Abstract
The gut is an important target organ for stress caused by severe insults such as sepsis, trauma, burn, shock, bleeding and infection. Severe insult to the gut is considered to have an important role in promoting infectious complications and multiple organ dysfunction syndrome. These are sequelae of interactions between deteriorated intestinal epithelium, the immune system and commensal bacteria. The gut is the “motor” of multiple organ failure, and now it is recognized that gut dysfunction is a causative factor in disease progression. The gut flora and environment are significantly altered in critically ill patients, and the number of obligate anaerobes is associated with prognosis. Synbiotic therapy is a combination of probiotics and prebiotics. Probiotic, prebiotic and synbiotic treatment has been shown to be a promising therapy to maintain and repair the gut microbiota and gut environment. In the critically ill, such as major abdominal surgery, trauma and ICU patients, synbiotic therapy has been shown to significantly reduce septic complications. Further basic and clinical research would clarify the underlying mechanisms of the therapeutic effect of probiotic/synbiotic treatment and define the appropriate conditions for use.
Keywords: Gut, Flora, Systemic inflammatory response syndrome, Short-chain fatty acids, Probiotics, Selective digestive decontamination, Motility
Introduction
The gut is an important target organ for various kinds of stress caused by severe insult such as sepsis, trauma, burn, shock, bleeding and infection [1]. Severe insult to the gut is considered to have an important role in promoting infectious complications and multiple organ dysfunction syndrome. These infectious complications and organ dysfunctions are related to factors including deteriorated intestinal epithelium, the immune system and commensal bacteria [2]. The gut is the “motor” of multiple organ failure, and now, gut dysfunction is recognized as a causative factor in the progression of diseases. However, neither the guidelines of the nutrition field nor those for the Surviving Sepsis Campaign have yet described a standard digestive tract treatment. Characterization of the intestinal microbiota and how alterations in the composition of the intestinal microbiota may be related to various clinical complications in critically ill patients is needed to provide a basis for such therapeutic recommendations [3, 4]. This review article summarizes some of the clinical findings on the characterization of the intestinal microbiota and the clinical outcomes of the application of probiotic and synbiotic therapy in intensive care unit (ICU) patients.
Gut Microbiota and Gut Immunity
The gut is the largest immune organ of the human body. The gut defense function involves three main components: intestinal flora, intestinal epithelium and the immune system in the gut [5]. The intestinal microbiota is widely acknowledged to play an important role in human health [6, 7]. Commensal gut flora has important and specific functions in metabolism, nutrition and protection against pathogens. The equilibrium between species of indigenous bacteria provides stability of the microbial population and maintenance of health within an individual under normal conditions. Distortions in the composition of this bacterial community or impaired homeostasis are often associated with pathological conditions. Previous animal studies have also shown a very low incidence of bacterial translocation when obligate anaerobes are maintained in the gut, suggesting that obligate anaerobic bacteria are the principal inhibitors of bacterial overgrowth and translocation of Escherichia coli and other potentially pathogenic bacteria [8]. This phenomenon is called “colonization resistance” [9]. The decreased obligate total anaerobic bacteria count could lead to decreased intestinal resistance to pathogens in critically ill patients.
The immune system in the gut (the gut-associated lymphoid tissue, GALT) is integral to the protection of the host because of its ability to distinguish between harmless antigens (food, commensal bacteria) and potential pathogens or harmful substances, as well as its influence and link with the systemic arm of the immune system [10, 11]. Maintaining the balance of gut flora is an important activity of the immune system and vice versa. Recent studies have shown that the human intestinal microflora contains at least 100 times as many genes of bacteria as the human genome and that humans can be “superorganisms,” whereby metabolism involves an amalgamation of microbial and human activities [12]. In this mutualistic relationship, there is immunological tolerance of many bacteria. In return, the commensal flora promotes colonization resistance against invasive pathogenic microbes. The importance of commensal gut flora to the immune system is exemplified by the recently developed germ-free mouse model. These mice not only have smaller Peyer’s patches and lamina propria but also a decrease of T cell and many other immune functions [13]. The role of the microbiota in the immune system has recently become better understood. Ivanov et al. [14] revealed that segmented filamentous bacteria induce Th17 cells. Atarashi et al. [15] revealed that Clostridium induces regulatory T cells. Dysbiosis of these bacteria may affect autoimmune diseases in animal studies [16]. Thus, the gut microbiota would help to shape the balance of immune regulatory (Treg) and proinflammatory (Th17) cells and modulate the immune status for the adaptive immune system [17]. These reports indicate that commensal gut bacteria have an important role in maintaining homeostasis. The delicate balance of commensal gut flora can be disrupted by invasive microorganisms that elicit a strong innate immune response resulting in an inflammatory reaction that leads to destruction of the intestinal barrier [18]. Impairment of the GALT can lead to increased susceptibility to infection, auto-immunity, allergy and excessive inflammation.
Short-chain fatty acids (SCFAs) consist of acetic, propionic and n-butyric acids with 2–4 carbon atoms [19]. Anaerobic metabolism of peptides and proteins by the microflora produces SCFAs that all have important functions in host physiology. SCFAs production by intestinal bacteria is regulated by many different host-related, environmental, dietary, and microbiological factors, such as substrate availability, bacterial species and composition of the microbiota [20]. SCFAs are utilized mainly by intestinal epithelial cells as energy substrates, and some are absorbed into the portal flow to the liver and utilized as systemic energy sources. They also affect the motility of the intestinal tract and increase intestinal blood flow. Especially, butyrate plays an important role in gene expression and possesses anti-inflammatory activity (e.g., inhibition of NF-κB, IL-12, and TNF-α) and increases in IL-10 [21]. SCFAs bind to the G-protein-coupled-receptor 43 (GPR43). Maslowski et al. [22] reported that GPR43-deficient mice showed exacerbated inflammation in models of colitis, arthritis and asthma. The stimulation of GPR43 by SCFAs therefore affects inflammatory response.
Gastrointestinal pH also has a significant impact on bacterial flora, absorption of vitamins and electrolytes and the activity of digestive enzymes [23]. In the stomach, the pH ranges from 1 to 3.5 during fasting, whereas ingestion of food, milk, or antacids may briefly increase pH to approximately 7. In the small intestine, pH increases to 5.5–6.5. This alkalization is probably due to secretion of bicarbonate and bile acids. The pH in the ascending colon is relatively low at around 5.6. This decrease from the ileum is mostly due to bacterial fermentation of non-digestible carbohydrates to SCFAs, which are weak acids. This change is caused by bicarbonate secreted into the colon in exchange with the uptake of SCFAs.
Characterization of Gut Microbiota and Prognosis in Severe Systemic Inflammatory Response Syndrome Patients: A Possible Prognostic Indicator for Complications?
Systemic inflammatory response syndrome (SIRS) is defined as the presence of two or more of the following conditions: abnormal body temperature, heart rate, respiratory rate and white blood cells counts [24]. SIRS is not a specific disease, but a syndrome that develops various kinds of critical illness. Some of the possible mechanisms have been developed from the viewpoint of gut pathobiology. First, bacterial translocation due to the loss of gut barrier function can cause systemic effects from bacteria and related toxins. Second, intestinal lymphatic mediators can cause excessive activation of neutrophils, endothelial injury and organ damage [25]. Third, gut immunity plays an important role in causing the imbalance between systemic inflammation and anti-inflammation [26].
Under conditions of critical illness, it is difficult to maintain normal gut flora. This is due not only to disease stresses such as trauma and burn but also to various invasive treatments, such as histamine H2 receptor blockers for bleeding prevention, catecholamines for blood pressure control, broad-spectrum antibiotics for target bacteria and mechanical respirators. Shimizu et al. [27] quantitatively evaluated the gut microflora and environmental changes in patients with severe SIRS. As shown in Table 1, severe SIRS patients had 100–10,000 times fewer total anaerobes, including “beneficial” Bifidobacterium and Lactobacillus, and 100 times more “pathogenic” Staphylococcus bacteria compared with healthy volunteers. These data demonstrated the disturbed balance of gut flora in critically ill patients. Total organic acids, acetic acid and butyric acid were significantly decreased in the SIRS patients when compared with healthy volunteers (Table 2). Butyrate, in particular, was almost diminished in the gut in critically ill conditions. Fecal pH was markedly increased in patients with severe SIRS in comparison with healthy volunteers (P < 0.05). These results showed the deterioration of the gut environment in the progression of SIRS.
Table 1.
Fecal flora in patients with severe systemic inflammatory response syndrome (SIRS)
| Fecal flora | SIRS patients | Normal |
|---|---|---|
| Total obligate anaerobes | 8.3 ± 2.3* | 10.5 ± 0.5 |
| Bacteroidaceae | 7.3 ± 3.0* | 10.1 ± 0.4 |
| Bifidobacterium | 4.8 ± 3.3* | 9.6 ± 0.7 |
| Clostridium | 2.1 ± 1.0 | 2.1 ± 0.7 |
| Veillonella | 3.1 ± 1.8* | 7.0 ± 1.2 |
| Total facultative anaerobes | 7.8 ± 1.4 | 7.5 ± 0.4 |
| Lactobacillus | 2.7 ± 1.5* | 5.0 ± 1.0 |
| Enterobacteriaceae | 4.1 ± 2.7* | 7.4 ± 0.8 |
| Enterococcus | 6.4 ± 2.5 | 7.0 ± 0.9 |
| Staphylococcus | 5.3 ± 1.7* | 2.7 ± 0.8 |
| Pseudomonas | 2.8 ± 1.4* | ND |
| Candida | 2.5 ± 1.0 | 2.0 ± 0.5 |
ND not detected, SD standard deviation
Log10counts/g feces. Data is given as mean ± SD
* P < 0.05 versus normal;
Table 2.
Fecal organic acid concentrations and pH in patients with severe systemic inflammatory response syndrome (SIRS)
| Organic acids | SIRS patients | Normal |
|---|---|---|
| Total organic acid | 30.3 ± 20.3* | 88.4 ± 21.2 |
| Succinic acid | 2.0 ± 2.5 | 0.9 ± 1.2 |
| Lactic acid | 3.8 ± 5.5 | 0.5 ± 0.3 |
| Formic acid | 1.7 ± 2.9 | 0.4 ± 0.3 |
| Acetic acid | 18.7 ± 15.9* | 50.8 ± 13.1 |
| Propionic acid | 2.5 ± 4.6* | 18.7 ± 6.8 |
| Isobutyric acid | 0.1 ± 0.5 | 1.1 ± 0.3 |
| Butyric acid | 0.9 ± 2.3* | 16.6 ± 6.7 |
| Isovaleric acid | 0.5 ± 1.9 | 1.4 ± 0.7 |
| Valeric acid | 0.1 ± 0.7 | 0.6 ± 0.4 |
| pH | 7.4 ± 0.6* | 6.6 ± 0.3 |
SD standard deviation
Organic acid (μ mol/g feces). Data as mean ± SD
* P < 0.05 versus normal
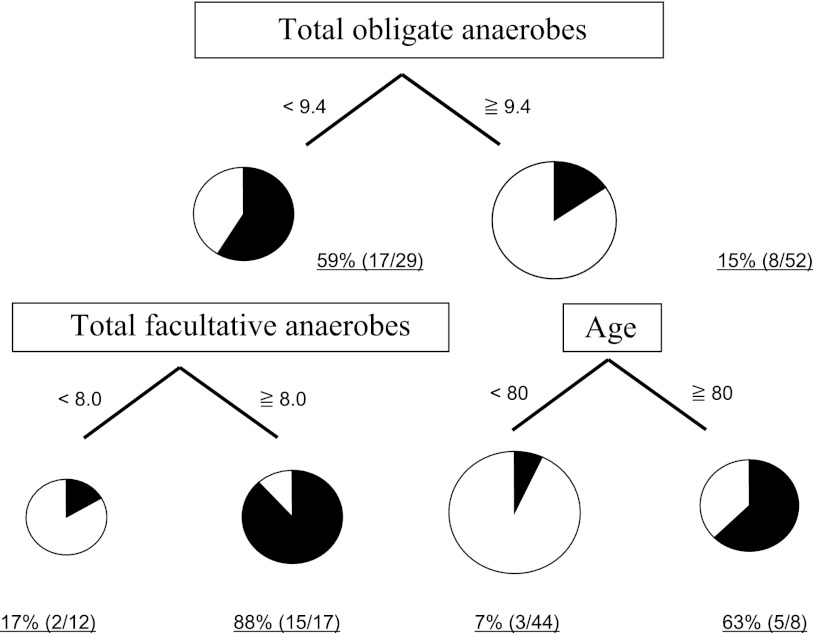
Of the many kinds of bacteria in the gut, the dominant factors for mortality were the numbers of total obligate anaerobes and total facultative anaerobes. In addition to the increase in pathogenic bacteria, the alteration of normal gut flora could be of great importance for the development of septic complications and mortality (Fig. 1). These results suggest that not only antibiotics but also probiotics that target normal gut flora would be an appropriate treatment [28]. Notably, in some clinical studies, the gut flora was maintained and improved by the administration of probiotics and synbiotics [29, 30]. These clinical changes were observed by anaerobic culture, RT-PCR and genome sequence methods [31, 32]. Gram-stained fecal bacteria can also be used as a quick bedside diagnostic marker for severe SIRS patients [33].
Fig. 1.
Mortality partitioned by total obligate anaerobes, total facultative anaerobes, and age using CART. Mortality is partitioned by the number of total obligate anaerobes 9.4 (log10CFU/g). In addition, mortality is partitioned 88 and 17 % by the number of total facultative anaerobes 8.0 (log10CFU/g). CART classification and regression trees, CFU colony-forming unit
Effects of Probiotic/Synbiotic Therapies in Severe Disease
Definition of Probiotics, Prebiotics, and Synbiotics
Probiotics are defined by the FAO/WHO as live microorganisms, which when administered in adequate amounts, confer a health benefit on the host and are widely used as a live microbial food supplement that can improve the intestinal microbial balance [34]. Probiotics, most commonly Lactobacillus and Bifidobacterium, have been shown to exert preventive effects in various diseases, such as acute diarrhea, antibiotic-induced diarrhea, necrotizing enterocolitis and campylobacter-induced enteritis [35]. Prebiotics are currently defined as a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon [36]. Galactooligosaccharides are one category of prebiotics, and contain growth-promoting factors for Bifidobacterium [37]. Synbiotics are generally considered as a combination of probiotics and prebiotics.
The mechanisms of probiotics have not yet been clarified, but one of the important factors is microorganism-host crosstalk such as microorganism-associated molecular patterns (MAMPs) of probiotics and pattern recognition receptors (PRRs) of the gastrointestinal mucosa [38]. MAMPs consist of flagellin, secreted proteins, lipopolysaccharide, lipoteichoic acid, peptidoglycan and other factors. The most well-known PRRs are Toll-like receptors (TLRs). For example, flagellins of the probiotic E. coli Nissle 1917 were shown to induce beta-defensin via TLR5 [39]. Peptidoglycan from microbiota translocates to the circulation and enhances the killing capacity of neutrophils via the Nod-like receptor molecule Nod1 [40]. These interactions may increase protection from infection by activation of immunity. In animal studies, probiotics possess potent anti-infectious activity against lethal STEC O157:H7 infection [41], MRSA [42], rotavirus, influenza and other infectious organisms [35].
Effect of Synbiotic Therapy Based on Gut Microbiota Alternation in Severe SIRS Patients
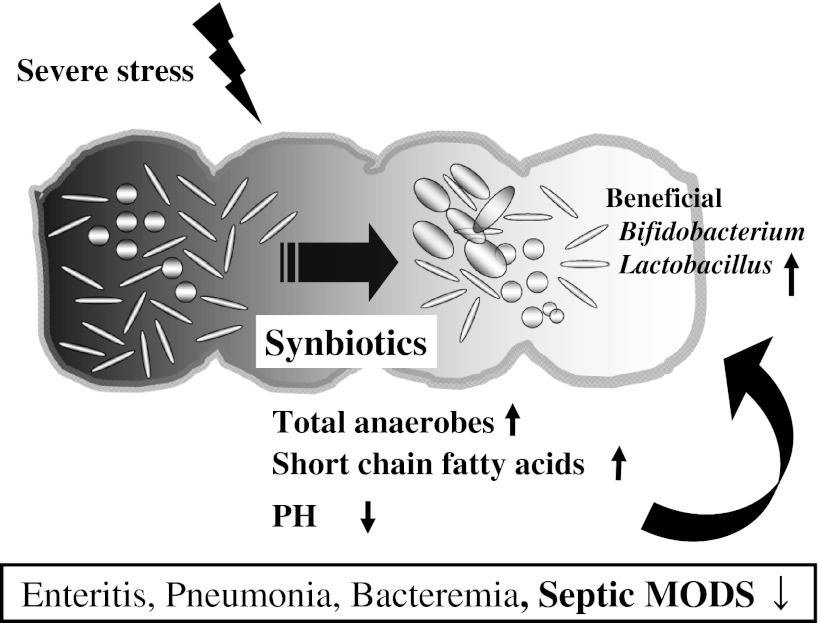
There have been few reports that showed the effects of synbiotics based upon the alteration of gut flora and environment in critically ill patients. Severe SIRS patients, who received Bifidobacterium breve strain Yakult and Lactobacillus casei strain Shirota as probiotics and galactooligosaccharides as prebiotics, had significantly greater levels of beneficial Bifidobacterium and Lactobacillus and SCFAs and lower incidence of infectious complications such as enteritis, pneumonia and bacteremia than those who received no synbiotics [29]. Synbiotics maintained the gut flora and environment and decreased the incidence of septic complications in patients with severe SIRS. The hypothesized mechanism of synbiotics is that their administration increases the levels of beneficial bacteria such as Bifidobacterium and Lactobacillus. The increased level of total anaerobes induces increased production of SCFAs in the gut. These environmental changes can help to maintain the gut flora. The beneficial alterations in gut flora and environment by synbiotics administration may enhance systemic immune function and decrease the incidence of septic complications such as enteritis, pneumonia and bacteremia in patients with severe SIRS (Fig. 2).
Fig. 2.
In patients under severe stress, “beneficial” bacteria decrease while “pathogenic” bacteria increase in the gut. The significant decrease in total anaerobes can cause the decreased production of short chain fatty acids, which in turn leads to the pH increase in the gut. These environmental changes can further induce the deterioration of the gut flora and a vicious circle leading to progression of systemic inflammatory response syndrome or infectious complications. MODS multiple organ dysfunction syndrome
Clinical Efficacy of Probiotics/Synbiotics Therapy for Severe Conditions/Disease
Abdominal Surgery
With regard to major abdominal surgery, several randomized controlled studies (Table 3) have been performed on patients receiving liver transplantation, hepatectomy for biliary cancer and pancreatoduodenectomy. Rayes et al. [43] compared the incidence of postoperative infections after 95 liver transplantations among three groups who were administered different enteral nutrition formulae: (a) standard formula plus selective bowel decontamination (SBD), (b) fiber-containing formula plus living Lactobacillus plantarum 299, and (c) fiber-containing formula plus heat-killed L. plantarum 299. The patients who received living lactobacillus plus fiber developed significantly fewer bacterial infections (13 %) than did the patients with SBD (48 %). Sugawara et al. [30] reported that in 101 patients with biliary cancer, the patients who received both preoperative and postoperative synbiotics with B. breve strain Yakult, L. casei strain Shirota and oligosaccharides for 2 weeks before and after surgery, had significantly fewer postoperative infections (12 %) compared with the patients who received only postoperative synbiotics (30 %). During the preoperative period, the numbers of Bifidobacterium and total organic concentrations measured in feces and NK activity increased significantly in the group receiving preoperative synbiotics in comparison with the group receiving postoperative synbiotics. These results showed that preoperative administration of probiotics and synbiotics would upregulate immune function and lead to a decrease in infectious complications.
Table 3.
Randomized controlled trials (RCTs) of probiotics and synbiotics for critically ill patients
| Years | Author (first) | Patient group | No. of probiotics, synbiotics/comparators | Probiotics/prebiotics | Outcomes (probiotics, synbiotics/comparators) | P value | |
|---|---|---|---|---|---|---|---|
| Abdominal surgery | |||||||
| 2002 | Rayes [43] | Liver transplantation | 31/32 | L. plantarum 299v | Infectious complications | 13 %/48 % | P < 0.05 |
| 2005 | Rayes [76] | Liver transplantation | 33/31 | Synbiotic2000 | Infectious complications | 3 %/48 % | P < 0.05 |
| 2005 | Kanazawa [30] | Hepatectomy for biliary carcinoma | 21/23 | BLO | Infectious complications | 19 %/52 % | P < 0.05 |
| 2006 | Sugawara [77] | Hepatectomy for biliary carcinoma | 40/41 | BLO | Infectious complications | 12 %/30 % | P < 0.05 |
| 2007 | Nomura [78] | Pacreatoduodenectomy | 30/34 | Bio-three | Infectious complications | 23 %/53 % | P < 0.05 |
| 2007 | Rayes [79] | Pacreatoduodenectomy | 40/40 | Synbiotic2000 | Infectious complications | 13 %/40 % | P < 0.05 |
| 2011 | Eguchi [80] | Living liver transplantation | 25/25 | BLO | Infectious complications | 4 %/24 % | P < 0.05 |
| 2011 | Usami [81] | Hepatectomy for liver cancer | 32/29 | BLO | Infectious complications | 0 %/17 % | P < 0.05 |
| ICU | |||||||
| 2004 | Jain [82] | ICU | 45/45 | Trevis | Infectious complications | 73 %/58 % | NS |
| 2005 | McNaught [83] | ICU | 52/51 | L. plantarum 299v | Infectious complications | 40 %/43 % | NS |
| 2008 | Forestier [45] | ICU | 102/106 | L. casei rhamnosus | VAP | 2.9 %/7.5 % | NS |
| 2009 | Knight [48] | ICU | 130/129 | Synbiotic2000FORTE | VAP | 9 %/13 % | NS |
| 2010 | Morrow [49] | ICU | 68/70 | L. rhamnosus GG | VAP | 19 %/40 % | P < 0.05 |
| Trauma | |||||||
| 2006 | Kotzampassi [44] | Trauma | 35/30 | Synbiotic2000FORTE | Infectious complications | 54 %/80 % | P < 0.05 |
| 2007 | Spindler-Vesel [45] | Trauma | 26/29 | Synbiotic2000FORTE | Pneumonia | 16 %/40 % | P < 0.05 |
| 2009 | Giamarellos-Bourboulis [46] | Trauma | 36/36 | Synbiotic2000FORTE | VAP | 41.7 %/44.4 % | NS |
| Acute pancreatitis | |||||||
| 2002 | Olah [50] | Acute pancreatitis | 22/23 | L. plantarum | Infectious complications | 4.5 %/30.4 % | P < 0.05 |
| 2007 | Olah [84] | Acute pancreatitis | 33/29 | Synbiotic2000 | Complications | 37 %/51 % | P < 0.05 |
| 2008 | Besselink [51] | Acute pancreatitis | 152/144 | Ecologic 641 | Infectious complications | 30 %/28 % | NS |
NS not significant, VAP ventilator-associated pneumonia
Ingredients of probiotics and synbiotics—
Synbiotic2000: Pediacoccus pentosaceus 5-33:3, Leuconostoc mesenteroides 32-77:1, Lactobacillus paracasei ssp. paracasei F19, L. plantarum 2632, fibers
Synbiotic2000FORTE: ten times contents as Synbiotic2000
BLO: B. breve strain Yakult, L. case strain Shirota, oligossacharides
Ecologic 641: L. acidophilus, L. casei, L. salivarius, L. lactis, B. bifidum, B. baterium lactis
Bio-three: Enterococcus faecalis T-110, Clostridium butyricum TO-A, Bacillus mesentericus TO-A
Trauma
In trauma, Kotzampassi et al. [44] reported that in 65 multiple trauma patients, the incidence of infectious complications in the synbiotics group decreased significantly more than those in the group without synbiotics (49 vs. 77 %). Inflammatory markers such as TNF-α and IL-6 also decreased in the synbiotics group. Spindeler-Vesel et al. [45] reported that in 113 trauma patients, the incidence of pneumonia in the synbiotics group decreased more than that in the group without synbiotics (16 vs. 40 %). The lactulose/mannitol intestinal permeability test showed significant decreases in the synbiotics group. Giamarellos-Bourboulis et al. [46] reported that in 72 multiple trauma patients, the incidence of ventilator-associated pneumonia (VAP) was not significantly different, but Acinetobacter baumannii was less identified as a bacterial cause of VAP in the synbiotics group than in the group without synbiotics. These results indicated that the administration of probiotics and synbiotics after trauma would stabilize the gut flora and attenuate inflammatory response and intestinal permeability, leading to prevention of pathogen colonization and infectious complications.
Ventilator-Associated Pneumonia
Knight et al. [47] reported that in 259 ventilator-assisted patients, the incidence of VAP in the synbiotics group was not significantly different from that in the non-synbiotics groups (9 vs.13 %). Forestier et al. [48] reported that in 208 ICU patients, the incidence of VAP in the probiotics group did not differ significantly from that of the control group (2.9 vs. 7.5 %). In the above two studies, there were no significant differences in oropharyngeal or gastric colonization. Contrarily, Morrow et al. [49] reported that in 138 ICU patients, the incidence of VAP with Lactobacillus rhamnosus GG decreased significantly more than those with no L. rhamnosus GG (19.1 vs. 40.0 %). In this study, probiotic administration significantly reduced oropharyngeal and gastric colonization. These changes indicated that the treatment could have effects on oropharyngeal or gastric colonization, and could correlate with the development of VAP. Changes in gut microbiota due to probiotics/synbiotics may have effects on immunity not only in the GALT but also in systemic organs.
Acute Pancreatitis
Oláh et al. [50] reported that the incidence of infectious complications with Lactobacillus plantarum decreased more than those without L. plantarum (4.5 vs. 30.4 %). In contrast, Besselink et al. [51] reported that mortality rates with six kinds of bacteria were significantly higher than those without these bacteria in the PROPATRIA study (16 vs. 6 %). The incidence of bowel ischemia was significantly higher in the probiotics group. However, in this study, the incidence of infectious complications showed no significant differences, and there was no bacteremia from the administered bacteria. In addition, this study has been criticized from multiple perspectives; the information was too optimistic, given that the research product had not been previously tested on humans, and the procedures for reporting serious adverse events did not conform to existing best practice. The Dutch Health Care Inspectorate, the Central Commission on Research Involving Human Participants, and the Food and Consumer Product Safety Authority all concluded that the study’s design, approval, and conduct had “major shortcomings” [52–54]. These studies suggest that the effect and safety of probiotics differ with the type of disease and the type and amount of administered bacteria. Further studies are needed to determine an appropriate therapy for acute pancreatitis.
In some cases such as sepsis, where the patient has suffered from severe stress even before admission, the number of obligate anaerobes associated with mortality would differ before the initiation of intestinal therapy. For example, elective surgery patients may undergo intestinal therapy before stress, whereas trauma or burn patients may have intestinal therapy administered after stress. The microbiota before intestinal therapy would be different with each disease because the periods of stress are different before intestinal therapy. However, most studies and meta-analyses mixed these disease situations together. Therefore, the results of intestinal therapy in critically ill patients should be considered with particular caution from the viewpoint of gut microbiota.
Several beneficial live microorganisms such as Lactobacillus, E. coli strain Nissle 1917 and Bifidobacterium have been reported. Although the mechanisms and systemic effects would be different with strains from the perspective of MAMPS and PRRs, the difference in effects among these strains has not been thoroughly delineated. Thus, further research is needed to clarify the mechanisms of each strain for appropriate use as probiotics in various diseases.
Antibiotics or Synbiotics for Critically Ill Patients?
Currently available digestive tract treatment strategies can be largely categorized into two types, namely, selective digestive decontamination (SDD) treatment and synbiotic treatment as mentioned earlier.
The effectiveness of SDD has been investigated in various clinical trials. Stoutenbeek et al. [55] first reported the effect of SDD in 1984 for 122 multiple trauma patients to reduce the total infection rate. In a randomized controlled study of 401 multiple-trauma patients, the use of polymyxin E, tobramycin and amphotericin B in the throat and gut throughout ICU treatment significantly reduced the incidence of infection, although the effect on mortality was not significant due to the underpowered study design.
For ICU patients, de Jonge et al. [56] compared the use of polymyxin E, tobramycin and amphotericin B with the control group with regard to the mortality rate and acquisition of resistant bacteria in a study of 934 ICU patients. In this study, SDD resulted in decreased mortality and lower detected levels of aerobic gram-negative bacilli. This finding was consistent with that by de Smet et al. [57] in a multicenter randomized cross-over trial involving 5,939 patients whereby both SDD and selective oropharyngeal decontamination (SOD) in ICU patients was shown to be effective in reducing mortality and gram-negative bacteria in comparison with the control. Furthermore, in critically ill burn patients, SDD was associated with significantly lower mortality rate and reduced pneumonia rate in comparison with placebo [58]. In a recent review, SDD reduced the incidence of VAP [59], gram-negative bloodstream infection [60], multiple organ dysfunction syndrome [61] and mortality in critically ill patients.
In contrast, negative aspects of SDD have also been reported. In settings with high levels of endemic, multidrug-resistant gram-negative bacteria or methicillin-resistant S. aureus, SDD was associated with increased selection of such pathogens [62–64]. In the investigation by Leone et al. [65] in 720 patients with multiple injuries, in the SDD group, methicillin-resistant S. epidermidis was detected with significantly higher frequency in comparison with the control. Bonten et al. [66] investigated 27 prospective randomized studies and six meta-analyses to reach the conclusion that the effectiveness of SDD has not been established and should not be recommended as a digestive tract treatment. Similarly, in the recent version of the Surviving Sepsis Campaign guidelines published in 2008, the opinions of the guidelines group was evenly split on the issue of SDD, with equal numbers weakly in favor of and against recommending the use of SDD. The committee therefore chose not to make a recommendation for the use of SDD specifically in severe sepsis [4].
In summary, results from both individual clinical studies and meta-analyses remain inconclusive with regard to the effectiveness of SDD as a digestive tract treatment. Despite its effectiveness in reducing pathogens, SDD also dramatically decreases obligate anaerobes in the gut, which can lead to severe imbalance of the intestinal microbiota. According to our own experience, SDD resulted in dramatic collapse of the microbiota and lethal bacteremia developed from the resulting enteritis [67]. In contrast to SDD, treatment using synbiotics appears to contribute to maintaining and repairing the environment and functions of the gut. Synbiotics is not only effective in reducing enteritis but also overall infectious complications and can be a promising treatment for critically ill patients. Additionally, synbiotic treatment exerts its effect in a physiological manner in the digestive tract. As for the safety of probiotics, although rare, there are a few adverse effects such as bacteremia, endocarditis and other complications [68, 69]. The pathogenic potential would be different with species or host conditions such as severe acute pancreatitis [54, 70]. Future clinical trials are expected to clarify whether appropriate intestinal therapies such as synbiotics and SDD can be used as standard digestive tract treatments in critically ill patients.
Gut Motility and the Limitations of Intestinal Therapy
Gut motility in critically ill patients is often disturbed by many factors, such as ischemia, analgesic drugs, adrenergic agents, fluid management and pre-existing illnesses such as diabetes [71]. This motor stasis leads to intolerance to enteral feeding, increased mucosal permeability for endoluminal mediators and bacteria and the development of SIRS. Montejo et al. [72] reported that enteral nutrition-related gastrointestinal complications in critically ill patients were present in 251 (62.8 %) of 400 patients and that enteral nutrition was withdrawn in 15.2 % of these patients. The complications included high gastric residuals (39 %), constipation (15.7 %), diarrhea (14.7 %), abdominal distention and vomiting and regurgitation. Patients with gastrointestinal complications had significantly higher mortality. In the ICU, it is well known that gut motility can easily decrease in abdominal surgery, head injury, spinal cord injury, burn and pancreatitis patients. In our retrospective study, mortality due to septic multiple organ dysfunction syndrome in patients with feeding intolerance (64 %) was significantly higher than that in patients without feeding intolerance (20 %), indicating that the patients with severe SIRS and gastrointestinal dysmotility have altered gut flora that would lead to an “undrained abscess.” These data indicated that intestinal dysmotility as a complication could be an indicative poor prognostic factor in patients with severe SIRS [73]. There are many prokinetic drugs reported, such as neostigmine, erythromycin, metoclopramide and Dai-kenchu-to, a famous Japanese herbal medicine [74, 75]. These prominent drugs are not always effective on our severe critically ill patients. This could be due to there being too much stress for the bowel to move, such that neither these drugs nor synbiotics can reach their destination to exert any effect.
Conclusions
The important role of the digestive tract has been well recognized as a therapeutic target in critically ill patients. However, to define an appropriate therapy, the relationship between the alteration of the gut microbiota and the specific clinical complications should be further clarified. Also a prognostic tool based on the alteration pattern of the gut microbiota should be developed and standardized to achieve timely treatment. In our current experience, probiotic/synbiotic treatment has been shown to be a promising therapy to maintain and repair the gut microbiota and gut environment and to significantly reduce septic complications in patients with severe SIRS. Finally, despite the promising clinical results with the use of these therapies, the mechanisms of action in the gastrointestinal tract remain undefined. Further clinical research that includes investigations of the microbiota and the underlying mechanisms of immune responses involved in the therapeutic effect should help to clarify the effectiveness of such therapies and define the appropriate conditions for use.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreymann KG, Berger MM, Deutz NE, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 5.Meddings J. The significance of the gut barrier in disease. Gut. 2008;57:438–440. doi: 10.1136/gut.2007.143172. [DOI] [PubMed] [Google Scholar]
- 6.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 7.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- 9.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. doi: 10.1128/AAC.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings JH, Antoine JM, Azpiroz F, et al. PASSCLAIM—gut health and immunity. Eur J Nutr. 2004;43:II118–II173. doi: 10.1007/s00394-004-1205-4. [DOI] [PubMed] [Google Scholar]
- 11.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 12.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 2005;35:1511–1520. doi: 10.1111/j.1365-2222.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 19.Rémésy C, Demigné C, Morand C. Metabolism of short chain fatty acids in the liver. In: Cummings JH, Rombeau JL, Sakata T, editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge: Cambridge University Press; 1995. pp. 171–190. [Google Scholar]
- 20.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 21.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 22.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- 24.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–244. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 26.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest. 2009;136:e28. doi: 10.1378/chest.09-2267. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu K, Ogura H, Goto M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60:126–133. doi: 10.1097/01.ta.0000197374.99755.fe. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu K, Ogura H, Hamasaki T, et al. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56:1171–1177. doi: 10.1007/s10620-010-1418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu K, Ogura H, Goto M, et al. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig Dis Sci. 2009;54:1071–1078. doi: 10.1007/s10620-008-0460-2. [DOI] [PubMed] [Google Scholar]
- 30.Kanazawa H, Nagino M, Kamiya S, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104–113. doi: 10.1007/s00423-004-0536-1. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura S, Maeda N, Miron IM, et al. Metagenomic diagnosis of bacterial infections. Emerg Infect Dis. 2008;14:1784–1786. doi: 10.3201/eid1411.080589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu K, Ogura H, Tomono K, et al. Patterns of gram-stained fecal flora as a quick diagnostic marker in patients with severe SIRS. Dig Dis Sci. 2011;56:1782–1788. doi: 10.1007/s10620-010-1486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FAO/WHO. Report, Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; October 1–4, 2001.
- 35.Nomoto K. Prevention of infections by probiotics. J Biosci Bioeng. 2005;100:583–592. doi: 10.1263/jbb.100.583. [DOI] [PubMed] [Google Scholar]
- 36.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 37.Ito M, Deguchi Y, Matsumoto K, Kimura M, Onodera N, Yajima T. Influence of galactooligosaccharides on the human fecal microflora. J Nutr Sci Vitaminol (Tokyo) 1993;39:635–640. doi: 10.3177/jnsv.39.635. [DOI] [PubMed] [Google Scholar]
- 38.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 39.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun. 2004;72:2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lkhagvadorj E, Nagata S, Wada M, et al. Anti-infectious activity of synbiotics in a novel mouse model of methicillin-resistant Staphylococcus aureus infection. Microbiol Immunol. 2010;54:265–275. doi: 10.1111/j.1348-0421.2010.00224.x. [DOI] [PubMed] [Google Scholar]
- 43.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74:123–127. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]
- 44.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30:1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 45.Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31:119–126. doi: 10.1177/0148607107031002119. [DOI] [PubMed] [Google Scholar]
- 46.Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma. 2009;67:815–821. doi: 10.1097/TA.0b013e31819d979e. [DOI] [PubMed] [Google Scholar]
- 47.Knight DJ, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35:854–861. doi: 10.1007/s00134-008-1368-1. [DOI] [PubMed] [Google Scholar]
- 48.Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008;12:R69. doi: 10.1186/cc6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 51.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 52.Sheldon T. Dutch probiotics study is criticised for its “design, approval, and conduct”. BMJ. 2010;340:c77. doi: 10.1136/bmj.c77. [DOI] [PubMed] [Google Scholar]
- 53.Expression of concern—probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:875–876. [DOI] [PubMed]
- 54.Morrow LE, Gogineni V, Malesker MA. Synbiotics and probiotics in the critically ill after the PROPATRIA trial. Curr Opin Clin Nutr Metab Care. 2012;15:147–150. doi: 10.1097/MCO.0b013e32834fcea8. [DOI] [PubMed] [Google Scholar]
- 55.Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984;10:185–192. doi: 10.1007/BF00259435. [DOI] [PubMed] [Google Scholar]
- 56.de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–1016. doi: 10.1016/S0140-6736(03)14409-1. [DOI] [PubMed] [Google Scholar]
- 57.de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 58.de La Cal MA, Cerda E, Garcia-Hierro P, et al. Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg. 2005;241:424–430. doi: 10.1097/01.sla.0000154148.58154.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2009:CD000022. [DOI] [PMC free article] [PubMed]
- 60.Silvestri L, van Saene HK, Milanese M, Gregori D, Gullo A. Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect. 2007;65:187–203. doi: 10.1016/j.jhin.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Silvestri L, van Saene HK, Zandstra DF, Marshall JC, Gregori D, Gullo A. Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: systematic review of randomized controlled trials. Crit Care Med. 2010;38:1370–1376. doi: 10.1097/CCM.0b013e3181d9db8c. [DOI] [PubMed] [Google Scholar]
- 62.Hammond JM, Potgieter PD, Saunders GL, Forder AA. Double-blind study of selective decontamination of the digestive tract in intensive care. Lancet. 1992;340:5–9. doi: 10.1016/0140-6736(92)92422-C. [DOI] [PubMed] [Google Scholar]
- 63.Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med. 1997;25:63–71. doi: 10.1097/00003246-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 64.Lingnau W, Berger J, Javorsky F, Fille M, Allerberger F, Benzer H. Changing bacterial ecology during a five-year period of selective intestinal decontamination. J Hosp Infect. 1998;39:195–206. doi: 10.1016/S0195-6701(98)90258-4. [DOI] [PubMed] [Google Scholar]
- 65.Leone M, Albanese J, Antonini F, Nguyen-Michel A, Martin C. Long-term (6-year) effect of selective digestive decontamination on antimicrobial resistance in intensive care, multiple-trauma patients. Crit Care Med. 2003;31:2090–2095. doi: 10.1097/01.CCM.0000079606.16776.C5. [DOI] [PubMed] [Google Scholar]
- 66.Bonten MJ, Krueger WA. Selective decontamination of the digestive tract: cumulating evidence, at last? Semin Respir Crit Care Med. 2006;27:18–22. doi: 10.1055/s-2006-933669. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu K, Nakagawa Y, Ogura H, et al. A case report of a severe alcoholic hepatitis with prominent increased white blood cells in blood and ascites. Kanzo. 2005;46:682–683 (in Japanese).
- 68.Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46:S104–S111; discussion S144–S151. [DOI] [PubMed]
- 69.Whelan K, Myers CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr. 2010;91:687–703. doi: 10.3945/ajcn.2009.28759. [DOI] [PubMed] [Google Scholar]
- 70.Asahara T, Takahashi M, Nomoto K, et al. Assessment of safety of lactobacillus strains based on resistance to host innate defense mechanisms. Clin Diagn Lab Immunol. 2003;10:169–173. doi: 10.1128/CDLI.10.1.169-173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fruhwald S, Holzer P, Metzler H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensive Care Med. 2007;33:36–44. doi: 10.1007/s00134-006-0452-7. [DOI] [PubMed] [Google Scholar]
- 72.Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447–1453. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu K, Ogura H, Asahara T, et al. Gastrointestinal dysmotility is associated with altered gut flora and septic mortality in patients with severe systemic inflammatory response syndrome: a preliminary study. Neurogastroenterol Motil. 2011;23:330–335. doi: 10.1111/j.1365-2982.2010.01653.x. [DOI] [PubMed] [Google Scholar]
- 74.Herbert MK, Holzer P. Standardized concept for the treatment of gastrointestinal dysmotility in critically ill patients—current status and future options. Clin Nutr. 2008;27:25–41. doi: 10.1016/j.clnu.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Endo S, Nishida T, Nishikawa K, et al. Dai-kenchu-to, a Chinese herbal medicine, improves stasis of patients with total gastrectomy and jejunal pouch interposition. Am J Surg. 2006;192:9–13. doi: 10.1016/j.amjsurg.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 76.Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation—a randomized, double-blind trial. Am J Transplant. 2005;5:125–130. doi: 10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 77.Sugawara G, Nagino M, Nishio H, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706–714. doi: 10.1097/01.sla.0000219039.20924.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nomura T, Tsuchiya Y, Nashimoto A, et al. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology. 2007;54:661–663. [PubMed] [Google Scholar]
- 79.Rayes N, Seehofer D, Theruvath T, et al. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg. 2007;246:36–41. doi: 10.1097/01.sla.0000259442.78947.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Ichikawa T, Kanematsu T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. Am J Surg. 2011;201:498–502. doi: 10.1016/j.amjsurg.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Usami M, Miyoshi M, Kanbara Y, et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J Parenter Enteral Nutr. 2011;35:317–328. doi: 10.1177/0148607110379813. [DOI] [PubMed] [Google Scholar]
- 82.Jain PK, McNaught CE, Anderson AD, MacFie J, Mitchell CJ. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin Nutr. 2004;23:467–475. doi: 10.1016/j.clnu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 83.McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–219. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Oláh A, Belágyi T, Pótó L, Romics L, Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology. 2007;54:590–594. [PubMed] [Google Scholar]