Abstract
Purpose
To analyse whether the permeability of the blood–brain barrier to the antimuscarinic drug trospium chloride is altered with ageing. This is a relevant question for elderly patients with overactive bladder syndrome who are treated with trospium chloride as the occurrence of adverse effects on the central nervous system (CNS) highly depends on the absolute drug concentration in the brain.
Methods
Trospium chloride at 1 mg/kg was intravenously administered to adult, middle-aged, and aged mice at 6, 12, and 24 months of age, respectively, and the absolute drug concentrations in the brain were analysed after 2 h. Furthermore, mRNA expression levels of relevant markers of blood–brain barrier integrity (occludin, claudin-5, and the drug efflux carrier P-glycoprotein) were analysed in brain samples from adult and aged mice.
Results
The absolute brain concentrations of the drug were identical in adult and middle-aged mice (13 ± 2 ng/g vs. 13 ± 2 ng/g) and were slightly, but significantly, lower in aged mice (8 ± 4 ng/g). The brain/plasma drug concentration ratios were not different between the age groups and demonstrated the generally low capability of trospium chloride in permeating the blood–brain barrier. Occludin, claudin-5, and P-glycoprotein showed identical mRNA expression levels in the brains of adult and aged mice.
Conclusion
Based on our in vivo data in a mouse model, we conclude that trospium chloride permeation across the BBB is not increased in ageing per se, and therefore, the occurrence of adverse CNS drug effects is also not expected to increase with ageing.
Keywords: Trospium chloride, Transport, Blood–brain barrier, P-glycoprotein, Ageing
Introduction
Antagonists of acetylcholine muscarinic receptors, such as darifenacin, fesoterodine, oxybutynin, propiverine, solifenacin, tolterodine, and trospium chloride (TCl), are the cornerstone of pharmacotherapy for the symptoms of overactive bladder (OAB) [1]. Apart from blocking muscarinic receptors in the bladder, most anticholinergic drugs can cross the blood–brain barrier (BBB) and antagonize the effects of acetylcholine in the central nervous system (CNS). In particular, blocking of muscarinic M1 receptors in the brain was found to be associated with cognitive impairment, dizziness, and sleep disturbance, which can be especially detrimental in older patients [2].
Most of the differences in CNS effects of antimuscarinic drugs can be explained by their different abilities to penetrate through the BBB. While most of the aforementioned antimuscarinic drugs are tertiary amines that are quite lipophilic, TCl is a highly polar quaternary amine [3]. Therefore, TCl showed much lower penetration across the BBB than other more lipophilic antimuscarinic drugs, at least in mice and rats [4, 5]. The low brain penetration of TCl was also confirmed in a recent study with older OAB patients who received extended-release trospium chloride 60 mg once daily over a 10-day period. In these patients, TCl showed normal peak plasma concentrations of 925 ± 478 pg/ml, but was assay undetectable in the cerebrospinal fluid (<40 pg/ml) [6].
Apart from their physicochemical properties, the brain penetration of antimuscarinic drugs also depends on their interaction with the drug-transporting P-glycoprotein (P-gp), which limits the entry of many drugs and xenobiotics into the brain by an efflux-based transport mechanism. Recently, we showed that penetration of TCl into the brain is significantly increased in P-gp-deficient knockout mice, indicating that P-gp normally restricts the entry of this drug into the brain [7].
However, the possibility that in elderly patients, who represent the majority of patients with OAB, the brain penetration of antimuscarinic drugs might be increased due to a histological or functional breakdown of the BBB or the down-regulation of P-gp has been discussed [2, 8]. However, until now, no clear experimental evidence has been found to show increased penetration of antimuscarinic drugs into the brain during ageing. Therefore, in the present study, we aimed to analyse the absolute brain concentrations of TCl after its administration to mice of different ages.
Materials and methods
Animals
For drug administration, seven aged (24 months), eight middle-aged (12 months), and three mature adult (6 months) male and female C57Bl/6N mice were used. For gene expression studies, we used seven aged (24 months) and eight adult (6 months) female C57Bl/6N mice as well as six aged (25 months) and four adult (4 months) male FVB mice. According to Flurkey et al., mice with 3–6 months of age have a life phase equivalent in humans of about 20–30 years. Mice with 10–14 months of age have a human age equivalent of 38–47 years and mice of 18–24 month of age correspond to humans with 59–69 years of age [9]. However, as the survival rate of mice around 28 month of age is only at about 50%, in the present study, we grouped 24/25-month-old mice to the group of aged mice. Nevertheless, several mice died before they reached the scheduled age of 24/25 months and even of 12 month. Therefore, the group size varied in the present study. All of the aged mice showed typical characteristics of ageing, including a sparse coat and decreased levels of general activity. The mice were housed in a specific pathogen-free (SPF) animal facility in isolated ventilated cages under a controlled temperature with a 12 h/12 h light/dark cycle. The mice had access to sterilized food and water ad libitum. All animal experiments were registered and approved by the local governmental administration and were conducted in accordance with the Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Drug preparation, administration, and tissue distribution
[3H]Trospium trifluoroacetate (42 Ci/mmol) was purchased from RC TRITEC AG (Teufen, Switzerland), and unlabelled TCl was kindly provided by Dr. R. Pfleger GmbH (Bamberg, Germany). The TCl was administered intravenously at a dosage of 1.0 mg/kg body weight (b.w.) in all experiments. For each animal, a mixture of [3H]trospium trifluoroacetate (2.5 μCi of the radiolabelled compound) and unlabelled TCl was prepared as described previously [7]. Due to the high excess of chloride in relation to trifluoroacetate in the drug preparation, this is further referred to as [3H]trospium chloride ([3H]TCl). Two hours after the administration of [3H]TCl, the mice were euthanized by cervical dislocation and the organs were removed and homogenized in 100–2,000 μl 0.05 M KOH, depending on the tissue weight. The levels of radioactivity in the blood and in the tissue homogenates were quantified by a Wallac 1409 liquid scintillation counter.
Gene expression analysis
The mice used for the gene expression studies had not been used in any other experiment and were euthanized by cervical dislocation. Brain samples were immediately extracted under sterile conditions and conserved in RNAlater solution (Applied Biosystems, Darmstadt, Germany). Then, RNA was isolated using the phenol/chloroform extraction method using TRI Reagent (Sigma-Aldrich, Taufkirchen, Germany). Genomic DNA was removed from the RNA preparations by DNase I digestion (Fermentas, St. Leon-Rot, Germany). The synthesis of cDNA was performed using the SuperScript III reverse transcriptase according to the manufacturer’s protocol (Invitrogen, Karlsruhe, Germany). For quantitative real-time PCR analysis, the following TaqMan Gene Expression Assays (Applied Biosystems) were used: Mm00440761_m1 for mouse mdr1a (P-gp), Mm00500912_m1 for mouse occludin, Mm00727012_s1 for mouse claudin-5, and Mm00607939_s1 for mouse beta-actin (endogenous control). Real-time quantitative PCR was performed with an ABI PRISM 7300 (Applied Biosystems). Each brain sample was tested in triplicate in a 96-well optical plate for all targets using 5 μl cDNA, 12.5 μl TaqMan Gene Expression Mastermix, 1.25 μl of the TaqMan Gene Expression Assay (Applied Biosystems) and 6.25 μl of water in each 25 μl reaction mixture. No-template controls used water instead of cDNA for PCR amplification. The plates were heated for 10 min at 95°C, and 40 cycles of 15 s at 95°C and 60 s at 60°C were subsequently applied. The relative expressions of the analysed genes were determined by subtracting the signal threshold cycle (C T) value of beta-actin from the C T value of each analysed gene (ΔC T).
Statistical analysis
All data are presented as the mean ± SD. The Student’s two-tailed unpaired t-test and one-way ANOVA followed by Bonferroni’s post hoc test were used to identify significant differences between the groups.
Results
Brain penetration and tissue distribution of [3H]TCl in aged mice
[3H]Trospium chloride was administered at a dosage of 1 mg/kg to adult (6 months, n = 3), middle-aged (12 months, male n = 4, female n = 4), and aged (24 months, male n = 2, female n = 5) C57Bl/6 N mice, and the absolute drug tissue concentrations were analysed 2 h after administration. Pooled data for each age group are shown in Fig. 1a, and an additional gender-specific subanalysis is given in Table 1. However, as most of the male aged mice (4 out of 6) died before they reached the scheduled age of 24 months, a statistical gender-specific subanalysis could not be performed in the group of aged mice. The absolute drug concentrations were highest in the liver (>1,000 ng/g) and the kidney (>500 ng/g) but showed no significant differences between the age groups in these organs (Fig. 1a). Drug concentrations among all organs analysed were by far lowest in the brain (≤13 ng/g). As shown in Fig. 1a, significantly lower drug concentrations were observed in the brain of the aged mice compared with the group of the adult mice (13 ± 2 ng/g vs. 8 ± 4 ng/g). However, in the gender-specific subanalysis, TCl concentrations in the brain showed no significant differences (P = 0.1086, Table 1). Furthermore, the brain/plasma concentration ratios were nearly identical among the age groups, namely 0.25 ± 0.01 for the adult mice, 0.29 ± 0.12 for the middle-aged mice, 0.24 ± 0.21 for the aged mice.
Fig. 1.
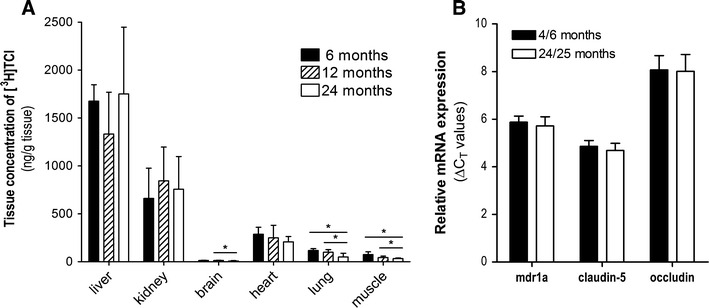
a Tissue concentrations of [3H]TCl in adult, middle-aged and aged mice after i.v. administration of 1 mg/kg body weight. Animals were euthanized 2 h after drug application, and organ drug concentrations were determined by liquid scintillation counting. Data represent means ± SD of three to eight animals per group. *Significantly different drug concentrations (P < 0.05). b Relative mRNA expression levels of the BBB markers mdr1a P-gp, claudin-5, and occludin in the brain of adult and aged mice analysed by quantitative real-time PCR
Table 1.
Drug concentrations of [3H]TCl in tissues (ng/g) and plasma (ng/ml) in adult, middle-aged and aged C57Bl/6N mice 2 h after i.v. administration of 1 mg/kg
| Adult (6 months) | Middle-aged (12 months) | Aged (24 months) | |||
|---|---|---|---|---|---|
| Male (n = 3) | Male (n = 4) | Female (n = 4) | Male (n = 2) | Female (n = 5) | |
| Brain | 13 ± 2 | 13 ± 2 | 13 ± 2 | 9 | 8 ± 5 |
| Liver | 1,675 ± 140 | 1,528 ± 344 | 1,137 ± 373 | 1,724 | 1,756 ± 696 |
| Kidney | 660 ± 258 | 713 ± 271 | 975 ± 332 | 279 | 948 ± 97 |
| Stomach | 701 ± 103 | 735 ± 230 | 935 ± 332 | 478 | 737 ± 752 |
| Lung | 117 ± 16 | 107 ± 30 | 89 ± 17 | 101 | 29 ± 20* |
| Spleen | 68 ± 12 | 117 ± 26 | 267 ± 271 | 91 | 55 ± 47 |
| Heart | 287 ± 60 | 273 ± 113 | 226 ± 129 | 221 | 202 ± 45 |
| Fat | 134 ± 149 | 56 ± 47 | 59 ± 35 | 22 | 24 ± 14 |
| Muscle | 74 ± 24 | 48 ± 6 | 39 ± 18 | 33 | 33 ± 6** |
| Blood | 52 ± 6 | 71 ± 14a | 40 ± 16 | 48 | 26 ± 11a, *** |
The data show means ± SD. Significant differences were analysed by one-way ANOVA followed by Bonferroni’s post hoc test except for the group of aged male mice due to the inadequate group size. * Significantly lower drug concentration compared with all other groups (P < 0.05); ** significantly lower drug concentration in aged female mice compared with adult male mice (P < 0.05); *** significantly lower concentration in aged female mice compared with middle-aged male mice (P < 0.05)
aBlood samples adequate for analysis could only be obtained for n = 3 mice in these groups
Apart from the brain, significantly lower drug concentrations were observed in the lung and muscle of the aged mice compared with the groups of adult and middle-aged mice (Fig. 1a). This was in part confirmed in the gender-specific subanalysis (Table 1). Furthermore, drug concentrations in the blood of the aged female mice were significantly lower compared with the adult male mice (26 ± 11 ng/ml vs. 71 ± 14 ng/ml) (Table 1). In addition to the comparison of the different age groups, the gender-specific subanalysis in the group of middle-aged mice allowed us to directly compare the tissue distribution and brain penetration of TCl between male and female mice (Table 1). Among all organs analysed, we found nearly or even identical drug concentrations in the male and female mice, particularly in the brain (13 ± 2 ng/g vs. 13 ± 2 ng/g).
P-gp expression pattern in adult and aged mice
Brain samples of 13 aged (24–25 months, male n = 6, female n = 7) and 12 adult (4–6 months, male n = 4, female n = 8) untreated mice were used for gene expression analysis of the drug efflux carrier P-gp (mdr1a) and the tight junction proteins claudin-5 and occludin by quantitative real-time PCR. Target mRNA expression was normalized to the expression of beta-actin. For each real-time PCR measurement, 50 ng of RNA was used per well and revealed very stable C T values for beta-actin of 18.32 ± 0.22 (total range of variation in the absolute C T values: 18.02–19.10). No significant differences occured in the beta-actin C T values between the different age groups. Therefore, beta-actin was a suitable gene for the normalization of the real-time quantification. As shown in Fig. 1b, the expression levels of mdr1a, claudin-5, and occludin showed no differences between the adult and aged mice. Furthermore, gender-specific subanalysis in both age groups revealed no differences in the expression levels between the male and female mice (data not shown).
Discussion
Older patients are more likely to experience adverse CNS effects under drug treatment for several reasons. In the case of anticholinergic drugs, these have been associated with cognitive deficits, behavioural changes, and sleep disturbance [10]. An increased permeability of the BBB may contribute to such vulnerabilities. In this regard, it was claimed that TCl is a safer anticholinergic alternative due to its generally low permeability across the BBB [11, 12]. However, the age-dependent penetration of trospium chloride into the brain has never been measured before in an experimental study.
In the CNS, different barriers exist between blood circulation and neural tissue that have to be considered for drug permeation into the brain: the blood–brain barrier (BBB), which is formed by cerebrovascular endothelial cells, and the blood–cerebrospinal fluid barrier (BCSFB), which is constituted by epithelial cells of the choroid plexus [13]. At these interfaces, a barrier function results from the combination of (I) a histological barrier formed by tight junction complexes that tightly connect the epithelial cells and thus greatly limit the paracellular flux of polar solutes, and (II) a functional barrier comprised of different transport systems such as P-gp, which regulate the solute flux across the barrier [14]. Since the surface area of the BBB is at least 5,000-fold greater than that of the choroid plexus [15], the BCSFB is only of marginal importance for the overall drug delivery into the brain. In contrast, absolute drug concentrations in the brain predominantly depend on the permeability of the BBB, and therefore, this barrier is the most relevant barrier in the CNS regarding adverse CNS effects under drug treatment [16].
Several factors can restrict the entry of drugs into the CNS including a highly polar surface area or a molecular weight of >450 Da [17]. Additionally, interactions with drug carriers at the BBB can facilitate or restrict drug entry into the brain by carrier-mediated uptake or efflux processes, respectively [16]. In the case of the anticholinergic drug TCl, brain penetration is highly restricted by the polar structure of the molecule and its low lipophilicity, as well as by a P-gp-mediated drug efflux in the endothelial cells of the BBB [7]. Therefore, compared with the highly lipophilic and uncharged drug oxybutynin, TCl showed 200-fold lower absolute drug concentrations in the brain when administered at an equal dosage to laboratory mice [4].
In recent reviews about OAB treatment with anticholinergic drugs, the fact that the BBB becomes more leaky with age and so might be more permeable to polar and P-gp-transported drugs such as TCl was discussed [2, 8]. Indeed, several age-related changes in the cerebral microvasculature have been reported, including a lower microvascular density, a smaller capillary lumen size, or gliofibrillar proliferation [18]. However, whether these changes actually affect drug penetration across the BBB has not been analysed. In animal studies with mice and rats, this question was addressed by studies that administered [14C]sucrose [19] or higher molecular weight compounds such as horseradish peroxidase [20]. In general, these reports showed no significant alteration in BBB permeability with ageing per se. Other studies that analysed the permeability of the BBB in older human subjects (most of them by measuring the albumin liquor/plasma ratio) produced inconsistent results [21]. Although some of these studies reported an elevation of the albumin liquor/plasma ratio with ageing, it must be emphasized that this ratio is a very artificial parameter for the assessment of absolute drug permeability across the BBB because: (I) alterations in this ratio primarily indicate a dysfunction of the BCSFB at the choroid plexus; (II) in terms of physicochemical properties and drug carrier interactions, albumin is not an appropriate surrogate for common drugs; and (III) an elevation of the albumin liquor/plasma ratio was previously shown to be associated with reduced cerebrospinal fluid production but not with increased albumin permeability at the BCSFB [22, 23]. Therefore, an increase in the albumin liquor/plasma ratio during ageing cannot simply be interpreted as an increased permeability of the BBB [24, 25].
However, a direct determination of the BBB drug permeability and of the absolute drug concentrations in the brain would require the analysis of brain material after drug administration, and therefore, such studies can generally not be carried out on human subjects. Therefore, we decided to analyse the age-dependent brain penetration of TCl in a mouse model, with animals ranging from 6 to 24 months of age. In these mice, the absolute drug concentrations were directly measured in the brain after a single-dose application and showed identical levels in the adult and middle-aged mice (13 ± 2 ng/g) and significantly lower levels in the aged mice (8 ± 4 ng/g). This slight decline in the brain concentration in the aged mice is probably due to the age-related changes in the cerebral microvasculature mentioned above. But due to the generally low drug concentrations in the brain compared to other organs and the relatively low degree (13 ± 2 ng/g vs. 8 ± 4 ng/g), this difference is not regarded as biologically meaningful with regard to the question of CNS side effects after treatment with antimuscarinic drugs. Furthermore, the brain/plasma concentration ratios for TCl were not different between the three age groups. Based on these data, it can be concluded that TCl permeation across the BBB is at least not increased with ageing per se.
We are aware of the potential limitations of the present study as the mouse, in terms of lifespan and metabolic activity, is a somewhat artificial model for the situation in human patients. Furthermore, this study was designed as a single-dose application study with single point detection and cannot directly be compared to a steady-state distribution after repeated drug application, which represents the situation in human patients. Nevertheless, our data are in good agreement with a recent clinical study in older OAB patients (≥65–75 years old) who received extended-release TCl treatment with 60 mg once daily over 10 days. These patients underwent memory testing using the Hopkins Verbal Learning Test-Revised and the Brief Visuospatial Memory Test-Revised, and showed no significant drug effects on learning or recall, which can be regarded as very sensitive parameters of adverse anticholinergic CNS effects. Furthermore, the TCl drug concentrations in the cerebrospinal fluid were assay undetectable (<40 pg/ml), with normal peak concentrations in plasma (~1 ng/ml) [6]. These data clearly indicate that in older OAB patients, there is no relevant penetration of TCl into the brain under a therapeutic dosage.
Apart from the polar drug TCl, brain penetration studies have previously been carried out using the more lipophilic drug verapamil. These studies, using [11C]verapamil positron emission tomography, found an increase in the distribution volume in certain, but not all, brain regions [26, 27]. As verapamil is a well-established substrate of the P-gp efflux transporter, it was suggested that P-gp might be functionally down-regulated with ageing. However, studies on human brain samples showed no influence of age on the cerebrovascular expression of the P-gp protein [28]. Even studies on P-gp expression at the BBB in rodents showed no down-regulation with age [29], including the present study where we found identical levels of mRNA expression of P-gp and the tight junction markers occludin and claudin-5 in mice of 4/6 and 24/25 months of age. We are aware of the fact that the protein expression level in particular of P-gp might be different, although the mRNA expression level is equal in adult and aged mice. But as we directly measured the drug entry into the brain which is significantly dependent on the functional expression of P-gp in the blood–brain barrier [7], we do not expect that the protein expression of P-gp is significantly up- or down-regulated in the aged mice.
However, it is possible that barrier functions in the CNS could be affected by several disease conditions and pathologies including multiple sclerosis, acute hypertension, cerebral ischaemia, diabetes, Alzheimer’s or Parkinson’s disease, and brain tumours or inflammation [13, 30, 31]. Under these conditions, impairment of the neurovascular barriers could range from transient opening of the tight junctions to chronic barrier breakdown [13, 21]. Additionally, the expression of drug efflux carriers such as P-gp at the BBB can be affected in certain diseased conditions such as Alzheimer’s and Parkinson’s disease, amyotrophic lateral sclerosis, and inflammatory processes [32]. Therefore, in patients with multiple co-morbidities, the brain penetration of peripherally acting antimuscarinic drugs might be increased.
In conclusion, we demonstrated in a mouse model that the absolute brain concentrations of the hydrophilic anticholinergic drug TCl were not increased with normal ageing. Furthermore, we found that, irrespective of the age of the mice, the brain was the organ with by far the lowest drug concentrations in the body of the mice, indicating that the BBB is a very effective permeation barrier against this drug. Based on our in vivo data, we conclude that TCl permeation across the BBB is not increased in ageing per se, and therefore, the occurrence of adverse CNS drug effects is also not expected to increase.
Acknowledgments
This study was kindly supported by Dr. R. Pfleger GmbH, Bamberg, Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007;100(5):987–1006. doi: 10.1111/j.1464-410X.2007.07205.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagg A, Verdejo C, Molander U. Review of cognitive impairment with antimuscarinic agents in elderly patients with overactive bladder. Int J Clin Pract. 2010;64(9):1279–1286. doi: 10.1111/j.1742-1241.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiedemann A, Schwantes PA. Antimuscarinic drugs for the treatment of overactive bladder: are they really all the same?—A comparative review of data pertaining to pharmacological and physiological aspects. Eur J Ger. 2007;9:29–42. [Google Scholar]
- 4.Geyer J, Gavrilova O, Schwantes U (2010) Differences in the brain penetration of the anticholinergic drugs trospium chloride and oxybutynin. UroToday Int J 3(1). doi:10.3834/uij.1944-5784.2010.02.12
- 5.Callegari E, Malhotra B, Bungay PJ, et al. A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br J Clin Pharmacol. 2011;72(2):235–246. doi: 10.1111/j.1365-2125.2011.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64(9):1294–1300. doi: 10.1111/j.1742-1241.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- 7.Geyer J, Gavrilova O, Petzinger E. The role of P-glycoprotein in limiting brain penetration of the peripherally acting anticholinergic overactive bladder drug trospium chloride. Drug Metab Dispos. 2009;37(7):371–374. doi: 10.1124/dmd.109.027144. [DOI] [PubMed] [Google Scholar]
- 8.Kay GG, Ebinger U. Preserving cognitive function for patients with overactive bladder: evidence for a differential effect with darifenacin. Int J Clin Pract. 2008;62(11):792–800. doi: 10.1111/j.1742-1241.2008.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flurkey K, Currer JM, Harrison DE. The mouse in aging research. In: Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A, editors. the mouse in biomedical research. 2. Massachusetts: Elsevier; 2007. pp. 637–672. [Google Scholar]
- 10.Klausner AP, Steers WD. Antimuscarinics for the treatment of overactive bladder: a review of central nervous system effects. Curr Urol Rep. 2007;8(6):441–447. doi: 10.1007/s11934-007-0046-0. [DOI] [PubMed] [Google Scholar]
- 11.Staskin DR. Overactive bladder in the elderly. A guide to pharmacological management. Drugs Aging. 2005;22(12):1013–1028. doi: 10.2165/00002512-200522120-00003. [DOI] [PubMed] [Google Scholar]
- 12.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Ärztebl Int. 2010;107(31–32):543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Ueno M, Nakagawa T, Wu B, et al. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17(12):1125–1138. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- 15.Crone C. The blood-brain barrier—facts and questions. In: Siesjö BK, Sorensen SC, editors. Ion Homeostasis of the Brain. Copenhagen: Munksgaard; 1971. pp. 52–62. [Google Scholar]
- 16.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89(11):1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::AID-JPS1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Clark DE. In silico prediction of blood-brain barrier permeation. Drug Discov Today. 2003;8(20):927–933. doi: 10.1016/S1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 18.Shah GN, Mooradian AD. Age-related changes in the blood-brain barrier. Exp Gerontol. 1997;32(4–5):501–519. doi: 10.1016/S0531-5565(96)00158-1. [DOI] [PubMed] [Google Scholar]
- 19.Wadhwani KC, Koistinaho J, Balbo A, Rapoport SI. Blood-nerve and blood-brain barrier permeabilities and nerve vascular space in Fischer-344 rats of different ages. Mech Ageing Dev. 1991;58(2–3):177–190. doi: 10.1016/0047-6374(91)90091-D. [DOI] [PubMed] [Google Scholar]
- 20.Rudick RA, Buell SJ. Integrity of blood-brain barrier to peroxidase in senescent mice. Neurobiol Aging. 1983;4(4):283–287. doi: 10.1016/0197-4580(83)90004-0. [DOI] [PubMed] [Google Scholar]
- 21.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease-systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2(8):506–511. doi: 10.1016/S1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 23.Chen RL, Chen CP, Preston JE. Elevation of CSF albumin in old sheep: relations to CSF turnover and albumin extraction at blood-CSF barrier. J Neurochem. 2010;113(5):1230–1239. doi: 10.1111/j.1471-4159.2010.06689.x. [DOI] [PubMed] [Google Scholar]
- 24.Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3–4):79–96. [PubMed] [Google Scholar]
- 25.Chen RL (2009) Is it appropriate to use albumin CSF/plasma ratio to assess blood brain barrier permeability? Neurobiol Aging. doi:10.1016/j.neurobiolaging.2008.08.024 [DOI] [PubMed]
- 26.Bartels AL, Kortekaas R, Bart J, et al. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30(11):1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Bauer M, Karch R, Neumann F, et al. Age dependency of cerebral P-gp function measured with (R)-[11C]verapamil and PET. Eur J Clin Pharmacol. 2009;65(9):941–946. doi: 10.1007/s00228-009-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelgesang S, Cascorbi I, Schroeder E, et al. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12(7):535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Warrington JS, Greenblatt DJ, von Moltke LL. The effect of age on P-glycoprotein expression and function in the Fischer-344 rat. J Pharmacol Exp Ther. 2004;309(2):730–736. doi: 10.1124/jpet.103.061234. [DOI] [PubMed] [Google Scholar]
- 30.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 31.Zeevi N, Pachter J, McCullough LD, Wolfson L, Kuchel GA. The blood-brain barrier: geriatric relevance of a critical brain-body interface. J Am Geriatr Soc. 2010;58(9):1749–1757. doi: 10.1111/j.1532-5415.2010.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapposelli S, Digiacomo M, Balsamo A. P-gp transporter and its role in neurodegenerative diseases. Curr Top Med Chem. 2009;9(2):209–217. doi: 10.2174/156802609787521544. [DOI] [PubMed] [Google Scholar]


