Graphical abstract
(a) PAK1 orchestrates mesalamine activity, (b) mesalamine inhibits PAK1; increases membranous E-cadherin and β-catenin; modulates cell adhesion
Abbreviations: CRC, colorectal cancer; IBD, inflammatory bowel diseases; UC, ulcerative colitis; AJ, adherens junction; PAK1, p21 activated kinase 1
Keywords: PAK1, Mesalamine, Beta-catenin, E-cadherin, Cell adhesion
Abstract
Mesalamine (5-ASA) is widely used for the treatment of ulcerative colitis, a remitting condition characterized by chronic inflammation of the colon. Knowledge about the molecular and cellular targets of 5-ASA is limited and a clear understanding of its activity in intestinal homeostasis and interference with neoplastic progression is lacking. We sought to identify molecular pathways interfered by 5-ASA, using CRC cell lines with different genetic background. Microarray was performed for gene expression profile of 5-ASA-treated and untreated cells (HCT116 and HT29). Filtering and analysis of data identified three oncogenic pathways interfered by 5-ASA: MAPK/ERK pathway, cell adhesion and β-catenin/Wnt signaling. PAK1 emerged as a consensus target of 5-ASA, orchestrating these pathways. We further investigated the effect of 5-ASA on cell adhesion. 5-ASA increased cell adhesion which was measured by cell adhesion assay and transcellular-resistance measurement. Moreover, 5-ASA treatment restored membranous expression of adhesion molecules E-cadherin and β-catenin. Role of PAK1 as a mediator of mesalamine activity was validated in vitro and in vivo. Inhibition of PAK1 by RNA interference also increased cell adhesion. PAK1 expression was elevated in APCmin polyps and 5-ASA treatment reduced its expression. Our data demonstrates novel pharmacological mechanism of mesalamine in modulation of cell adhesion and role of PAK1 in APCmin polyposis. We propose that inhibition of PAK1 expression by 5-ASA can impede with neoplastic progression in colorectal carcinogenesis. The mechanism of PAK1 inhibition and induction of membranous translocation of adhesion proteins by 5-ASA might be independent of its known anti-inflammatory action.
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the rectum that may extend to more proximal parts of the colon in a continuous fashion. Histopathology shows an infiltrate of neutrophils within the lamina propria and within mucosal crypts leading to epithelial destruction [1]. The primary cause(s) of UC is unknown, however, genetic and environmental factors are involved [2]. Patients with UC are at risk of developing colorectal cancer (CRC). Colon carcinogenesis involves multiple mutations or epigenetic modifications followed by changes in gene expression. These changes require several years leaving open a window of opportunity to prevent the transition from normal to malignant cells.
Sulfasalazine, a drug used in the treatment of chronic gut inflammation, was developed to combine antibacterial and anti-inflammatory effects [3]. The anti-inflammatory potential of its metabolite mesalamine (5-amino salicylic acid, 5-ASA), has been investigated in studies involving the arachidonic acid cascade, cyclooxygenase and lipoxygenase metabolites [4,5]. Although,the therapeutic efficacy of mesalamine in inducing and maintaining remission in UC is well established its role in prevention of colitis-associated carcinogenesis remains controversial [6,7]. Some authors have found inhibitory effects on the growth of colorectal cancer cells and in β-catenin signaling [8].
At the cellular level, 5-ASA reduces oxidative stress [9], inhibits cell cycle progression through activation of a replication checkpoint [10], decreases transcriptional activity of NF-κB [11] while activating PPAR-gamma [12] and interfering with the canonical Wnt pathway [13,14]. Though several potential mechanisms of its action have been investigated, a clear understanding of its interference with cancer development is lacking [8]. Such understanding may help to identify key regulators of colon homeostasis and carcinogenesis. To this end, we identified both known (Wnt-β-catenin) and novel (cell adhesion and MAPK/ERK) cellular mechanisms regulated by mesalamine using differential gene expression analysis. PAK1 (p21-activated kinase-1) turned out to be the consensus target of 5-ASA, orchestrating all three pathways. In this study we investigated the functional relevance of PAK1 as a mediator of mesalamine activity in vitro and in vivo.
2. Materials and methods
2.1. Cell lines and reagents
Human colorectal carcinoma cell lines HCT116, HT29 (obtained from ATCC) were grown in IMDM (Gibco/Invitrogen, Lofer, Austria) containing 10% fetal bovine serum (FBS; Biochrom, Berlin, Germany). 5-ASA (>99.9% pure; a generous gift from Shire Inc., Eysins, Switzerland) was dissolved in the culture medium (pH adjusted to 7.2 with NaOH) as described earlier [10]. IPA3 (Sigma–Aldrich) was dissolved in DMSO.
2.2. Microarray and data analysis
Accession number: The data is open to public at Array Express: http://www.ebi.ac.uk/microarray-as/ae/; experiment ID E-TABM-768
Total RNA was linearly amplified and fluorescently labeled using modified protocol of Low RNA Input Linear Amplification Kit (Agilent Technologies). The modifications to the protocol allow for incorporation of aminoallyl-UTP (Epicenter Biotechnologies) into amplified RNA and subsequent labeling by coupling with DY-547 NHS-ester and DY-647 NHS-ester fluorescent dyes (Dyomics). Fluorescently labeled cRNA from control and treated cells were co-hybridized to Human OneArray oligonucleotide microarrays (Phalanx Biotech). Four hybridizations in two replicas were carried out for each time interval (8 and 24 h) for CRC cell lines HCT116 and HT29. Microarrays were scanned with confocal microarray scanner (ScanArray Express, Perkin Elmer), extraction of spot intensities was done using QuantArray software (Packard Biochip). Data was further processed (normalization, filtering) in R statistical environment (http://www.R-project.org) and R Bioconductor [15] and analyzed with MEV software. Significantly up- and down-regulated genes were determined with SAM algorithm implemented in MEV (DFCI, JCVI, and the University of Washington). Gene expressions that were not different between 8 and 24 h were selected to represent the change induced by the treatment (5-ASA, 20 mM) in both the cell lines. Analysis of microarray data was performed using various available programs, including Pathway Studio Ariadne software (Ariadne, Rockville, MD, http://www.ariadnegenomics.com), Onto-Compare (Intelligent Systems and Bioinformatics Laboratory, Wayne State University, http://vortex.cs.wayne.edu), and MAPPFinder (Gladstone Institutes, University of California, http://www.genmapp.org/and http://www.gladstone.ucsf.edu).
2.3. Cell fractionation, Western blotting and antibodies
Cytosolic and membrane fractions were collected as described elsewhere [16]. Briefly, cells were grown in 15 cm dishes and were collected with 400 μl cold hypotonic buffer (10 mM Tris–HCl pH 7.5, 0.2 mM MgCl2, with protease and phosphatase inhibitors (Roche) with a cell scraper. The extract was dounce homogenized and kept on ice for 30 min. The extract was spun at 15,000 × g for 45 min at 4 °C, and the supernatant was collected as the cytosolic fraction. The pellet was washed twice in hypotonic buffer and then resuspended by vortexing in 50 μl lysis buffer (150 mM NaCl, 20 mM Tris–HCl pH 7.5, 1% Triton X-100, and proteinase inhibitors). The extract was vortexed for 10 seconds every 10 min and kept on ice for 30 min. Afterwards, the supernatant was collected as the membrane fraction. Both fractions were incubated with Laemmli sample buffer containing 10% β-mercaptoethanol at 95 °C for 10 min and then analyzed by western blot. Protein concentrations were measured by Bradford assay (Bio-Rad). Proteins were separated by SDS-PAGE and immunoblotted onto a PVDF membrane. The protein bands were visualized with anti-rabbit or anti-mouse antibodies coupled to horseradish peroxidase using the ECL kit (Amersham) or with IRDye coupled antibodies (either or both mouse/rabbit; LI-COR) and scanned on Odyssey imager (LI-COR Biotechnology). Primary antibodies used were as follows: monoclonal antibody, anti-E-cadherin, anti-β-catenin (BD Transduction Laboratories), Rad 6 (Zymed), pan-actin, calnexin, Lamin B1 (Santa Cruz Biotech), FRZB, alpha-tubulin, anti-Oct1, Anti-Fibrillarin (Abcam), phospho-β-catenin, PAK1, Na,K-ATPase, Phospho-p44/42 MAPK, and p44/42 MAPK (Cell signaling).
2.4. Cell adhesion assay
Cell adhesion assay was modified and performed as described previously [17,18]. Cells were treated with 5-ASA for 24 h (5–20 mM; as indicated in figures), washed in PBS, counted and plated equally (40–50,000 cells/well) in 24-well plates for attachment. After 30 min of incubation, each plate was washed with PBS until no floating cells remained and then replaced with the fresh medium (without 5-ASA) and MTT reagent. This washing step is critical for the cell attachment assay. After 4 h, medium was removed and the remaining precipitates were dissolved in DMSO/ethanol mixture (50/50, v/v). The experiment was repeated three times, and for each condition, four wells were scored.
2.5. Transcellular resistance measurement
Real time quantitative technique electric cell-substrate impedance sensing (ECIS) was utilized for measuring cell attachment [18]. The 96 well ECIS plate (Applied Biophysics, 96W10E+) was pre-coated with fibronectin (10 μg/ml; Sigma, F2006-1MG) for 1 h at 37 °C in a CO2 Incubator with 5% CO2. Then 80,000 Caco-2 cells (Sigma, 86010202) per well were plated in 200 μl Dulbeco‘s modified Eagle Medium (DMEM, 1% L-Glutamine, 1% Penicillin/Streptomycin, 10% FCS; PAA, E15-009) and incubated overnight. On the next day the plate was connected with the ECISz instrument (Applied Biophysics) and measured with an AC current of 4 kHz. The substances: control (DMEM), 5-ASA 1 mM and 5 mM were pre- incubated in the 37 °C Incubator with 5% CO2 for 1 h in a 96well plate. Then the experiment was paused and after removal of the old medium the substances were added to the Caco-2 cells. ECIS plate was re-connected with the instrument and the measurement was continued. Afterwards the impedance (ohm) was normalized by setting the time point 0 h at 1.
2.6. Immunofluorescence microscopy
Cells were fixed in methanol and immunostaining was performed using antibodies against β-catenin (clone 14/BD Transduction Laboratories) and E-cadherin (clone 36/BD Transduction Laboratories). For protein visualization AlexaFluor 488 and 568 antibodies (Invitrogen) were used. Nuclear staining was performed using Vectashield with DAPI (Vector laboratories) for mounting. Images were scanned at 40× magnification on a LSM 510 (Zeiss). Digital images were processed with Zeiss LSM Browser.
2.7. Luciferase reporter assay
HT29 or HCT116 cells seeded in 6-well plates at 5 × 105 cells/well were transfected with 2 μg of the TCF reporter pTOPFLASH or pFOPFLASH (gift of Dr. Paiva, Yale University) and co-transfected with 30 ng of pCMV-Renilla luciferase (Promega, Madison, WI, USA) per well using Lipofectamine 2000 reagent (Invitrogen Life Technologies) for 6 h. Cells were then treated with 5-ASA (20 mM) for 8 h and 24 h and corresponding cell lysates were subjected to dual luciferase reporter assay (Roche). Fluorescence of luciferase levels was measured on a luminometer (Bio-Rad Laboratories).
2.8. Chromatin Immunoprecipitation (ChIP) assay
Cells were treated with 20 mM of 5-ASA for 24 h, washed and fixed with 1% formaldehyde. β-catenin immunopellets were then separated onto chromatin DNA and protein fractions. Chromatin in formaldehyde-fixed cell lysates was sonicated to an average size of 500 bp. Cell lysates were clarified by centrifugation at 20,800 × g for 10 min at 4 °C and incubated with primary antibody overnight at 4 °C. Secondary rabbit anti-mouse IgG was then added for 6 h. Immunocomplexes were captured with BSA/glycogen-blocked protein A Sepharose (Calbiochem), washed, and the bead pellet was resuspended in 100 μl of TE (pH 8.0). RNA was digested for 30 min at 37 °C with 50 μg of RNase A (Roche). SDS was added to 0.25% and proteins were digested with 250 μg of proteinase K (Roche) for 12 h at 37 °C. Formaldehyde cross-links were reversed at 65 °C for 6 h. Samples were phenol/chloroform-extracted, and the DNA was precipitated in 100% ethanol. Samples were corrected for input DNA and values obtained were normalized to IgG control. DNA fraction was subjected to semi-quantitative PCR with primers specific for the human c-Myc, c-Net, Cdx1 and Cyclin D1 promoter regions and selected from qPrimerDepot database. GoTaq Hot Start DNA polymerase (Promega; 2.5 U/100 μl reactions) was used in 1x Green Flexi buffer/2 mM MgCl2 (Promega). CHIP reactions were performed using negative control IgG and RNA pol II antibody and GAPDH control primers (ChIP-IT Control Kit-Human, Active Motif).
2.9. RNA interference
For PAK1 siRNA transfection, cells (HCT116 and HT 29) were plated at a density of 1 × 105 cells per well in a six-well plate and were transfected using 50 nM and 100 nM of PAK1 siRNA duplex (dose was selected after titrating 10–100 nM duplex RNA) with siRNA transfection reagent (Santa Cruz). Fresh medium was added 24 h after transfection. Adhesion assay was performed 48 h post transfection. siRNA oligonucleotides were purchased from Dharmacon (catalog number D-003521-03, Accession Numbers: NM_002576, target sequence CAUCAAAUAUCACUAAGUC). Control siRNA-A: sc-37007 (Santa Cruz Biotechnology, CA) was prepared based on the manufactures instructions.
2.10. Animals and experimental procedure
Animal studies were performed under the Austrian Animal Experiments Regulation and approved by the animal experiment committee in accordance with the institutional good scientific practice guidelines. 4–6 week old heterozygous female and male C57BL/6J-ApcMin/+ mice (Jackson Laboratories) were fed with either 2500 mg/kg 5-ASA (A3537, Sigma–Aldrich) mixed into the chow or with a control diet (C1000, Altromin). The 5-ASA dose corresponds to the intake of 3 g/day in humans. After 12 weeks the mice were euthanized, the whole gut dissected and coiled up to a Swiss-roll. The intestine was fixed in neutral buffered formalin (10%) for 24 h and embedded in paraffin. Serial tissue cuts (4 μm) were H&E stained and analyzed histopathologically by a blinded pathologist. Tumors were counted regarding size (small < 0.3 mm, medium < 1 mm, large polyp >1 mm).
2.11. Immunohistochemistry
Immunohistochemistry analysis was done on paraffin-embedded mouse intestine. Briefly, slides were dried, de-waxed in xylol and rehydrated using a decreasing alcohol series. After blocking of endogenous peroxidase with 15% H202 in Methanol, antigen retrieval was performed in 10 mM citrate buffer, pH 6. Subsequently slides were blocked in 2% horse serum, 3% BSA in Tris-buffer and endogenous IgG was blocked with Vector M.O.M. Blocking Reagent. Staining was performed using Avidin–biotin complex method. Briefly, antibody against PAK1 (#2602, Cell signaling) or E-cadherin (clone 36/BD Transduction Laboratories) or c-Myc (9E10 abcam; 1:1000 dilution) was incubated 4 °C (overnight), followed by incubation with biotinylated anti-mouse/rabbit antibody and avidin–biotin-HRP complex. Staining was visualized using DAB and nuclear counterstaining was performed using hematoxylin. Slides were dehydrated and embedded in Histofluid. A four grade scoring system as described previously (18) was used for scoring immunoreactivity (0/1/2/3/x where x = 25 and % cells with no staining= 0; 1 = weak; 2 = moderate; 3 = strong; with maximum reactivity score or IRS = 12). Averaged IRS score of two independent investigators was used for the analysis. APCmin sections were evaluated from five untreated and five 5-ASA treated mice. For all antibodies used, staining with only secondary antibody was also performed to rule out nonspecific binding.
2.12. Statistical analysis
All in vitro experiments were independently performed three times each. Statistical analysis was performed by ANOVA (including linear contrasts for trend analysis). Post hoc analysis was performed by independent t-test provided that global ANOVA test was statistically significant. p value < 0.05 was considered significant. SPSS software (version 17) was used for all analyses.
3. Results
3.1. 5-ASA interferes with multiple signaling pathways
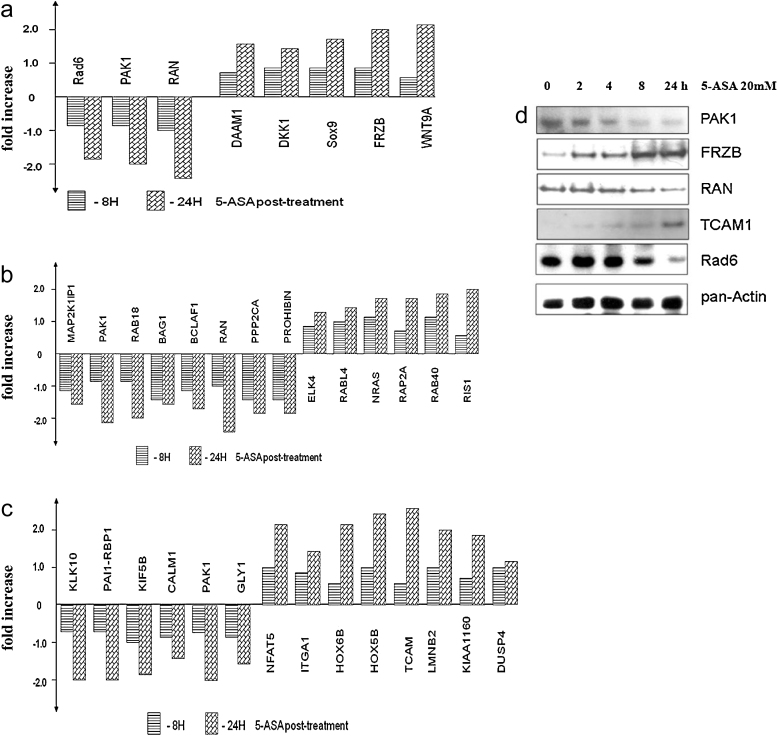
Differential gene expression was tested in colorectal cell lines (HT29 and HCT116) treated with 20 mM 5-ASA (8 and 24 h) as described in the methods. These cell lines were selected for their complementary differences in gene mutations like p53, β-catenin and APC [19,20]. Microarray data was further normalized, filtered and analyzed with MEV software. Almost 300 up- or down-regulated genes were selected (http://www.ebi.ac.uk/arrayexpress/; experiment ID E-TABM-768; some of the genes differentially regulated by 5-ASA are listed in Table 1). Clustering was performed using log2 ratio (red/green) and selected genes were subjected to bio-computational pathway analysis (as described in Section 2) that revealed involvement of genes in three pathways: canonical Wnt signaling (Fig. 1a), Ras/MAP kinase (Fig. 1b) and cell adhesion (Fig. 1c). Validation of selected 5-ASA targets was confirmed by Western blotting (Fig. 1d). Out of these three signaling pathways interfered by 5-ASA, Wnt/β-catenin has been previously investigated [13,14]. In our experiments, western blot with MAPK antibodies confirmed downregulation of this pathway by 5-ASA in HCT116 and HT29 (data not shown). However, it remained unclear how 5-ASA regulates genes implicated in the cell adhesion. The analysis did not provide any clear mechanism. We further investigated the effect of 5-ASA on cell adhesion and its underlying mechanism.
Table 1.
5-ASA regulated genes and their tumorigenic properties.
| Gene name | Function | Folds difference |
|---|---|---|
| Rad6 | DNA damage and repair protein that is overexpressed in many cancers; transcriptional target of the TCF/β-catenin pathway | −1.85 |
| PAK1 | Increased Pak-1 expression was shown in malignant progression of human colorectal carcinoma. It interferes with Wnt/β-catenin,MAPK pathway, cell migration and adhesion. | −2.1 |
| RAN (ras-related nuclear protein) | Regulator of Wnt signaling, possibly involved in colorectal carcinogenesis. | −2.7 |
| DAAM1 (disheveled associated activator of morphogenesis 1) | DAAM1 mediates transduction of disheveled-dependent WNT/PCP signals to the RhoA signaling cascade and interferes with JNK signaling pathway through MAPKKKs and MAPKK4/7. It regulates cell morphogenesis and actin dynamics through Rho GTPases and Src. | 1.65 |
| DKK1 | DKK1, extracellular Wnt inhibitor, is transcriptionally silenced by hypermethylation in colon cancer cell lines. Restoration of DKK-1 function reduces colony formation and tumor growth inhibition. | 1.55 |
| Sox9 | Strong SOX9 expression was shown to be an independent adverse prognosticator in colorectal cancer. In CRC cells SOX9 inhibits overexpression of carcinoembrionic antigen that protects cells against apoptosis and contributes to carcinogenesis. | 1.7 |
| FRZB (frizzled-related protein B) | The loss of FrzB in gastric cancer suggests that it acts as a tumor suppressor. Lost of FRZB function or gene polymorphism may contribute to in the development of colorectal cancer. | 2.1 |
| WNT9A | WNT protein member that plays a role in carcinogenesis. Depletion of WNT9A increases cell proliferation. WNT9A promoter methylation and decreased gene expression occurs frequently in primary colon cancers and acute lymphoid leukemias. | 2.2 |
| NFAT5 (nuclear factor of activated T-cells) | NGAT5 belongs to a NFAT family of transcription factors that exhibit strong anti-tumor-promoting activity. | +2.2 |
| ITGA1 (adhesion molecule integrin α 1) | Member of the integrin family, has been linked with epithelial cell motility, cellular survival and carcinoma invasion, hallmarks of metastatic tumors. It expression correlates with the NFAT activity. This protein heterodimerizes with the beta 1 integrin subunit to form a cell-surface receptor for collagen and laminin. The heterodimeric receptor is involved in cell–cell adhesion and may play a role in inflammation and fibrosis. | +1.6 |
| Dusp4 | Downregulation of Dusp26 contributes to malignant phenotypes of glioma. | +1.5 |
| PAI-1-RBP (plasminogen activator inhibitor-1 mRNA binding protein) | PAI-1 belongs to a family of factors associated with the invasion of gastric cancer cells through the basement membrane. | −1.9 |
| Lamin B | Lamins are upstream regulators of an actin dynamics pathway that contribute to the loss of cell adhesion, leading to enhanced cell motility and consequently increased invasive potential within certain tumors. | −2.0 |
| KIF5B | KIFs, microtubule-directed motor proteins that participate in subcellular transport of several cancer-related proteins, including the ß-catenin-cadherin(s) complex and APC. Dusp26 is a regulator of the KIF3. | −1.84 |
| GLY1 (1 N-glycanase 1) | GLY1 modifies E-cadherin within a complex of N-glycans. Removal of this complex increases interaction of E-cadherin-β-catenin complexes with actin cytoskeleton and destabilizes adherens junctions (AJs). | −1.6 |
| CALM1 (Calmodulin 1) | Member of calcium-modulated proteins that functions in growth and cell cycle as well as in signal transduction and the synthesis and release of neurotransmitters. Calmodulin and dependent protein phosphatises are overexpressed in many tumors. | −1.8 |
| KLK10 (kallikrein 10) | KLK10 expression is up-regulated in CRC and GC and higher expression of KLK10 closely correlates with advanced disease stage, which predicts a poorer prognosis. | −2.0 |
| BCLAF1 (BCL2-associated transcription factor 1) | It binds to DNA in vitro and represses transcription. Overexpression of Bclaf1 was shown to result in apoptosis and transcriptional repression. | −1.7 |
| BAG1 (BCL2-associated athanogene) | Predominantly expressed in colon carcinoma tissues, related to the malignant degree, infiltration and metastasis of colon carcinoma. | −1.6 |
| MAP2K1IP1 | Part of a MAPK scaffold complex that modulates the Raf-MKK1/2-ERK1/2 pathway. Upregulated during cancer progression. | −1.5 |
| RAB18 | Encodes antigen that was found preferentially in cancer patients. | −1.95 |
| PPP2CA | Encodes Raf-mediated protein from MAPK cascade, overexpressed in cancers. | −1.85 |
| PROHIBITIN (PHB) | Encodes mitochondrial protein that activates Raf/MEK/ERK pathway by active Ras and is therefore involved in cancer pathways. It modulates epithelial cell adhesion and migration. PHB directly interacts with C-Raf. In PHB silenced cells, the adherent junctions are stabilized. | −1.9 |
| ELK4 | One of the Ets transcription factors implicated in various aspects of tumor progression. | 1.4 |
| NRAS (neuroblastoma RAS viral (v-ras) oncogene homolog) | N-Ras(G12D), RAS homolog, confers resistance to apoptosis. | 1.75 |
| RAP2A | RAS family member regulates androgen sensitivity in human prostate cancer cells by antagonizing PSA expression. Plays a role in cell migration and adhesion. | 1.7 |
Fig. 1.
Differential regulation of genes by 5-ASA. Two cell lines (HCT116, HT29) treated with 20 mM 5-ASA (8, 24 h) were used in the analysis. The graphs represent expression of genes representative of the three major pathways obtained after microarray data analyses as described in methods. (a) Wnt/β-catenin (b) MAPK signaling (c) Cell adhesion. (d) Validation of some targets by Western blot. 5-ASA inhibited PAK1 that mediates all three pathways. Pan-actin was used as a loading control.
3.2. 5-ASA increases intercellular cell adhesion
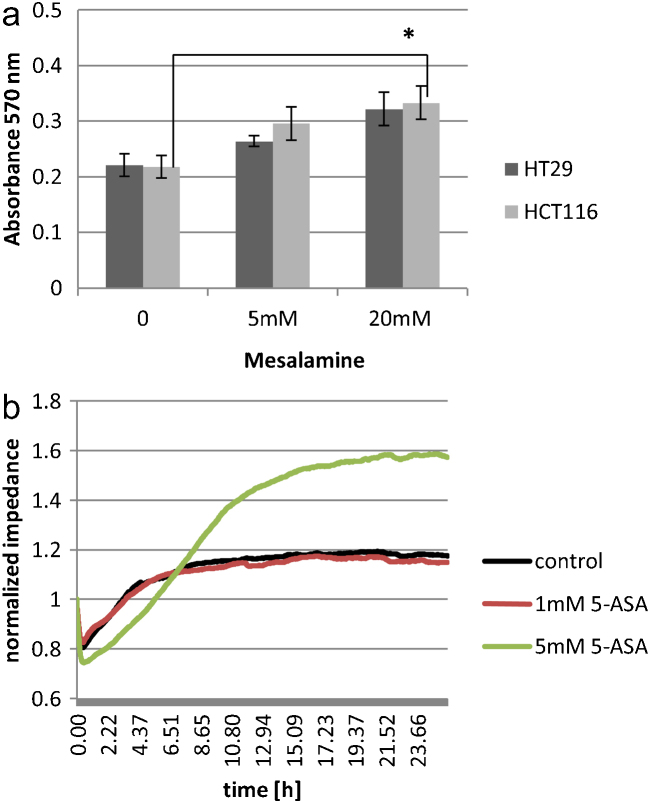
Cell adhesion was increased in both HT29 and HCT116 cells upon treatment with 5-ASA (Fig. 2a). Statistical analysis revealed that observed increase in the cell adhesion was less pronounced in HT29 compared to HCT116. Interestingly, this effect was not specific to colon epithelial cells as treatment with 5-ASA also proved to enhance cell adhesiveness in HeLa cells. Increase in cell adhesion was observed only by 5-ASA and not its inactive metabolite N-acetyl-5-ASA (data not shown).
Fig. 2.
5-ASA increases cell adhesion. (a) Adhesion of HCT116 and HT29 upon 5-ASA treatment (20 mM; 24 h). The graph is representative of one of the three independent experiments, ±SD of quadruplicate samples. Statistical analysis revealed that 5-ASA increases cell adhesion in a dose-dependent manner (p = 0.005 for the linear contrast). This effect was more pronounced in HCT116 cells. (b) Electric cell substrate impedance sensing (ECIS) assay to monitor cell adhesion upon 5-ASA treatment. The graph shows an increase in impedance upon 5-ASA treatment. The cell attachment data was collected using AC current at 4000 Hz.
Electric cell-substrate impedance sensing (ECIS) technology is a sensitive technique to monitor and quantify cell adhesion and barrier function of cell monolayer [21]. We measured impedance upon 5-ASA treatment on Caco-2 cells which have been successfully utilized for such assays [22]. An increase in impedance with time was observed upon 5-ASA treatment (Fig. 2b). Low doses of 5-ASA were used in this assay, as cells must proliferate to form a monolayer, and 5-ASA is shown to cause cell cycle arrest in a dose dependent manner [10]. The measurements were taken at low frequency as it responds strongly to the changes between the cells. Therefore, an increase in intercellular resistance is an indirect measurement of stronger cell-to-cell contacts. This data demonstrated that 5-ASA activity increased intercellular cell adhesion.
3.3. 5-ASA induces membranous expression of E-cadherin and β-catenin
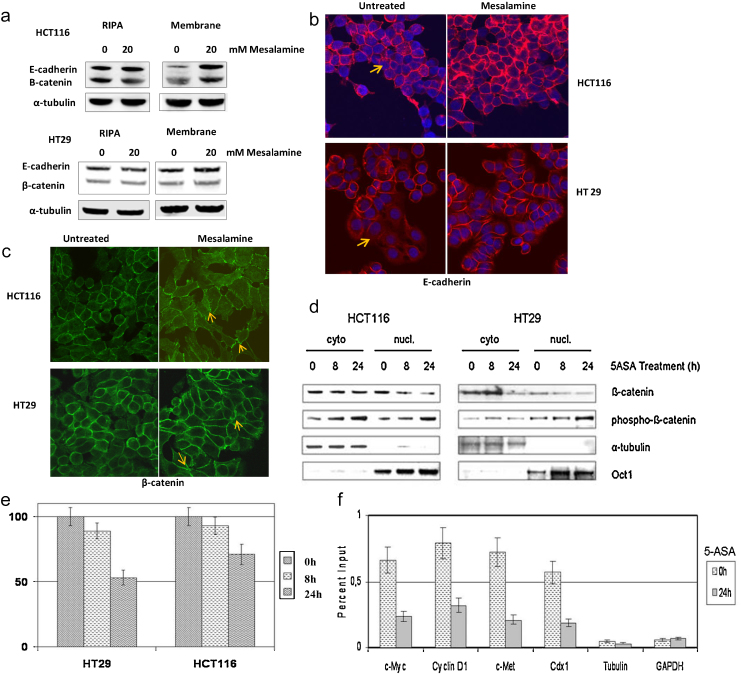
Anchoring cellular junctions that lead to adhesion can occur at the point of cell-to-cell contact (AJs, adherens junctions; desmosomes) or cell-to-matrix contact (focal adhesions). In epithelia, the AJ protein complex consists of E-cadherin that directly interacts with p120 catenin and β-catenin, which in turn binds alpha-catenin [23]. Moreover, cadherins also interfere with the Wnt/β-catenin pathway by sequestering β-catenin and promoting cell adhesion [24]. This led us to examine E-cadherin expression and localization upon 5-ASA treatment. Western blot analysis was performed on the whole cell extract and membrane fractions. Interestingly, E-cadherin expression remained unchanged in whole cell extracts in the presence of 5-ASA treatment (Fig. 3a). This indicated that 5-ASA activity is unlikely to alter its de novo synthesis. However, we observed an increase in membranous E-cadherin (Fig. 3a) in both cell lines examined. This effect was more pronounced in HCT116 cells. Immunofluorescent staining showed that untreated cells expressed less E-cadherin on the cell membrane and 5-ASA treatment induced E-cadherin localization at cell-to-cell contacts or AJs (Fig. 3b). As was observed with western blotting, the effect was more prominent in HCT116 cells compared to HT29. This observation was consistent with the previous observation in cell adhesion assay.
Fig. 3.
5-ASA recruits E-cadherin and β-catenin to the cell membrane. (a) Western blot analyses of E-cadherin and β-catenin in total (RIPA) or membrane fractions of 5-ASA treated cells. The data is representative of three independent experiments (b) Immunofluorescent detection of E-cadherin expression. Arrows indicate low levels of membranous E-cadherin in untreated cells. (c) Immunofluorescent detection of β-catenin. Arrows indicate AJs. Cells were grown on coverslips and treated with or without 5-ASA (20 mM) for 24 h. An increase in intercellular membranous E-cadherin and β-catenin was observed upon 5-ASA treatment. The experiment was performed three times. (d) Protein profiles of cytoplasmic and nuclear β-catenin and phospho-β-catenin in cells treated with 5-ASA for indicated time intervals. (e) 5-ASA inhibits β-catenin signaling in CRC cells. Luciferase reporter assay was performed in cells transfected with the TCF reporter pTOPFLASH or pFOPFLASH and co-transfected with pCMV-Renilla luciferase. Following treatment with 5-ASA for 8 h and 24 h, luciferase was measured using a luminometer. All samples were taken in quadruplicates and independently repeated 2 times (p < 0.05). (f) CHIP assay showing downregulation of transcriptional targets of β-catenin on 5-ASA treatment. Error bars represent SEM.
As β-catenin directly interacts with E-cadherin in forming AJs, we also examined the expression and membranous localization of β-catenin in 5-ASA treated cells. Western blot showed a similar increase in the membranous fraction of β-catenin as previously observed with E-cadherin (Fig. 3a). Immunofluorescent staining of β-catenin demonstrated its localization at AJs in 5-ASA treated cells (Fig. 3c).
We also assessed nuclear and cytoplasmic expression levels of β-catenin and phospho-β-catenin (at N-terminal serine33/37 and threonine 41) in HCT116 and HT29 cells. Upon treatment with 5-ASA, a decrease in nuclear β-catenin and an increased phosphorylation of β-catenin in both nuclear and cytoplasmic fractions was observed (Fig. 3d). To further examine, whether β-catenin localization at AJs was associated with the decrease in nuclear Wnt/β-catenin signaling through TCF, we performed a β-catenin/TCF induced transactivation assay using a reporter plasmid (pTOPFlash or pFOPFlash). 5-ASA treatment suppressed transcription of the reporter in both HT29 and HCT116 cells (Fig. 3e). We performed chromatin-immunoprecipitation, for 5-ASA-induced alterations in Wnt target genes by near verified β-catenin/TCF binding elements. Reduced β-catenin binding at the promoter region of Wnt/β-catenin target genes was seen for c-Myc, c-Met, Cdx1 and Cyclin D1 (Fig. 3f). These findings support the notion that 5-ASA inhibits β-catenin transcriptional activity, modulates its N-terminal phosphorylation and promotes intercellular cell adhesion through its membranous translocation. Overall, these observations elucidated a novel role of 5-ASA in cell adhesion through inhibition of β-catenin signaling and recruitment of adhesion proteins at AJs.
3.4. Depletion of PAK1 increases cell adhesion and membranous localization of β-catenin and E-cadherin
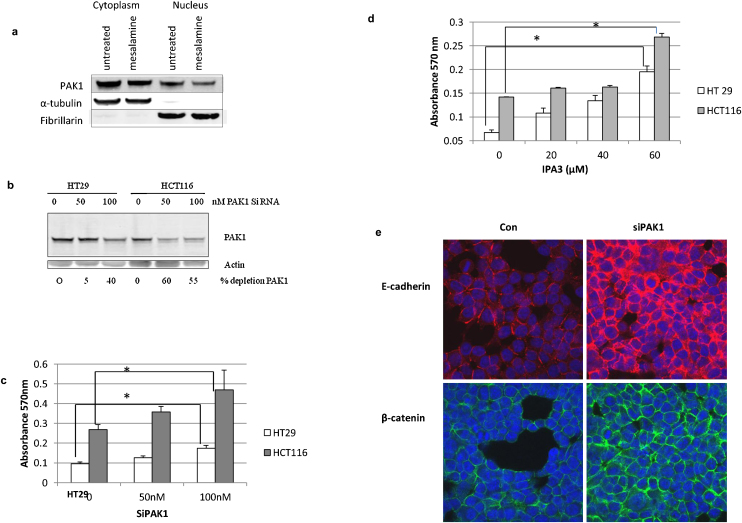
In the three molecular pathways identified by microarray analysis, PAK1, a serine/threonine kinase and a Rac/Cdc42 effector protein, was found to be the common factor (Fig. 1). In colon cancer, PAK1 expression levels are elevated [25,26]. Recently, a direct interaction and phosphorylation of β-catenin by PAK1 was shown to be required for full activation of canonical Wnt signaling [25]. Taken together, these observations link PAK1 activity in both MAPK signaling and the Wnt/β-catenin pathway disrupted by 5-ASA. However, the role of PAK1 in 5-ASA mediated cell adhesion was unclear. Treatment with 5-ASA inhibits expression of PAK1 (Fig. 1d). As localization and activation of PAK1 is critical in regulating its function, we examined the effect of 5-ASA on PAK1 expression in cell fractions (Fig. 4a). Nuclear PAK1 is associated with advanced stages of CRC [27] and we also observed high PAK1 expression in the nuclear fraction of untreated cells. 5-ASA treatment reduced PAK1 expression in both the cytoplasm and nucleus, with the effect more pronounced in the nuclear fraction.
Fig. 4.
PAK1 mediates 5-ASA effects. (a) Western blot showing inhibition of PAK1by 5-ASA in cytoplasmic and nuclear fractions. Fibrillarin and tubulin were used as control for purity of cellular fractions. The blot represents one of the three independent experiments (b) Western blot to analyze PAK1 knockdown using siRNA. PAK1 depletion shows decreased level of PAK1 protein upon increasing siRNA in CRC cells. (c) The effect of PAK1 depletion (by siRNA) on cell adhesion. Linear trend analysis by ANOVA revealed a significant dose-dependent effect on cell adhesion upon silencing PAK1 (p = 0.032) which was more pronounced in HCT116 cells. (d) The effect of IPA3 on cell adhesion. A dose dependent increase in cell adhesion was observed upon pharmacological inhibition of PAK1 kinase activity. Linear trend analysis by ANOVA showed statistically significant dose dependence (p < 0.001) (e) PAK1 depletion increases membranous localization of E-cadherin and β-catenin. HCT116 cells stained with anti-E-cadherin or β-catenin were analyzed by confocal microscopy. The experiment was repeated three times.
To further verify if 5-ASA acts through inhibition of PAK1, we assessed the effect of PAK1 depletion on cell adhesion. Using siRNA against PAK1 (Fig. 4b), we observed an increase in the cell adhesion in HCT116 and HT29 cells (Fig. 4c) similar to what was observed upon 5-ASA treatment (Fig. 2a). Notably, compared to HT29, HCT116 cells were more susceptible to PAK1 inhibition. HT29 is mutated in APC and in the absence of evidence; it remains speculative if APC mutation modulates PAK1 expression in these cells. We also utilized a biochemical approach to inhibit PAK1 kinase activity by IPA3 [28]. As was observed with PAK1 silencing, cell adhesion increased in a dose dependent manner in IPA3 treated cells (Fig. 4d). This observation substantiated that PAK1 interferes with cell adhesion in colon epithelial cells.
To determine, if silencing of PAK1 has similar molecular effects like 5-ASA, we performed E-cadherin immunostaining in PAK1-depleted cells (Fig. 4e). Increased membranous E-cadherin confirmed the functional role of PAK1 silencing in establishing cell-to-cell contacts. Sub-cellular localization of β-catenin upon PAK1 depletion was also examined using immunofluorescence microscopy. PAK1-depleted cells showed an increased membranous expression of β-catenin at cellular junctions (Fig. 4e). The effect of siPAK1 on membranous increase of E-cadherin and β-catenin was also confirmed by western blot (data not shown). A similar membranous increase in E-cadherin and β-catenin was observed in the HT29 cells transfected with siPAK1 (data not shown). We observed that in siPAK1 cells, β-catenin expression was reduced compared to control. This is consistent with its role in inhibiting β-catenin activity [29]. An increased interaction of E-cadherin and β-catenin at cell contacts was also observed in PAK1 depleted cells. The data thus uncovered the functional role of PAK1 inhibition by 5-ASA in cell adhesion.
Altogether, these results provide novel insights for 5-ASA activity (through inhibition of PAK1) in E-cadherin trafficking and cell adhesion. We conclude that increased membranous expression of junctional molecules (E-cadherin and β-catenin) by 5-ASA contributes to intercellular adhesion, which could potentially impede with neoplastic progression and restore epithelial integrity in the context of UC.
3.5. In vivo effects of 5-ASA activity on PAK1 and E-cadherin expression in APCmin mouse model
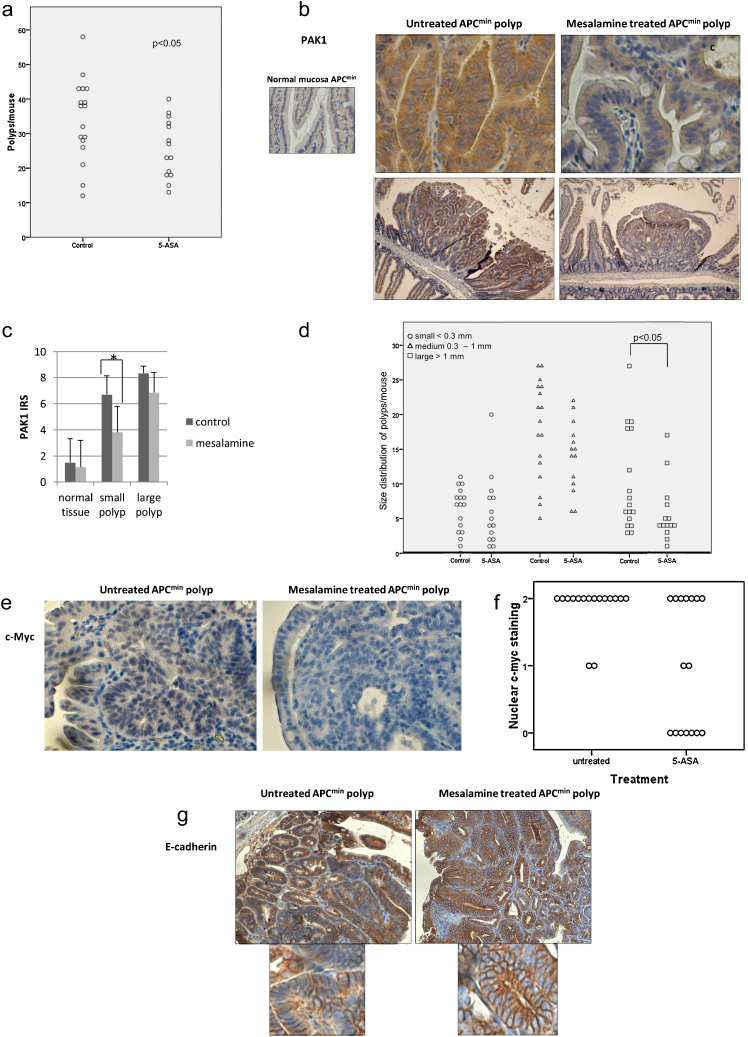
The immunofluorescence data and western blot analysis in PAK1-depleted cells indicated that inhibition of PAK1 (by siRNA or 5-ASA) was sufficient for membranous accumulation of β-catenin and E-cadherin. With emergence of PAK1 as a consensus target of mesalamine and as a mediator of multiple signaling pathways (altered in CRC cells), our data suggests that 5-ASA counteracts PAK1 activity in the colon. Inactivating mutations in the adenomatous polyposis coli (APC) gene predispose to CRC by constitutive activation of the β-catenin/Tcf complex [30]. However, not all studies in literature agree with the efficacy of 5-ASA in reducing polyp formation in APCmin mouse model [31]. We hypothesized that PAK1 contributes to β-catenin signaling in APCmin mice and its inhibition by 5-ASA could interfere with APCmin polyposis. We utilized this mouse model to test the hypothesis. Analysis of polyp formation in the 5-ASA treated group showed significant reduction in polyp number (Fig. 5a). Immunohistochemistry was performed to examine PAK1 expression in the polyps from control and 5-ASA treated mice. In the untreated group, PAK1 expression was found to be enhanced in the tissue sections from APCmin polyps (Fig. 5b) and not in normal mucosa. This observation indicated that PAK1 expression is modulated by APCmin mutation and activated β-catenin signaling in the polyps. 5-ASA treatment of mice reduced PAK1 expression in such APCmin polyps (Fig. 5b). Immunoreactive scores (IRS) of PAK1 staining revealed a statistically significant trend toward elevated PAK1 expression from normal mucosa through small polyps and large polyps (Fig. 5c), emphasizing the role of PAK1 signaling in tumor progression. Moreover, IRS revealed that 5-ASA treatment significantly reduced PAK1 expression, an effect that was more pronounced in small polyps. This observation also suggests that, inhibition of PAK1 by 5-ASA attenuates progression of neoplasia. This was also reflected in the significant reduction of number of large polyps by 5-ASA (Fig. 5d). In control group, small polyps proliferated further to become large polyps. 5-ASA treatment was effective in reducing PAK1 expression in small polyps and thereby impeding with tumor progression into large polyps. This was reflected in the reduction of large polyps in 5-ASA treated group and no significant change in small polyps compared to untreated controls. To confirm the effect of 5-ASA in the reduction of β-catenin/TCF signaling, we examined c-Myc (β-catenin target gene) expression in these polyps. In control mice, c-Myc expression was found to be highly elevated in all the polyps examined. The difference between treated and control polyps was visible using higher dilutions of c-Myc antibody. 5-ASA treatment significantly reduced nuclear c-Myc expression (Fig. 5e and f) indicating inhibition of β-catenin transcriptional activity. Immunohistochemical analysis was also performed for Ki-67 expression as a marker of proliferation. There was no significant difference between the control and 5-ASA treated polyps (data not shown). However, TUNEL staining revealed a significant increase in apoptosis in 5-ASA treated polyps compared to controls (data not shown, separate manuscript). Mutations in tumor suppressor gene APC stabilizes β-catenin and promotes its transcriptional activity. We therefore propose that inactivated APC and activated Wnt/β-catenin signaling stimulate PAK1 expression, which is downregulated by 5-ASA activity.
Fig. 5.
Effect of 5-ASA in vivo. (a) Intestinal polyps per mouse in untreated and 5-ASA treated APCmin mice. The total number of polyps significantly decreased upon treatment with 5-ASA for 12 weeks. For statistical analysis the nonparametric Mann–Whitney and Wilcoxon test was performed to compare untreated vs 5-ASA treated group (b) PAK1 expression was elevated in APCmin polyps (left) and 5-ASA activity effectively reduced its expression (right). (c) Immunoreactivity score (IRS) of PAK1 expression in APCmin polyps. Linear contrast analysis by ANOVA confirmed that 5-ASA treatment significantly reduced PAK1 expression (p = 0.015). This trend was most pronounced in small polyps (p = 0.029) (d) Number of APCmin polyps separated for size. There was a significant reduction in large polyps upon 5-ASA treatment. (e) Nuclear c-myc expression in polyps from untreated and 5-ASA treated APCmin mice. Treatment with 5-ASA siginficantly decreased nuclear c-myc staining within the polyps (p < 0.01). (f)Single polyp, represented by a single dot in the plot (n = 16), were scored for nuclear c-myc expression within the polyp (0, no staining; 1, low staining intenstiy; 2, medium staining instensity). Representative pictures were imaged at 200× magnification. (g) In APCmin polyps, E-cadherin was heterogeneously expressed in the epithelium that was distorted in the architecture (left). 5-ASA treatment was effective in restoring homogeneity of E-cadherin expression as well as its membranous localization. This was also reflected in re-organization of epithelial architecture (right). Image magnifications 100×.
We also performed E-cadherin and β-catenin immunostaining in APCmin polyps in mice with and without 5-ASA in their diet. We observed high expression of β-catenin in the polyps and no significant change was observed in the membranous expression levels, in either of the groups (data not shown). Immunohistochemistry analysis showed that E-cadherin expression was hetrogenous in APCmin polyps (Fig. 5g). The effect of 5-ASA was visible through induction of homogenous expression of E-cadherin (Fig. 5g) along with enhanced membranous expression of E-cadherin. E-cadherin is considered as a tumor suppressor gene and this effect of 5-ASA might contribute to its chemopreventive activity.
Taken together, the in vitro and in vivo data demonstrated a novel activity of mesalamine, altering cellular signaling cascades mediated by PAK1.
4. Discussion
Gene expression profiling in 5-ASA treated cells identified three cellular signaling mechanisms (Fig. 1), some of which (Wnt/β-catenin pathway) were recognized previously, others (cell adhesion and ERK/MAP kinase pathways) emerged as novel targets of 5-ASA. Cdc42/Rac1 effector molecule PAK1, was found to be the common mediator of these pathways and hence, a consensus target of 5-ASA. Cells treated with 5-ASA showed an increased intercellular adhesion that was measured by ECIS. Overall, the data demonstrated that 5-ASA increases cell adhesion; inhibits PAK1; downregulates β-catenin signaling and promotes membranous localization of E-cadherin and β-catenin. Molecular effects of 5-ASA activity were also mimicked by PAK1 inhibition and demonstrated its role in cellular adhesion. This study shows novel mechanism of 5-ASA activity in establishing cell-to-cell contacts, which is critical for mucosal barrier integrity in the setting of chronic gut inflammation and restoring epithelial homeostasis, impeding carcinogenesis. In vivo data suggest a novel role of PAK1 in APCmin polyposis. 5-ASA counteracted polyp formation, possibly through inhibition of PAK1. This study suggests that 5-ASA activity may have wider implications in its therapeutic efficacy, through interference with cellular signaling that might be independent of its anti-inflammatory action.
In the dynamic complex of AJ, the cytoplasmic domain of E-cadherin forms a complex with β-catenin which is also shared by canonical Wnt signaling pathway. Activation of Wnt/β-catenin signaling is vital for gut homeostasis particularly for the maintenance of intestinal crypt integrity. Activation of this pathway inhibits cadherin-catenin mediated cell adhesion through sequestration of β-catenin. Inhibition of β-catenin signaling by 5-ASA was previously shown to be partially mediated by upregulation of mu-protocadherin belonging to the cadherin superfamily. In this study, mu-protocadherin was shown to inhibit β-catenin signaling by sequestering it to the plasma membrane [24]. Here, we show that 5-ASA facilitates recruitment of both E-cadherin and β-catenin at cell-to-cell contacts with consequential increase in intercellular adherence. Our findings are also supported by another study that examined molecular effects of 5-ASA in patients with sporadic colorectal adenomas [32]. Besides downregulation of β-catenin signaling, the authors found a significant increase in E-cadherin membranous expression in the 5-ASA treated samples compared to placebo. These observations emphasize the role of 5-ASA in establishing intestinal architecture which is lost during chronic inflammation and in neoplasia. The ambiguity of microarray data opened up several possibilities of 5-ASA activity in cell adhesion and mucosal healing. As we focused primarily on the mechanism of E-cadherin and β-catenin regulation by 5-ASA, we cannot rule out the potential involvement of other proteins implicated in epithelial barrier function and mucosal integrity (such as tight junction proteins, desmosomal proteins) that might be modulated by 5-ASA. Nevertheless, this study provides a ground to further investigate the molecular mechanisms of 5-ASA in mucosal healing in UC and in colorectal carcinogenesis.
As a molecular mediator, PAK1 regulates diverse cellular mechanisms; it is critical in coordinating growth promoting signaling pathways [24]; has been implicated in the regulation of Wnt [25] and Ras signaling, and has a role in cytoskeleton dynamics and cell motility [26]. Phosphorylation of MEK1 by PAK1 in Ras-MAPK signal transduction is crucial [27]. We verified the role of PAK1 as a mediator of 5-ASA activity in cell adhesion. The data showed that inhibition of PAK1 (by siRNA) also resulted in membranous localization of β-catenin and E-cadherin, as was observed with 5-ASA. Previously, PAK1 was shown to contribute in the destabilization of cell-to-cell contacts in keratinocytes [33]. Membranous translocation of β-catenin upon siPAK1 corroborates with findings by Zhu et al. (37). Their study demonstrated PAK1 kinase activity contributes to nuclear localization and signaling of β-catenin, whereas our data shows that inhibition of PAK1 promotes β-catenin activity in cell adhesion at the membrane. We also observed direct interaction of PAK1 and β-catenin in colon epithelial cells (data not shown). Our in vivo studies indicated that PAK1 activity is associated with APCmin polyposis. Whether, the observed up-regulation of PAK1 in APC mutant mice is a cause or consequence of activated β-catenin signaling, needs further investigation. Additional mutations such as KRAS are implicated in APC polyposis [34] which might activate PAK1. In our study, 5-ASA reduced number of APCmin polyps and this corroborated with reduction in PAK1 expression that increased with the size of polyps. We propose that PAK1 inhibition by 5-ASA impedes with APCmin polyposis. We observed a difference in the efficacy of 5-ASA in reducing number of polyps in APCmin mice compared to a previous report from Ritland et al. [31]. This difference might be attributed to the drug concentrations used. In this study 5-ASA corresponded to a dosage range of 100–1920 mg/kg/day and we used 2500 mg/kg (corresponding to 3 gram/day in humans). In recent years, PAK1 has been generating interest as an oncogenic kinase [25,26,35] and attempts are in progress to identify selective PAK1 inhibitors for cancer therapy and its prevention [36,37]. Since, PAK1 emerged as a common mediator linking cellular mechanisms disrupted by 5-ASA; it is also likely to be an indirect target of 5-ASA. This study suggests the regulation of E-cadherin/β-catenin/PAK1 axis by 5-ASA. PI3 K/Akt signaling cooperates with Wnt/β-catenin signaling during inflammation [38] and it is tempting to speculate that 5-ASA regulates PAK1 through interference with this pathway. The possibility that 5-ASA targets a molecule upstream of PAK1 responsible for its activation cannot be ruled out. Deregulation and genetic association of Rac1 (Rho GTP-binding protein that can activate PAK1) with UC has been recently identified [39]. Recent studies provide evidence for association of PAK1 in sporadic colon cancer and we propose that as a mediator of 5-ASA activity, it contributes to colitis and inflammation driven colon cancer. We are currently examining the regulation of PAK1 in UC and in inflammation driven colon cancer. It remains to be investigated if 5-ASA acts through interference with either upstream activators of PAK1 or other cellular signaling pathways, to validate if PAK1 can be used as a biomarker of 5-ASA activity.
Disclosures
CG has research collaboration with Shire Pharmaceuticals and received research support, lecturing or consulting honoraria from Ferring and Dr Falk Pharma.
Competing interests
None.
Acknowledgments
Grant Support: The financial support by the Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development is gratefully acknowledged. This study was supported in part by the Austrian Science Fund (FW18270 to CG). We would like to acknowledge Ce-M-M research center for molecular medicine (The Austrian Academy of Sciences) for using their core facility (confocal microscopy).
References
- 1.Moehle C., Ackermann N., Langmann T., Aslanidis C., Kel A., Kel-Margoulis O. Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J Mol Med (Berl) 2006;84:1055–1066. doi: 10.1007/s00109-006-0100-2. [DOI] [PubMed] [Google Scholar]
- 2.Cho J.H., Brant S.R. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704–1712. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svartz N., Sulfasalazine: I.I. Some notes on the discovery and development of salazopyrin. Am J Gastroenterol. 1988;83:497–503. [PubMed] [Google Scholar]
- 4.Stolfi C., Fina D., Caruso R., Caprioli F., Sarra M., Fantini M.C. Cyclooxygenase-2-dependent and -independent inhibition of proliferation of colon cancer cells by 5-aminosalicylic acid. Biochem Pharmacol. 2008;75:668–676. doi: 10.1016/j.bcp.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Horn H., Preclik G., Stange E.F., Ditschuneit H. Modulation of arachidonic acid metabolism by olsalazine and other aminosalicylates in leukocytes. Scand J Gastroenterol. 1991;26:867–879. doi: 10.3109/00365529109037024. [DOI] [PubMed] [Google Scholar]
- 6.Velayos F.S., Terdiman J.P., Walsh J.M. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein C.N., Nugent Z., Blanchard J.F. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in IBD: a population based study. Am J Gastroenterol. 2011;106:731–736. doi: 10.1038/ajg.2011.50. [DOI] [PubMed] [Google Scholar]
- 8.Lyakhovich A., Gasche C. Systematic review: molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol Ther. 2010;31:202–209. doi: 10.1111/j.1365-2036.2009.04195.x. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie S.M., Doe W.F., Buffinton G.D. 5-aminosalicylic acid prevents oxidant mediated damage of glyceraldehyde-3-phosphate dehydrogenase in colon epithelial cells. Gut. 1999;44:180–185. doi: 10.1136/gut.44.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luciani M.G., Campregher C., Fortune J.M., Kunkel T.A., Gasche C. 5-ASA affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology. 2007;132:221–235. doi: 10.1053/j.gastro.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan L.J., Mays D.C., Huntoon C.J., Bell M.P., Pike M.G., Sandborn W.J. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 12.Rousseaux C., Lefebvre B., Dubuquoy L., Lefebvre P., Romano O., Auwerx J. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J.B., Lee G., Managlia E., Grimm G.R., Dirisina R., Goretsky T. Mesalamine inhibits epithelial beta-catenin activation in chronic ulcerative colitis. Gastroenterology. 2010;138:595–605. doi: 10.1053/j.gastro.2009.10.038. 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bos C.L., Diks S.H., Hardwick J.C., Walburg K.V., Peppelenbosch M.P., Richel D.J. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/beta-catenin pathway activity. Carcinogenesis. 2006;27:2371–2382. doi: 10.1093/carcin/bgl071. [DOI] [PubMed] [Google Scholar]
- 15.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard S., Deroo T., Fujita Y., Itasaki N. A positive role of cadherin in Wnt/beta-catenin signalling during epithelial-mesenchymal transition. PLoS ONE. 2011;6:e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni J., Hollander D. Applications of the MTT assay to functional studies of mouse intestinal intraepithelial lymphocytes. J Clin Lab Anal. 1996;10:42–52. doi: 10.1002/(SICI)1098-2825(1996)10:1<42::AID-JCLA7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Sawhney R.S., Cookson M.M., Sharma B., Hauser J., Brattain M.G. Autocrine transforming growth factor alpha regulates cell adhesion by multiple signaling via specific phosphorylation sites of p70S6 kinase in colon cancer cells. J Biol Chem. 2004;279:47379–47390. doi: 10.1074/jbc.M402031200. [DOI] [PubMed] [Google Scholar]
- 19.Morin P.J., Sparks A.B., Korinek V., Barker N., Clevers H., Vogelstein B. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou J.A., Noda M., Rowan A., Dixon C., Chinery R., Jawhari A. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin–catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo C.M., Keese C.R., Giaever I. Impedance analysis of MDCK cells measured by electric cell-substrate impedance sensing. Biophys J. 1995;69:2800–2807. doi: 10.1016/S0006-3495(95)80153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basuroy S., Sheth P., Kuppuswamy D., Balasubramanian S., Ray R.M., Rao R.K. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 23.Gumbiner B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 24.Parenti S., Ferrarini F., Zini R., Montanari M., Losi L., Canovi B. Mesalazine inhibits the beta-catenin signalling pathway acting through the upregulation of mu-protocadherin gene in colo-rectal cancer cells. Aliment Pharmacol Ther. 2010;31:108–119. doi: 10.1111/j.1365-2036.2009.04149.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu G., Wang Y., Huang B., Liang J., Ding Y., Xu A. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene. 2011 doi: 10.1038/onc.2011.294. [DOI] [PubMed] [Google Scholar]
- 26.Carter J.H., Douglass L.E., Deddens J.A., Colligan B.M., Bhatt T.R., Pemberton J.O. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- 27.Qing H., Gong W., Che Y., Wang X., Peng L., Liang Y. PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumour Biol. 2012;33:985–994. doi: 10.1007/s13277-012-0327-1. [DOI] [PubMed] [Google Scholar]
- 28.Deacon S.W., Beeser A., Fukui J.A., Rennefahrt U.E., Myers C., Chernoff J. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He H., Shulkes A., Baldwin G.S. PAK1 interacts with beta-catenin and is required for the regulation of the beta-catenin signalling pathway by gastrins. Biochim Biophys Acta. 2008;1783:1943–1954. doi: 10.1016/j.bbamcr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Korinek V., Barker N., Morin P.J., van W.D., de W.R., Kinzler K.W. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 31.Ritland S.R., Leighton J.A., Hirsch R.E., Morrow J.D., Weaver A.L., Gendler S.J. Evaluation of 5-aminosalicylic acid (5-ASA) for cancer chemoprevention: lack of efficacy against nascent adenomatous polyps in the Apc(Min) mouse. Clin Cancer Res. 1999;5:855–863. [PubMed] [Google Scholar]
- 32.Munding J., Ziebarth W., Pox C.P., Ladigan S., Reiser M., Huppe D. The influence of 5-aminosalicylic acid on the progression of colorectal adenomas via the ss-catenin signaling pathway. Carcinogenesis. 2012;33:637–643. doi: 10.1093/carcin/bgr306. [DOI] [PubMed] [Google Scholar]
- 33.Lozano E., Frasa M.A., Smolarczyk K., Knaus U.G., Braga V.M. PAK is required for the disruption of E-cadherin adhesion by the small GTPase Rac. J Cell Sci. 2008;121:933–938. doi: 10.1242/jcs.016121. [DOI] [PubMed] [Google Scholar]
- 34.Phelps R.A., Chidester S., Dehghanizadeh S., Phelps J., Sandoval I.T., Rai K. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh N., Liu K.H., Baldwin G.S., He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta. 2010;1803:1106–1113. doi: 10.1016/j.bbamcr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Kichina J.V., Goc A., Al-Husein B., Somanath P.R., Kandel E.S. PAK1 as a therapeutic target. Expert Opin Ther Targets. 2010;14:703–725. doi: 10.1517/14728222.2010.492779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eswaran J., Li D.Q., Shah A., Kumar R. Molecular pathways: targeting p21-activated kinase 1 signaling in cancer – opportunities, challenges, and limitations. Clin Cancer Res. 2012;18:3743–3749. doi: 10.1158/1078-0432.CCR-11-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee G., Goretsky T., Managlia E., Dirisina R., Singh A.P., Brown J.B. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. doi: 10.1053/j.gastro.2010.05.037. 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muise A.M., Walters T., Xu W., Shen-Tu G., Guo C.H., Fattouh R. Single nucleotide polymorphisms that increase expression of the guanosine triphosphatase RAC1 are associated with ulcerative colitis. Gastroenterology. 2011;141:633–641. doi: 10.1053/j.gastro.2011.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]