapsing-remitting multiple sclerosis. Eur Neurol. 2011;66:368–370. doi: 10.1159/000334107. [DOI] [PubMed] [Google Scholar]
- 102.Filippi M, Wolinsky JS, Comi G, CORAL Study Group Effects of oral glatiramer acetate on clinical and MRI-monitored disease activity in patients with relapsing multiple sclerosis: a multicentre, double-blind, randomised, placebo-controlled study. Lancet Neurol. 2006;5:213–220. doi: 10.1016/S1474-4422(06)70327-1. [DOI] [PubMed] [Google Scholar]
- 103.De Stefano N, Filippi M, Confavreux C, et al. The results of two multicenter, openlabel studies assessing efficacy, tolerability and safety of protiramer, a high molecular weight synthetic copolymeric mixture, in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:238–243. doi: 10.1177/1352458508098269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 509 kb)