Abstract
Magnetic resonance imaging is an established tool in the management of multiple sclerosis (MS). Loss of blood brain barrier integrity assessed by gadolinium (Gd) enhancement is the current standard marker of MS activity. To explore the complex cascade of the inflammatory events, other magnetic resonance imaging, but also positron emission tomographic markers reviewed in this article are being developed to address active neuroinflammation with increased sensitivity and specificity. Alternative magnetic resonance contrast agents, positron emission tomographic tracers and imaging techniques could be more sensitive than Gd to early blood brain barrier alteration, and they could assess the inflammatory cell recruitment and/or the associated edema accumulation. These markers of active neuroinflammation, although some of them are limited to experimental studies, could find great relevance to complete Gd information and thereby increase our understanding of acute lesion pathophysiology and its noninvasive follow-up, especially to monitor treatment efficacy. Furthermore, such accurate markers of inflammation combined with those of neurodegeneration hold promise to provide a more complete picture of MS, which will be of great benefit for future therapeutic strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0155-4) contains supplementary material, which is available to authorized users.
Keywords: Multiple sclerosis, Inflammation, Imaging biomarkers, Blood brain barrier, Contrast agent, Edema
Magnetic resonance imaging (MRI) has emerged as an indispensable tool in multiple sclerosis (MS) [1, 2], with applications ranging from early diagnosis [3] to use as a surrogate marker for efficacy in therapeutic trials [4]. Currently, a large battery of MRI methods is being developed (measurement of atrophy, T1 hypointensity, iron mapping, spectroscopy, diffusion tensor, magnetization transfer ratio, imaging of cortical anomalies, and so forth) to provide quantitative information on the severity of the neurodegenerative component of MS [5] that is thought to be an important substrate of long-term irreversible disability. By contrast, gadolinium (Gd) enhancement of lesions is the only reference method to assess blood brain barrier (BBB) permeability that reflects the inflammatory component of MS [6], and which is the predominant pathogenic mechanism in the early stages of approximately 85% of patients with a relapsing remitting form of MS. Although Gd is a powerful marker, limiting active inflammation to Gd-enhanced lesions is an oversimplification, as Gd can lack sensitivity, specificity, and does not address the cellular and edematous components that accompany active neuroinflammation [7] (Fig. 1). Furthermore, Gd cannot address subtle, but disseminated, inflammation that contributes to clinical progression together with neurodegenerative axonal loss. Consequently, other markers of active neuroinflammation are being developed in experimental animal models, and some have already been translated to human studies. These new markers of active inflammation aim to reflect the BBB permeability more accurately than Gd and to assess the cellular and edematous components of active lesions. In this article, we will give an overview of imaging markers of active inflammation in MS and we will discuss their sensitivity and specificity. We will further briefly review their potential relevance, particularly in terms of therapeutic implications.
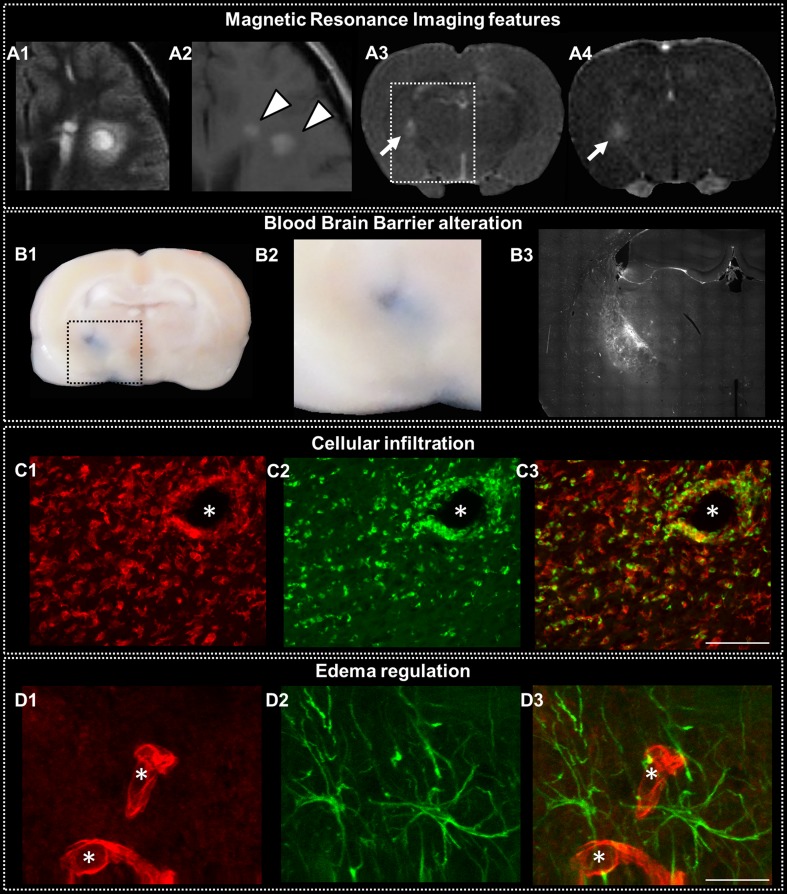
Fig. 1.
Active lesions in multiple sclerosis (MS). Features of MS active lesions in the human brain (A1, A2) and the rat brain (A3, A4, B, C, and D). Active MS lesions in the human are surrounded by blurry Fluid Attenuated Inversion Recovery (FLAIR) hypersignal related to edema (A1) and show gadolinium (Gd) enhancement (A2, arrowheads). A focal MS-like lesion induced in the rat brain shows the same MR features on T2-weighted image (A3, arrow) and T1-weighted image after Gd injection (A4) 3 days after its induction. Histological study of such focal prototypical MS lesion in the rat shows blood brain barrier (BBB) alteration as assessed by extravasation of Evans blue injected 2 h prior sacrifice (B1 and B2, enlarged view of the square dotted area) and serum protein extravasation depicted with IgG staining (B3, staining at the same level as the white rectangular dotted area delineated on T2). Cellular infiltration appears as perivascular infiltration (*indicates a capillary lumen) of recruited macrophages stained with Iba1 (C1, red) and ED1 (C2, green), whereas some of these cells could also represent amoeboid microglia with a fully activated profile. Some resident activated microglia Iba+, but ED1- are also seen (C3, merged image). Interstitial edema is difficult to depict histologically, but it is revealed by a strong expression of molecules involved in water regulation, such as aquaporin 4 (D1, red), which is expressed at the BBB level on the astrocyte end feet (D2, green; D3, merged image). Scale bars, 25 μm
Imaging BBB Alteration
Pathological Substrate
Vascular endothelial activation, induced by proinflammatory cytokines Th1, such as interferon-γ, tumor necrosis factor-α, interleukin-1β, and interleukin-6, is an early modification characterized by the expression of several endothelial cell adhesion molecules (CAMs) (intercellular CAM 1 [ICAM1], vascular CAM 1 [VCAM1], platelet endothelial CAM 1 [PECAM1], E selectin, P selectin) [8], which has shown to be important in the development of MS and its animal model, experimental autoimmune encephalomyelitis (EAE) [9]. Thereafter, BBB permeability increases with the direct effects of cytokines/chemokines, as well as indirect leukocyte-mediated injury [10]. The finalities are a decreased expression of the tight junction proteins [11], which in turn increases paracellular permeability, and an increase in the expression of caveolin (especially cav-1), which is involved in the transcellular passage [12].
Imaging Markers of BBB Alteration
Gd Imaging
Gd enhancement is currently the reference standard to detect active neuroinflammatory lesions in MS patients [1, 2]. As a small molecular weight contrast agent, Gd is thought to diffuse passively within the interstitium along a concentration gradient via a BBB that has lost its integrity [8]. Local Gd enhancement has been correlated with histological markers of BBB breakdown accompanying actively inflamed lesions in EAE [13] (Fig. 1, panel B).
The sensitivity of Gd to detect active inflammatory lesions may vary according to the physicochemical characteristics of the contrast agent (e.g., relaxivity, concentration) and to the acquisition protocol (i.e., delay between injection and image acquisition, parameters of the postinjection T1-wi acquisition) [6]. In addition to these considerations, the sensitivity of Gd enhancement as a marker of inflammation is considered imperfect. First, Gd may miss active lesions with mild changes in permeability [14]. Second, Gd may be particularly insensitive to certain forms of inflammation, such as activated microglia that may develop with a mainly intact BBB [15]. Alternatively, regarding the highly dynamic process of inflammation, Gd may miss the steps characterized by transendothelial inflammatory cell infiltration without accompanying BBB disruption [7].
With respect to the specificity of Gd enhancement as a marker of inflammation, 1 should consider that Gd enhancement parallels a significant BBB leakage that is mainly associated with active inflammation. Nevertheless, it does not measure inflammation per se and, in particular, cell infiltration and turnover. Consequently, it can only be considered as a nonspecific and indirect marker of active inflammation. Thus, incomplete restoration of tight junction integrity and BBB function in inactive, noninflamed, chronic lesions [16] could be falsely classified by Gd enhancement.
Other Markers of BBB Alteration
The sensitivity and specificity issues of Gd as a marker of active inflammation have led to alternative Magnetic Resonance (MR) markers to be explored. Gadofluorine M (Gf) (Bayer Shering Pharma AG, Berlin, Germany) is an amphiphilic Gd complex with a molecular weight of 1528 g/mol and a concentration of 250 mmol Gd/l [17]. The exact mechanism of Gf entry into the central nervous system (CNS) is still unclear, but Gf binds to albumin and is thought to enter the extracellular space along the disturbed BBB. Thereafter, Gf can be trapped within the interstitium owing to its high affinity to extracellular matrix proteins [18]. This could lead to a higher local concentration that is better visualized than Gd, which will re-diffuse back to intravascular space along the reversed concentration gradient induced by rapid renal excretion. In an established animal model of MS, Gf enhancement showed significant increase in sensitivity compared to Gd because most enhanced lesions did not show Gd uptake, and a 100% specificity for histologically confirmed active inflammatory lesions [19].
Another strategy to assess BBB damage is based on superparamagnetic MR contrast agents that are nanoparticles consisting of a core of insoluble iron oxides solubilized by coating with hydrophilic polymers [20, 21]. The classification of the particles is mainly based on their size. Ultra-small superparamagnetic iron oxides (USPIO) (20 nm on average) and superparamagnetic iron oxides (SPIO) (50-150 nm on average) are the most commonly used agents and are considered as cellular markers (see as follows) [20, 21]. Very small superparamagnetic iron oxide particles (VSOPs) are a subclass composed of citrate-coated iron oxide nanoparticles and characterized by a hydrodynamic size of approximately 7 mm and a short half-life due to their anionic surface [20]. Such extremely small VSOPs have been shown to provide sensitivity to the BBB breakdown [22] by demonstrating T2* hypointensity foci in regions without Gd enhancement in an EAE model [23]. As histology showed a diffuse extracellular accumulation within the connective tissue in the vicinity of inflammatory plaques, the authors concluded that VSOP could diffuse through an altered BBB providing an augmented sensitivity to subtle alteration compared to Gd because of the induction of a strong susceptibility effect on T2*-wi [23]. VSOP could also provide information on the cellular component similarly to the more frequently used (and larger) nanoparticles (see as follows) as they are efficiently phagocytized, and also found within macrophages on immunofluorescent stainings [23].
BBB alteration could also be assessed with molecular imaging contrast agents that are obtained by conjugating ligands (antibodies, proteins, peptides, and so forth) to MR contrast agents (contrastophore), such as iron oxide nanoparticles [24]. This technology has been used to target endothelial activation molecules, as it could occur in advance of the BBB breakdown [25–28], thus increasing the sensitivity and specificity to neuroinflammation. Nevertheless, lack of sensitivity for endothelial targets present in small quantities and problems of specificity due to circulating nontargeting contrast agents were major issues when conjugating ligands to classical USPIO/SPIO iron oxide particles. Another important caveat is that such targeted contrast agents could be phagocytosed before (or after) reaching their target molecule, precluding specificity. More promising results were obtained by using very large iron oxide particles called micron-sized particles of iron oxide (MPIO) as a contrastophore. Because of the large amount of iron conveyed, MPIO cause a local magnetic field inhomogeneity extending for a distance roughly 50 times their physical diameter allowing single particle detection [29] and increasing sensitivity. Furthermore, MPIO have very short (<5 minute) half-life in the circulation generating minimal vascular background contrast [30], whereas their size and incompressible nature preclude translocation across the endothelium, which is all favoring specificity for endothelial targets. By conjugating anti-VCAM1 antibody to MPIO, McAteer et al. [31] demonstrated the in vivo delineation of activated cerebral blood vessels at a time when pathology was otherwise undetectable [31, 32]. In EAE animals, presymptomatic lesions could be quantified using VCAM-MPIO when they were undetectable by Gd in histologically confirmed areas of endothelial activation [33] with high specificity, as demonstrated through blocking experiments [31].
Imaging the Cellular Component of Inflammation
Pathological Substrate
BBB disruption may facilitate inflammatory cell migration in active MS lesions [7], but cellular infiltration is not simply the consequence of the BBB breakdown because it requires additional signaling through CAMs and chemokines [34]. Although rolling, leukocytes show a chemokine-dependent activation and firm arrest mediated by the binding of leukocyte integrins (i.e., Leukocyte function-associated molecule-1 (LFA-1), macrophage glycoprotein associated with complement receptor function (Mac-1), and Very late antigen-4 (VLA-4)) to endothelial cell adhesion molecules [34]. Subsequently, leukocytes migrate and cross the endothelial barrier (i.e., diapedesis) by transmigrating through individual endothelial cells via a transcellular pore [35]. In a murine model of MS, most leukocytes crossed the BBB transcellularly while tight junctions were intact [36]. Figure 1, panel C, illustrates cell infiltration in an acute MS lesion.
Imaging Markers of the Cellular Component of Inflammation
SIO and USPIO Particles
SPIO containing several iron oxide crystallite cores and monocrystalline USPIO are the most widely used particles to target neuroinflammation [20, 21]. These nanoparticles have their specific uptake by the monocyte-macrophage system in common, and can be used to track macrophage infiltration by MRI after systemic injection owing to the high r1 and r2 relaxivities of the iron-loaded macrophages [37, 38]. Approximately 24 h after their intravenous injection, free SPIO/USPIO are cleared from the circulation, and signal alterations are thought to arise from the capture of particles by circulating phagocytic cells that are attracted to inflammatory lesions [39]. Although SPIO/USPIO-enhancements most probably reflect the entrance into the CNS of labeled monocytes, which have taken-up USPIO in the circulating blood, in this interpretation it should be noted that the final demonstration of the mechanism for their entry in MS lesions is still debated [37]. Partial “passive,” free, nonmacrophage-related USPIO extravasation through damaged BBB could be possible [40, 41], but is probably minor and followed by macrophage/microglia uptake in situ within the parenchyma, which allows for consideration of iron oxide particles as a marker of inflammatory cell content. Alternatively, ex vivo iron oxide-labeling of inflammatory cells and their tracking by MRI after cell transfer could be used to increase cellular specificity, but this is a much more complex strategy than systemic injection, and it was only used in a few articles [42, 43].
Animal models of MS have shown that signal alterations observed after SPIO/USPIO administration were mainly correlated with the intracellular localization of iron oxide particles in cells with the typical morphological features of macrophages [44, 45]. Following these first experimental studies, a mismatch between Gd-enhanced and USPIO-enhanced lesions was observed that demonstrated the BBB leakage shown by Gd enhancement and the cellular infiltration shown by USPIO enhancement could be distinct [46, 47]. More recent studies further support SPIO/USPIO as a cellular marker [48, 49] and systematic comparison of SPIO-enhancement with Gf as 1 of the most sensitive marker of BBB breakdown (see previously), confirmed that macrophage infiltration and leakage of the BBB for humoral factors are independent events [50].
USPIO-enhanced MRI has been translated to MS patients, and all studies have consistently demonstrated discrepancies between the BBB leakage shown by Gd enhancement and the cellular infiltration shown by USPIO enhancement [51–53], in line with animal experiments (Fig. 2). Combining Gd and USPIO led to an increase in both the number of lesions and the number of patients considered to have active disease compared with the use of Gd alone [53]. If each enhanced lesion (Gd and/or USPIO) is truly related to some component of inflammation (increased BBB permeability and/or macrophage infiltration), then combining Gd and USPIO can increase the sensitivity of MR to disease activity. One potential explanation is that the combination of Gd and USPIO detects lesions at different stages in the natural history of the disease. Longitudinal data showed that USPIO could precede Gd enhancement [52]. Thus, initially, a new MS lesion may be characterized by cell infiltration without accompanying BBB disruption [7, 36], which could correspond to the USPIO-only lesions. At a later stage, while cellular recruitment continues or decreases, many proinflammatory mediators (cytokines) increase the BBB permeability [10], which could correspond to lesions enhancing with Gd (Gd + USPIO or Gd-only lesion).
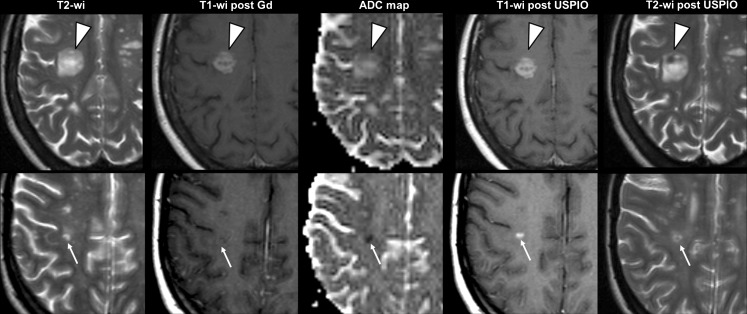
Fig. 2.
Discrepant imaging profiles of active lesions. Images from 2 relapsing-remitting multiple sclerosis (MS) patients included in the European ultra-small superparamagnetic iron oxide (USPIO) study [53]. Axial T2-wi, T1-wi after a few minutes after gadolinium (Gd; Dotarem, Guerbet, France) injection and ADC maps obtained 7 (top row) and 8 (bottom row) days following a clinical relapse. Immediately after these first MR scans, ultra-small superparamagnetic iron oxide particles (Sinerem, Guerbet, France) were injected over 30 minutes and second MR scans were repeated 24 h later to assess T1-wi post-USPIO and T2-wi post-USPIO. The first patient (top row) showed a large MS lesion (arrowheads) with active features, assessed by Gd enhancement, reflecting blood brain barrier (BBB) breakdown, which was associated with USPIO enhancement (seen both on T1-wi and T2-wi), reflecting concomitant macrophage invasion, and high apparent diffusion coefficient (ADC) values probably related to ongoing vasogenic edema. The second patient (bottom row) also showed an MS lesion (arrows) that could be classified as active, according to USPIO enhancement (seen both on T1-wi and T2-wi) reflecting macrophage invasion while the BBB was not macroscopically altered (no Gd enhancement). The significance of the associated low ADC values is still debated, but could reflect hypoxic damage and the close association with USPIO enhancement could suggest toxic products from activated macrophages as an inducer of hypoxic-like conditions
Alternatively, the USPIO and Gd patterns may detect active lesions of different types and/or severity rather than different stages, which would provide more specific information on the lesion activity or severity. In accordance with this hypothesis, we reported subtle USPIO ring-like enhancements on T1-wi in certain lesions of patients with a progressive form of the disease [53]. This pattern may represent the residual inflammatory activity of progressive MS, particularly microglial activation that can be trapped behind an intact BBB or is associated with low-grade BBB damage [54]. Another group has also shown moderate diffuse signal modification after USPIO, which is reported to disclose subtle and diffuse inflammatory activity in the normal-appearing brain parenchyma [55]. In contrast to this pattern of subtle USPIO-only enhancement, we provided the first evidence that lesions enhancing with both contrasts could represent a subgroup with more aggressive features [53] (e.g., such a pattern, see Fig. 2). The USPIO + Gd lesions were the largest, potentially reflecting the spatial extension of more active lesions. In addition, the USPIO + Gd lesions were more prone to continue enhancing for a long period of time than the other enhancement patterns, which may represent long-lasting inflammatory foci after severe tissue disruption. This finding can be related to the pathogenic roles of proinflammatory macrophages that directly attack myelin and collaborate with the other immune components [56]. Such results are consistent with our previous animal experiments in which USPIO enhancement at the beginning of EAE was associated with the severity of acute and chronic tissue damage, including axonal loss [57]. In this interpretation, it should be noted that such results on the predictive value of USPIO were all obtained with the same particle (Ferumoxtran-10; Sinerem, Guerbet, France) and remain to be validated in lager cohorts and with other particles. In particular, the macrophage M2 immunomodulatory subpopulations, as opposed to M1 proinflammatory macrophages, are important to initiate repair mechanisms and could be targeted by some SPIO/USPIO particles. Although this assumption requires further research to be validated, the current data indicate that combining USPIO and Gd could help overcome some limitations of the Gd-only approach by detecting more lesions (increased sensitivity) and differentiating patterns that may represent the pathological heterogeneity of MS lesions (increased specificity).
Other Markers of Cellular Infiltration
Myeloperoxidase (MPO), 1 of the most abundant enzymes secreted primarily by macrophages/microglia [58], can be used as a marker of cellular infiltration. Chen et al. [59] developed a Gd-based contrast agent that is radicalized when converted by MPO in the presence of hydrogen peroxide. These intermediate compounds can combine to form dimers and oligomers, as well as cross-link proteins containing tyrosine residues. These MPO-mediated changes increase the molecular size of the contrast agent shortening the T1 and increasing enhancement [60]. This prototype MPO-activatable paramagnetic sensor reaches the CNS through degraded BBB, but induces increased signal through MPO-mediated activation. In an EAE model, this sensor was more sensitive than conventional Gd, allowing the detection and confirmation of more, smaller, earlier, and less severe lesions with more subtle BBB breakdown [61]. Furthermore, unlike conventional nonspecific Gd, MPO imaging not only indicates BBB breakdown, but also the presence of actual inflammation [61].
Visualization of cells labeled by the stable fluorine isotope 19F constitutes a new “positive” contrast agent to track inflammatory cells [62]. 19F is MR active and exhibits sensitivity close to the 1H nucleus. Perfluorocarbons (PFCs) are nontoxic, biologically stable, provide a high payload of 19F nuclei and can be emulsified into nanoparticles resulting in a high concentration of 19F atoms to serve as fluorine markers [63]. PFCs can be used to label cells ex vivo before tracking using in vivo19F-MRI. Alternatively, PFCs can be injected intravenously, with emulsified particles being taken up by circulating monocytes/macrophages and transported to areas of inflammation similarly to the SPIO/USPIO approach. Because of their size, intravenously administrated PFC nanoparticles do not leak through the BBB, but provide insight into cell infiltration [64]. The main advantage of this strategy is its excellent degree of specificity resulting from the lack of any 19F background while dark areas following SPIO/USPIO injection can sometimes be difficult to attribute to these nanoparticles or to other inhomogeneities associated with anatomy that forms a background signal [62]. In combination with anatomical 1H-MRI, “hot spot” 19F images can be superimposed on 1H images generated in the same imaging session [65]. The technique was applied to monitor neuroinflammation in a focal model of nerve inflammation [66] and in neuroinflammation associated with ischemia in photothrombosis model [64, 67]. However, no data are yet available in an MS model, probably because of sensitivity constraints related to low concentration of cells. Current restrictions for 19F/1H-MRI concern are mainly its sensitivity, but this may be overcome by improving imaging hardware, imaging sequences, as well as cell labeling procedures in the future [62].
Assessing cellularity with noncontrast MR methods is highly challenging because it implies to be able to disentangle the contribution of cells to the overall MR signal arising from the other components of the tissue. By using diffusion basis spectrum imaging in cuprizone-treated mice, Wang et al. [68] separated different diffusion components within each voxel and identified a restricted isotropic component reported to represent cellularity. This restricted diffusion component strongly correlated with DAPI-positive nucleus counts and was separated from the contribution of edema, demyelination, and axonal injury associated with acute inflammation [68]. These results are promising, even if obtained on a cuprizone model that does not attempt to mimic MS as a disease because it primarily reflects demyelination with only reactive inflammation and without much contribution from the adoptive immune system [69]. An alternative approach comes from very high-field magnets (7Tesla) that enable high-resolution quantitative imaging of the local field shift caused by compounds, such as iron. The observation of peripheral phase rings undetectable with classical magnitude T2 images in approximately 8% of lesions of MS patients scanned at 7T [70] raised the hypothesis of visualizing iron-rich macrophages using a method highly sensitive to endogenous iron without any need of exogenous contrast agent, such as SPIO/USPIO. A comparative MR histological study also reported iron-rich activated macrophages/microglia at the edges of active lesions as contributors of peripheral R2* changes [71]. Nevertheless, the recent observation that ring-phase lesions remained unchanged for 2.5 years of follow-up [72] challenges the notion that such lesions reveal the presence of acute activated iron-rich macrophages, and further studies are needed to understand if MR techniques sensitive to iron can help address inflammatory cellularity.
Positron emission tomography (PET) imaging may provide an alternative to MR for cellular markers of disease activity. Microglial activation upregulates the expression of the 18 kDa translocator protein (TSPO) that is involved in the release of proinflammatory cytokines during inflammation [73, 74]. TSPO is also expressed in invading blood-borne cells, such as macrophages [73, 74] and its upregulation, reflecting active neuroinflammation, can be detected in vivo with PET and selective radioligands, such as [11C]-PK11195, the most commonly used TSPO probe [73, 74]. [11C]-PK11195 showed the highest overlap with plaques enhanced after gadolinium that are likely to contain a high amount of infiltrating mononuclear cells [75, 76]. Furthermore, increased [11C]-PK11195 signal was also found well beyond focal lesions supporting sensitivity of this marker to a widespread subtle inflammatory process, such as activation of microglia that is underappreciated with other methods [75, 77]. The sensitivity of [11C]-PK11195 also allowed depicting significant cortical microglial activation in MS patients that was correlated with clinical disability [78]. In response to [11C]-PK11195 low signal-to-noise ratio and difficulties in quantification, new TPSO radioligands ([11C]-DAA1106, [18F]-FEDAA1106, [11C]-PBR28, [18F]-PBR111, and [18F]-DPA714 amongst others) have been developed [79, 80], offering a better signal-to-noise ratio and greater promise in quantifying translocator protein binding [81] while still requiring further evaluation.
Imaging the Edematous Component
Pathological Substrate
Edema has been the focus of relatively few studies in MS, although the BBB dysfunction in active lesions inevitably allows an increase in the passage of plasma proteins and subsequent water into the extracellular compartment defining vasogenic edema [82]. This is classically opposed to cytotoxic or cellular edema that results from abnormal water uptake by injured cells, especially astrocytes [82]. Vasogenic edema accompanying active inflammatory MS lesions is usually mild, but can be marked in some tumefactive cases [83]. Although water entry parallels BBB permeability [82], its resolution after BBB closure implies the aquaporin 4 (AQP4) water channel as a major pathway for water clearance back to the blood or cerebrospinal fluid [84, 85] (Fig. 1, panel D). AQP4 demonstrated relevance to MS pathophysiology in such inflammatory edema clearance [86], but also in astrocyte migration for postinsult gliosis [87, 88], and in direct inflammation severity [89, 90]. Implication of AQP4 is still more direct in the pathogenesis of neuromyelitis optica [91].
Imaging Markers of the Edematous Component
Although blurry, T2 hypersignal surrounding enhanced lesions is thought to represent vasogenic edema associated with active lesions (Fig, 1, panels A1 and A2), T2 hypersignal is not specific and heterogeneous pathological substrates all lead to similar appearance of hyperintensity on T2 [1, 2]. Despite such lack of specificity, Shinohara et al. [92] showed that, in a proper context, MS lesions exhibiting moderate but not extreme hypointensity on T1-wi and hyperintensity on T2-wi, which reflected vasogenic edema, allowed the accurate classification of active lesions without the need of contrast injection owing to a voxel-level prediction algorithm.
Using T2 distribution by separating the multiple T2 relaxation times that contribute to the overall signal, authors have aimed to separate the biological components contributing to the mean T2 signal [93]. A short T2 component has retained most of the attention as a potential in vivo marker for myelin, but T2 relaxation studies have also helped to understand the contribution of edema and demyelination of similarly appearing T2 lesions [94, 95]. At the time of this writing, the method still requires long acquisition time and it still lacks sensitivity and specificity to separate intracellular and extracellular water and to accurately identify lesions with significant vasogenic edema, which are supposed to be active ones [96].
MRI can also provide quantitative maps of water molecular diffusion through the diffusion-weighted imaging technique [97]. A summary parameter, the apparent diffusion coefficient (ADC), is obtained from the integration of all the microscopic displacement distributions of the water molecules present in a voxel, and it allows inference to be made in regard to the microstructure of the tissue [97]. Although the precise underlying mechanisms explaining ADC variations are still debated, it is known that changes in the volume fractions of the intracellular and extracellular spaces (i.e., cytotoxic or vasogenic edema) always lead to variations in the ADC [97]. Active inflammatory lesions typically demonstrate increased ADC values that have been postulated to be due to vasogenic edema accompanying the active phase of inflammation [98] (Fig. 2, upper row). Nevertheless, membrane breakdown through demyelination and axonal loss also increase ADC values [99] that consequently cannot be specific enough of the acute neuroinflammation. MS lesions exhibiting restricted diffusion (low ADC) are particular cases that could be of interest [100]. The significance of such a pattern is still to be elucidated, but it could be a marker of acute inflammation providing more specific information on acute lesion heterogeneity (Fig. 2, lower row). MS exhibiting low ADC values could reflect the histological type III pattern described by Lucchinetti and Lassmann [101], which was characterized by oligodendrocyte dystrophy and apoptosis reflecting hypoxic white matter damage as a phathogenetic component of the lesion [102], although histopathological correlations are still lacking. Low ADC lesions, if close to type III pattern, could result from severe and profound inflammatory processes, which is substantiated by a central spot with low ADC values observed in focal EAE model during the first days after lesion induction, at a time and place in which the cytokine concentration is at its maximum level [103].
New diffusion methods, such as the diffusion basis spectrum, could overcome some limitations of the simple ADC approach by resolving the multiple diffusion components that participate with the overall signal. As already discussed for cellularity (see previously), Wang et al. [68] recently applied the method to an inflammatory model in mice brains and showed that it was able to distinguish the contribution of different microscopic alterations, including vasogenic edema.
Other than imaging edema itself, in vivo markers of molecular actors, such as AQP4, could be of great interest in regard to its important roles that could be therapeutically targeted [104]. Our group was the first to suggest that diffusion-weighted imaging could be a marker of not only edema (and cell density), but also of AQP4 expression reflecting the intensity of transmembrane water passage through AQP4 [105]. Badaut et al. [106] confirmed the direct relationship between AQP4 expression and ADC measurements with AQP4 inhibition experiments. Further studies are needed to understand if a subcomponent of the diffusion signal (with diffusion spectrum analysis) could more specifically reflect AQP4 expression in prospect of its monitoring in vivo.
Relevance to MS and its Therapy
Support in Diagnosis
Early diagnosis after a clinically isolated syndrome can be achieved through the definition of MR criteria to show disease dissemination in space and time [3]. Current MR markers of active inflammation are especially relevant in this strategy. In particular, the coexistence of Gd-enhancing and nonenhancing lesions can be considered as indicative of dissemination in time and can prompt a diagnosis after single MR imaging [107]. Such early diagnosis allows early treatment and can slow progression to clinically definite MS [108], but also the long-term progression of the disease [109]. Alternative strategies (previously described) to assess disease activity with better sensitivity and specificity than Gd could improve the detection of active lesions and still accelerate diagnosis with potential impact in terms of therapy.
Insights into Pathogenesis
MR markers of active inflammation can improve our understanding of plaque formation, which in turn can help to define new therapeutic targets and to understand drug mechanisms of action [110]. For example, Gd has helped to identify the pivotal role of the BBB in lesion formation [1]. More recent analyses helped to further our understanding of lesion development showing results consistent with an expanding wave of inflammation recruiting additional vessels outward from a central vein [111]. Thanks to serial monitoring of individual lesions with a duration of time with new markers, it was possible to visualize endothelial activation with VCAM1 expression [33] and cellular infiltration in advance of BBB breakdown [52]. This has helped to identify parts of the cascade to therapeutically target the earliest events. More sensitive markers may further help to find new targets. For example, Gf has demonstrated for the first time in vivo the involvement of circumventricular organs during EAE, assuming that the function of the circumventricular organs “gates to the brain” for immune cells can be altered in MS pathophysiology [112]. Sensitive and specific markers of inflammation are also needed to address the complex relationship between inflammation/neurodegeneration and disability, and markers with specificity toward the most aggressive component of inflammation could aid in understanding how a significant amount of inflammation could be needed to expose the degenerative tendency [113].
Monitoring Therapy
Gd enhancement, the current imaging marker of disease activity (together with the identification of new or enlarged T2 lesions [114]), has been widely used as the primary endpoint in phase II trials (surrogate marker) regarding its capacity to measure treatment efficacy earlier, more conveniently, and more sensitively than with the clinical evaluations that are used in phase III trials [1]. Such screening of potential treatment using MR surrogate markers usually results in an important reduction of the trial duration and the size of the samples while allowing the investigators to anticipate the clinical effect of a given treatment [115]. Meta-analysis of randomized, placebo-controlled, clinical trials of relapsing-remitting MS found that the effects of therapies on such MR markers correlate with the effects on relapses [116] and, to a lesser degree, with Expanded Disability Status Scale (EDSS) [117], demonstrating the usefulness of MR imaging to monitor treatment and anticipate their clinical impact. Nevertheless, the use of Gd as a surrogate marker is debated [118], as other studies have shown that the relationship between Gd enhancement and the relapse rate was only marginal [119, 120], thus concluding that the criteria for Gd surrogacy was not fulfilled. The weak correlation found so far between Gd enhancement and disease progression is problematic when it comes to following therapeutic efficacy [119]. This may at least partially result from the lack of sensitivity and, more than anything, the lack of specificity of Gd for the most destructive aspects of active neuroinflammation. Alternative markers previously discussed could overcome some limitations of Gd to screen treatment efficacy in preclinical development, and in the early steps of clinical development, through their increased sensitivity and specificity to neuroinflammation. As a proof of principle, USPIO [45, 121, 122] and the MPO sensor [123] have been efficiently used to monitor treatment efficacy in EAE animal models and PFCs have been also used to monitor anti-inflammatory therapy, but not in the brain so far [62].
For day-to-day decisions regarding the treatment of an individual, the strategy is mainly based on the clinical status of the patient, but MR criteria are also used, especially in escalating immunotherapy, to select nonresponsive patients with a very active MS [124]. The greater availability of alternative treatment options reviewed in this issue of Neurotherapeutics will warrant methods to determine individual treatment efficacy. Although repetitive MR follow-up has been the focus of criticism, particularly in stable patients [119], additional MR (surrogate) markers for disease activity are helpful when an insufficient treatment response is clinically suspected [124], and USPIO or other markers could aid in determining responsiveness and therefore personalize treatment decisions.
Translation to Clinical Use
Most Gd alternative strategies to assess active neuroinflammation are based on novel contrast agents and translational to human and clinical practice requires full toxicological testing. To date, iron oxide nanoparticles are the most advanced, as some particles are already approved for applications other than brain imaging. SPIO, such as ferumoxides (Endorem®, Guerbet in Europe and Feridex®, AMAG Pharmaceuticals Inc. in the USA and Japan), are approved by the United States Food and Drug Administration for MR imaging of liver tumors [125] and USPIO ferumoxytol (Feraheme, AMAG Pharmaceutical Inc.) is also Food and Drug Administration-approved for iron replacement therapy in patients with chronic renal failure. Another USPIO, ferumoxtran-10 (Sinerem, Guerbet, Europe, also known as Combidex, AMAG Pharmaceuticals Inc., USA and Japan), is still investigational, although it has completed phase III trials [126] and its safety profile assessed from pooled clinical studies in 1777 adults did not show major adverse effects [127]. Despite this, there is still no authorization for iron oxide nanoparticles use outside the experimental setting specifically for CNS exploration. There are some ongoing concerns; the first concern are the functional consequences of cell labeling by iron oxide nanoparticles. It seems that uptake of the contrast agents by macrophages is not associated with cell activation [128], but the labeling of other cell types than macrophages could change their physiological properties [129]. The second concern is for the long-term fate of iron carried by nanoparticles in the brain, as could be argued that any additional iron introduction could be pejorative in the context of neuroinflammation [130]. We found no evidence of a prolonged increase of iron in USPIO-enhanced lesions as a T1 or T2* effect was no longer detectable at 6 months (before USPIO re-injection) in lesions that were enhanced at baseline [53]. Nevertheless, we are aware that a more sensitive method is required to address the issue of long-term iron increases in USPIO-enhanced lesions. Furthermore, although there is no evidence of any adverse effects of USPIO on iron metabolism parameters [127], more work is needed to investigate this issue in more detail. For the other agents, PFCs have not been licensed yet for human use in 19F-MRI, but certain types of PFC have already been clinically evaluated as artificial blood substitutes, and these were well-tolerated, [131]. Similarly, MPO sensors were only used in animal studies, but these Gd-based contrast agents showed high stability and low toxicity [60], suggesting suitability for translational into human studies. Other MR strategies with Gf or MPIO cross-linked agents are farther away from human use, despite elegant animal proof-of-concept studies [19, 31], as there lacks data for their nontoxicity and biodegradable properties. PET tracers [74] can usually be applied in humans more rapidly due to the very small efficient doses and their absence of toxicity (certain macrophage/microglia tracers have already been used in humans [74]). Nevertheless, the PET technique may have restricted usefulness in MS because of the related cost and the need to limit radioisotope exposure. In this context, the MR noncontrast approach or the evolution toward higher magnetic fields previously described could become popular.
Conclusion
Gd is the current reference standard to assess the inflammatory component of MS. Regarding the complex cascade of events resulting in the formation of an acute inflammatory lesion such as cell recruitment and edema formation, other markers of active inflammation described in this review are being developed. At the same time, markers of the consequences of acute inflammatory, such as matrix digestion by metalloproteinase, acute demyelination, and axonal injury, are under investigation. These MR and PET markers of neuroinflammation will join the growing toolbox to assess the neurodegenerative component of MS, especially axonal loss and incomplete remyelination. The combination of these approaches could provide in vivo sensitive and specific assessment of MS components to reveal a more complete picture of MS that will increase our diagnostic performance, our understanding of pathophysiology, and our ability to accurately follow the course of the disease with ultimate consequences for therapeutic development and monitoring.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
We thank Philip Robinson for critical reading of the manuscript. This work was supported by ARSEP Foundation, “Fondation pour l’aide à la recherche sur la Sclérose en Plaques” and the Translational Research and Advanced Imaging Laboratory (TRAIL) laboratory of excellence at Bordeaux University to T.T. We declare no real or perceived conflict of interest. Full conflict of interest disclosures are available in the electronic supplementary material for this article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Filippi M, Rocca MA. MR imaging of multiple sclerosis. Radiology. 2011;259:659–681. doi: 10.1148/radiol.11101362. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi R, Thompson AJ, Rocca MA, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7:615–625. doi: 10.1016/S1474-4422(08)70137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DH, Altmann DR, Chard DT. Advances in imaging to support the development of novel therapies for multiple sclerosis. Clin Pharmacol Ther. 2012;91:621–634. doi: 10.1038/clpt.2011.349. [DOI] [PubMed] [Google Scholar]
- 5.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 6.Lovblad KO, Anzalone N, Dorfler A, et al. MR imaging in multiple sclerosis: review and recommendations for current practice. AJNR Am J Neuroradiol. 2010;31:983–989. doi: 10.3174/ajnr.A1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frohman EM, Racke MK, Raine CS. Multiple sclerosis — the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 8.Waubant E. Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. Dis Markers. 2006;22:235–244. doi: 10.1155/2006/709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yednock TA, Cannon C, Fritz LC, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 10.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 11.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol. 2008;115:635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 12.Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol. 2007;114:459–469. doi: 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins CP, Munro PM, MacKenzie F, et al. Duration and selectivity of blood-brain barrier breakdown in chronic relapsing experimental allergic encephalomyelitis studied by gadolinium-DTPA and protein markers. Brain. 1990;113(pt 2):365–378. doi: 10.1093/brain/113.2.365. [DOI] [PubMed] [Google Scholar]
- 14.Soon D, Tozer DJ, Altmann DR, Tofts PS, Miller DH. Quantification of subtle blood-brain barrier disruption in non-enhancing lesions in multiple sclerosis: a study of disease and lesion subtypes. Mult Scler. 2007;13:884–894. doi: 10.1177/1352458507076970. [DOI] [PubMed] [Google Scholar]
- 15.Bradl M, Lassmann H. Progressive multiple sclerosis. Semin Immunopathol. 2009;31:455–465. doi: 10.1007/s00281-009-0182-3. [DOI] [PubMed] [Google Scholar]
- 16.Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol. 2007;33:86–98. doi: 10.1111/j.1365-2990.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 17.Misselwitz B, Platzek J, Weinmann HJ. Early MR lymphography with gadofluorine M in rabbits. Radiology. 2004;231:682–688. doi: 10.1148/radiol.2313021000. [DOI] [PubMed] [Google Scholar]
- 18.Meding J, Urich M, Licha K, et al. Magnetic resonance imaging of atherosclerosis by targeting extracellular matrix deposition with Gadofluorine M. Contrast Media Mol Imaging. 2007;2:120–129. doi: 10.1002/cmmi.137. [DOI] [PubMed] [Google Scholar]
- 19.Bendszus M, Ladewig G, Jestaedt L, et al. Gadofluorine M enhancement allows more sensitive detection of inflammatory CNS lesions than T2-w imaging: a quantitative MRI study. Brain. 2008;131:2341–2352. doi: 10.1093/brain/awn156. [DOI] [PubMed] [Google Scholar]
- 20.Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wuerfel J, Tysiak E, Prozorovski T, et al. Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur J Neurosci. 2007;26:190–198. doi: 10.1111/j.1460-9568.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 23.Tysiak E, Asbach P, Aktas O, et al. Beyond blood brain barrier breakdown — in vivo detection of occult neuroinflammatory foci by magnetic nanoparticles in high field MRI. J Neuroinflammation. 2009;6:20. doi: 10.1186/1742-2094-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grenier N, Brader P. Principles and basic concepts of molecular imaging. Pediatr Radiol. 2011;41:144–160. doi: 10.1007/s00247-010-1835-z. [DOI] [PubMed] [Google Scholar]
- 25.Sibson NR, Blamire AM, Bernades-Silva M, et al. MRI detection of early endothelial activation in brain inflammation. Magn Reson Med. 2004;51:248–252. doi: 10.1002/mrm.10723. [DOI] [PubMed] [Google Scholar]
- 26.Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 27.Tsourkas A, Shinde-Patil VR, Kelly KA, et al. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug Chem. 2005;16:576–581. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 28.Schneider C, Schuetz G, Zollner TM. Acute neuroinflammation in Lewis rats — a model for acute multiple sclerosis relapses. J Neuroimmunol. 2009;213:84–90. doi: 10.1016/j.jneuroim.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro EM, Skrtic S, Sharer K, et al. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Yanasak N, Schumacher A, Hu TC. Temporal and noninvasive monitoring of inflammatory-cell infiltration to myocardial infarction sites using micrometer-sized iron oxide particles. Magn Reson Med. 2010;63:33–40. doi: 10.1002/mrm.22175. [DOI] [PubMed] [Google Scholar]
- 31.McAteer MA, Sibson NR. von Zur Muhlen C, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med. 2007;13:1253–1258. doi: 10.1038/nm1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serres S, Anthony DC, Jiang Y, et al. Systemic inflammatory response reactivates immune-mediated lesions in rat brain. J Neurosci. 2009;29:4820–4828. doi: 10.1523/JNEUROSCI.0406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serres S, Mardiguian S, Campbell SJ, et al. VCAM-1-targeted magnetic resonance imaging reveals subclinical disease in a mouse model of multiple sclerosis. Faseb J. 2011;25:4415–4422. doi: 10.1096/fj.11-183772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008;b20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 37.Petry KG, Boiziau C, Dousset V, Brochet B. Magnetic resonance imaging of human brain macrophage infiltration. Neurotherapeutics. 2007;4:434–442. doi: 10.1016/j.nurt.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckmann N, Cannet C, Babin AL, et al. In vivo visualization of macrophage infiltration and activity in inflammation using magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:272–298. doi: 10.1002/wnan.16. [DOI] [PubMed] [Google Scholar]
- 39.Bendszus M, Stoll G. Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging. J Neurosci. 2003;23:10892–10896. doi: 10.1523/JNEUROSCI.23-34-10892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engberink RD, Blezer EL, Hoff EI, et al. MRI of monocyte infiltration in an animal model of neuroinflammation using SPIO-labeled monocytes or free USPIO. J Cereb Blood Flow Metab. 2008;28:841–851. doi: 10.1038/sj.jcbfm.9600580. [DOI] [PubMed] [Google Scholar]
- 41.Oude Engberink RD, Blezer EL, Dijkstra CD, et al. Dynamics and fate of USPIO in the central nervous system in experimental autoimmune encephalomyelitis. NMR Biomed. 2010;23:1087–1096. doi: 10.1002/nbm.1536. [DOI] [PubMed] [Google Scholar]
- 42.Anderson SA, Shukaliak-Quandt J, Jordan EK, et al. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann Neurol. 2004;55:654–659. doi: 10.1002/ana.20066. [DOI] [PubMed] [Google Scholar]
- 43.Stoll G, Bendszus M. Imaging of inflammation in the peripheral and central nervous system by magnetic resonance imaging. Neuroscience. 2009;158:1151–1160. doi: 10.1016/j.neuroscience.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 44.Dousset V, Delalande C, Ballarino L, et al. In vivo macrophage activity imaging in the central nervous system detected by magnetic resonance. Magn Reson Med. 1999;41:329–333. doi: 10.1002/(SICI)1522-2594(199902)41:2<329::AID-MRM17>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Floris S, Blezer EL, Schreibelt G, et al. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain. 2004;127:616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 46.Dousset V, Ballarino L, Delalande C, et al. Comparison of ultrasmall particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis. AJNR Am J Neuroradiol. 1999;20:223–227. [PMC free article] [PubMed] [Google Scholar]
- 47.Rausch M, Hiestand P, Baumann D, Cannet C, Rudin M. MRI-based monitoring of inflammation and tissue damage in acute and chronic relapsing EAE. Magn Reson Med. 2003;50:309–314. doi: 10.1002/mrm.10541. [DOI] [PubMed] [Google Scholar]
- 48.Chin CL, Pai M, Bousquet PF, et al. Distinct spatiotemporal pattern of CNS lesions revealed by USPIO-enhanced MRI in MOG-induced EAE rats implicates the involvement of spino-olivocerebellar pathways. J Neuroimmunol. 2009;211:49–55. doi: 10.1016/j.jneuroim.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Baeten K, Hendriks JJ, Hellings N, et al. Visualisation of the kinetics of macrophage infiltration during experimental autoimmune encephalomyelitis by magnetic resonance imaging. J Neuroimmunol. 2008;195:1–6. doi: 10.1016/j.jneuroim.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Ladewig G, Jestaedt L, Misselwitz B, et al. Spatial diversity of blood-brain barrier alteration and macrophage invasion in experimental autoimmune encephalomyelitis: a comparative MRI study. Exp Neurol. 2009;220:207–211. doi: 10.1016/j.expneurol.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 51.Dousset V, Brochet B, Deloire MS, et al. MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am J Neuroradiol. 2006;27:1000–1005. [PMC free article] [PubMed] [Google Scholar]
- 52.Vellinga MM, Oude Engberink RD, Seewann A, et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain 2008;131:800-807. [DOI] [PubMed]
- 53.Tourdias T, Roggerone S, Filippi M, et al. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2012;264:225–233. doi: 10.1148/radiol.12111416. [DOI] [PubMed] [Google Scholar]
- 54.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vellinga MM, Vrenken H, Hulst HE, et al. Use of ultrasmall superparamagnetic particles of iron oxide (USPIO)-enhanced MRI to demonstrate diffuse inflammation in the normal-appearing white matter (NAWM) of multiple sclerosis (MS) patients: an exploratory study. J Magn Reson Imaging. 2009;29:774–779. doi: 10.1002/jmri.21678. [DOI] [PubMed] [Google Scholar]
- 56.Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;c32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 57.Brochet B, Deloire MS, Touil T, et al. Early macrophage MRI of inflammatory lesions predicts lesion severity and disease development in relapsing EAE. Neuroimage. 2006;32:266–274. doi: 10.1016/j.neuroimage.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 58.Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. [PubMed] [Google Scholar]
- 59.Chen JW. Querol Sans M, Bogdanov A Jr., Weissleder R. Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates. Radiology. 2006;240:473–481. doi: 10.1148/radiol.2402050994. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez E, Nilges M, Weissleder R, Chen JW. Activatable magnetic resonance imaging agents for myeloperoxidase sensing: mechanism of activation, stability, and toxicity. J Am Chem Soc. 2010;132:168–177. doi: 10.1021/ja905274f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JW, Breckwoldt MO, Aikawa E, Chiang G, Weissleder R. Myeloperoxidase-targeted imaging of active inflammatory lesions in murine experimental autoimmune encephalomyelitis. Brain. 2008;131:1123–1133. doi: 10.1093/brain/awn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoll G, Basse-Lusebrink T, Weise G, Jakob P. Visualization of inflammation using (19) F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:438–447. doi: 10.1002/wnan.1168. [DOI] [PubMed] [Google Scholar]
- 63.Janjic JM, Ahrens ET. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flogel U, Ding Z, Hardung H, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bulte JW. Hot spot MRI emerges from the background. Nat Biotechnol. 2005;23:945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 66.Weise G, Basse-Luesebrink TC, Wessig C, Jakob PM, Stoll G. In vivo imaging of inflammation in the peripheral nervous system by (19)F MRI. Exp Neurol. 2011;229:494–501. doi: 10.1016/j.expneurol.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 67.Weise G, Basse-Lusebrink TC, Kleinschnitz C, et al. In vivo imaging of stepwise vessel occlusion in cerebral photothrombosis of mice by 19F MRI. PLoS One. 2011;6:e28143. doi: 10.1371/journal.pone.0028143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denic A, Johnson AJ, Bieber AJ, et al. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64:707–713. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 71.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602–3615. doi: 10.1093/brain/awr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bian W, Harter K, Hammond-Rosenbluth KE, et al. A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler 2012. doi:10.1177/1352458512447870 [DOI] [PubMed]
- 73.Banati RB. Visualising microglial activation in vivo. Glia. 2002;40:206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- 74.Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia 2012. doi:10.1002/glia.22357 [DOI] [PMC free article] [PubMed]
- 75.Banati RB, Newcombe J, Gunn RN, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(pt 11):2321–2337. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- 76.Debruyne JC, Versijpt J, Van Laere KJ, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10:257–264. doi: 10.1046/j.1468-1331.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- 77.Versijpt J, Debruyne JC, Van Laere KJ, et al. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler. 2005;11:127–134. doi: 10.1191/1352458505ms1140oa. [DOI] [PubMed] [Google Scholar]
- 78.Politis M, Giannetti P, Su P, et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology. 2012;79:523–530. doi: 10.1212/WNL.0b013e3182635645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 80.Kiferle L, Politis M, Muraro PA, Piccini P. Positron emission tomography imaging in multiple sclerosis-current status and future applications. Eur J Neurol. 2011;18:226–231. doi: 10.1111/j.1468-1331.2010.03154.x. [DOI] [PubMed] [Google Scholar]
- 81.Abourbeh G, Theze B, Maroy R, et al. Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [(1)(8)F]DPA-714. J Neurosci. 2012;32:5728–5736. doi: 10.1523/JNEUROSCI.2900-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nag S, Manias JL, Stewart DJ. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009;118:197–217. doi: 10.1007/s00401-009-0541-0. [DOI] [PubMed] [Google Scholar]
- 83.cLucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 2008;131:1759-1775. [DOI] [PMC free article] [PubMed]
- 84.Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Saadoun S, Papadopoulos MC. Aquaporin-4 in brain and spinal cord oedema. Neuroscience. 2010;168:1036–1046. doi: 10.1016/j.neuroscience.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Tourdias T, Mori N, Dragonu I, et al. Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J Neuroinflammation. 2011;8:143. doi: 10.1186/1742-2094-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saadoun S, Papadopoulos MC, Watanabe H, et al. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 88.Auguste KI, Jin S, Uchida K, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. Faseb J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 89.Li L, Zhang H, Verkman AS. Greatly attenuated experimental autoimmune encephalomyelitis in aquaporin-4 knockout mice. BMC Neurosci. 2009;10:94. doi: 10.1186/1471-2202-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. Faseb J. 2011;25:1556–1566. doi: 10.1096/fj.10-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratelade J, Verkman AS. Neuromyelitis optica: Aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol 2012;44:1519-30. [DOI] [PMC free article] [PubMed]
- 92.Shinohara RT, Goldsmith J, Mateen FJ, Crainiceanu C, Reich DS. Predicting breakdown of the blood-brain barrier in multiple sclerosis without contrast agents. AJNR Am J Neuroradiol 2012;33:1586-90. [DOI] [PMC free article] [PubMed]
- 93.Ropele S, Langkammer C, Enzinger C, Fuchs S, Fazekas F. Relaxation time mapping in multiple sclerosis. Expert Rev Neurother. 2011;11:441–450. doi: 10.1586/ern.10.129. [DOI] [PubMed] [Google Scholar]
- 94.Laule C, Vavasour IM, Moore GR, et al. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J Neurol. 2004;251:284–293. doi: 10.1007/s00415-004-0306-6. [DOI] [PubMed] [Google Scholar]
- 95.Vavasour IM, Laule C, Li DK, et al. Longitudinal changes in myelin water fraction in two MS patients with active disease. J Neurol Sci. 2009;276:49–53. doi: 10.1016/j.jns.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 96.MacKay AL, Vavasour IM, Rauscher A, et al. MR relaxation in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:1–26. doi: 10.1016/j.nic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 98.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol. 1999;20:1491–1499. [PMC free article] [PubMed] [Google Scholar]
- 99.Le Bihan D. The "wet mind": water and functional neuroimaging. Phys Med Biol. 2007;52:R57–R90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- 100.Balashov KE, Lindzen E. Acute demyelinating lesions with restricted diffusion in multiple sclerosis. Mult Scler 2012. doi:10.1177/1352458512445407 [DOI] [PMC free article] [PubMed]
- 101.Lucchinetti C, Bruck W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::AID-ANA3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 102.Lassmann H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci. 2003;206:187–191. doi: 10.1016/S0022-510X(02)00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tourdias T, Hiba B, Raffard G, et al. Adapted focal experimental autoimmune encephalomyelitis to allow MRI exploration of multiple sclerosis features. Exp Neurol. 2011;230:248–257. doi: 10.1016/j.expneurol.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 104.Papadopoulos MC, Verkman AS. Potential utility of aquaporin modulators for therapy of brain disorders. Prog Brain Res. 2008;170:589–601. doi: 10.1016/S0079-6123(08)00446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tourdias T, Dragonu I, Fushimi Y, et al. Aquaporin 4 correlates with apparent diffusion coefficient and hydrocephalus severity in the rat brain: a combined MRI-histological study. Neuroimage. 2009;47:659–666. doi: 10.1016/j.neuroimage.2009.04.070. [DOI] [PubMed] [Google Scholar]
- 106.Badaut J, Ashwal S, Adami A, et al. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab 2011;31:819-31. [DOI] [PMC free article] [PubMed]
- 107.Rovira A, Swanton J, Tintore M, et al. A single, early magnetic resonance imaging study in the diagnosis of multiple sclerosis. Arch Neurol. 2009;66:587–592. doi: 10.1001/archneurol.2009.49. [DOI] [PubMed] [Google Scholar]
- 108.Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007;370:389–397. doi: 10.1016/S0140-6736(07)61194-5. [DOI] [PubMed] [Google Scholar]
- 109.Trojano M, Pellegrini F, Fuiani A, et al. New natural history of interferon-beta-treated relapsing multiple sclerosis. Ann Neurol. 2007;61:300–306. doi: 10.1002/ana.21102. [DOI] [PubMed] [Google Scholar]
- 110.Yong VW. Differential mechanisms of action of interferon-beta and glatiramer aetate in MS. Neurology. 2002;59:802–808. doi: 10.1212/WNL.59.6.802. [DOI] [PubMed] [Google Scholar]
- 111.Gaitan MI, Shea CD, Evangelou IE, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–29. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wuerfel E, Infante-Duarte C, Glumm R, Wuerfel JT. Gadofluorine M-enhanced MRI shows involvement of circumventricular organs in neuroinflammation. J Neuroinflammation. 2010;7:70. doi: 10.1186/1742-2094-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Compston A. Making progress on the natural history of multiple sclerosis. Brain. 2006;129:561–563. doi: 10.1093/brain/awl034. [DOI] [PubMed] [Google Scholar]
- 114.Bonzano L, Roccatagliata L, Mancardi GL, Sormani MP. Gadolinium-enhancing or active T2 magnetic resonance imaging lesions in multiple sclerosis clinical trials? Mult Scler. 2009;15:1043–1047. doi: 10.1177/1352458509106610. [DOI] [PubMed] [Google Scholar]
- 115.Smith JJ, Sorensen AG, Thrall JH. Biomarkers in imaging: realizing radiology's future. Radiology. 2003;227:633–638. doi: 10.1148/radiol.2273020518. [DOI] [PubMed] [Google Scholar]
- 116.Sormani MP, Bonzano L, Roccatagliata L, et al. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009;65:268–275. doi: 10.1002/ana.21606. [DOI] [PubMed] [Google Scholar]
- 117.Sormani MP, Bonzano L, Roccatagliata L, et al. Surrogate endpoints for EDSS worsening in multiple sclerosis. A meta-analytic approach. Neurology. 2010;75:302–309. doi: 10.1212/WNL.0b013e3181ea15aa. [DOI] [PubMed] [Google Scholar]
- 118.Sormani MP, Filippi M, De Stefano N, Ebers G, Daumer M. MRI as an outcome in multiple sclerosis clinical trials. Neurology. 2009;73:1932–1933. doi: 10.1212/WNL.0b013e3181bd6b8f. [DOI] [PubMed] [Google Scholar]
- 119.Daumer M, Neuhaus A, Morrissey S, Hintzen R, Ebers GC. MRI as an outcome in multiple sclerosis clinical trials. Neurology. 2009;72:705–711. doi: 10.1212/01.wnl.0000336916.38629.43. [DOI] [PubMed] [Google Scholar]
- 120.Held U, Heigenhauser L, Shang C, Kappos L, Polman C. Predictors of relapse rate in MS clinical trials. Neurology. 2005;65:1769–1773. doi: 10.1212/01.wnl.0000187122.71735.1f. [DOI] [PubMed] [Google Scholar]
- 121.Deloire MS, Touil T, Brochet B, et al. Macrophage brain infiltration in experimental autoimmune encephalomyelitis is not completely compromised by suppressed T-cell invasion: in vivo magnetic resonance imaging illustration in effective anti-VLA-4 antibody treatment. Mult Scler. 2004;10:540–548. doi: 10.1191/1352458504ms1090oa. [DOI] [PubMed] [Google Scholar]
- 122.Rausch M, Hiestand P, Foster CA, et al. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: clinical implications for ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2004;20:16–24. doi: 10.1002/jmri.20057. [DOI] [PubMed] [Google Scholar]
- 123.Forghani R, Wojtkiewicz GR, Zhang Y, et al. Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target. Radiology. 2012;263:451–460. doi: 10.1148/radiol.12111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wiendl H, Toyka KV, Rieckmann P, et al. Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J Neurol. 2008;255:1449–1463. doi: 10.1007/s00415-008-0061-1. [DOI] [PubMed] [Google Scholar]
- 125.Ros PR, Freeny PC, Harms SE, et al. Hepatic MR imaging with ferumoxides: a multicenter clinical trial of the safety and efficacy in the detection of focal hepatic lesions. Radiology. 1995;196:481–488. doi: 10.1148/radiology.196.2.7617864. [DOI] [PubMed] [Google Scholar]
- 126.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 127.Bernd H, De Kerviler E, Gaillard S, Bonnemain B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest Radiol. 2009;44:336–342. doi: 10.1097/RLI.0b013e3181a0068b. [DOI] [PubMed] [Google Scholar]
- 128.Hsiao JK, Chu HH, Wang YH, et al. Macrophage physiological function after superparamagnetic iron oxide labeling. NMR Biomed. 2008;21:820–829. doi: 10.1002/nbm.1260. [DOI] [PubMed] [Google Scholar]
- 129.Schafer R, Ayturan M, Bantleon R, et al. The use of clinically approved small particles of iron oxide (SPIO) for labeling of mesenchymal stem cells aggravates clinical symptoms in experimental autoimmune encephalomyelitis and influences their in vivo distribution. Cell Transplant. 2008;17:923–941. doi: 10.3727/096368908786576480. [DOI] [PubMed] [Google Scholar]
- 130.Levine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2004;1012:252–266. doi: 10.1196/annals.1306.021. [DOI] [PubMed] [Google Scholar]
- 131.Riess JG. Perfluorocarbon-based oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)