ABSTRACT
Carotid intima-media thickness (CIMT) can be reliably determined in vivo by carotidian ultrasound and is an accessible and reliable method to assess subclinical atherosclerosis. Available epidemiological data showed that CIMT is significantly correlated with future cardiovascular events. However it has limited value to help risk stratification on top of standard risk-derived functions such as Framingham risk score. It is particularly useful in individuals classified as being at intermediate or high risk by the presence of multiple conventional risk factors.
CIMT has a class IIa (LOE: B) reccommendation for cardiovascular risk assessment according to the practice guidelines published in 2010, emphasizing the presence of high risk if the common carotid artery intima–media thickness is above the 75th percentile. There is no indication to measure IMT in patients with full-blown atherosclerotic carotid disease, although carotidian ultrasound still remains a very useful tool to assess the severity of disease even in these subjects.
Progression of CIMT (also associated with increasing age) can be delayed by some drugs (statins, colestipol and niacin) and by risk factors modification. However, there is no consistent data demonstrating a link between progression of CIMT and coronary and cerebral events. Subsequently, studies using CIMT progression as primary outcome to indicate the influence of a certain therapy on cardiovascular risk are inherently misleading as suggested in the recently published ACC/AHA Guidelines.
Keywords: rheumatoid arthritis, inflammation, metabolic syndrome, accelerated atherosclerosis
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease that has a silent course for a few decades before symptoms and atherothrombotic complications occur (1); in this stage the disease already has major and poorly reversible histopathological consequences. Atherosclerosis progression is associated with inflammatory changes from its early stages to the most advanced, accompanied by thrombotic events (2). Considering the demonstrated relationship between inflammation and atherosclerosis it is of uttermost importance to identify early markers for the disease, such as inflammatory factors identifiable in serum or endothelial dysfunction and thickening in order to presumably prevent disease progression.
Carotid intima-media thickness (CIMT) is such a marker that can be used to identify subclinical atherosclerotic disease. Because it can be measured in a relatively simple and noninvasive way, it is well suited for use in large-scale population studies (3). ❑
ULTRASOUND METHODS TO ASSESS CAROTID INTIMA - MEDIA THICKNESS
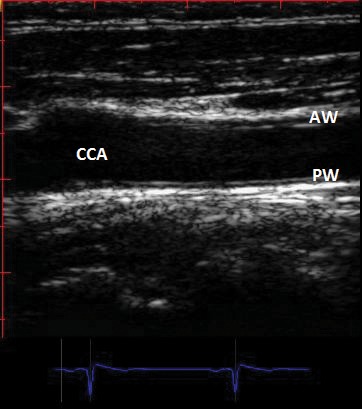
In 1986 Italian investigators compared direct measurements of arterial wall thickness of 18 human aorta and common carotid arteries by gross and microscopic examination with B mode real-time imaging of the same specimens (4). They described a characteristic B mode image of the arterial wall composed of two parallel echogenic lines separated by a hypoechoic space (Figure 1). The space between the two lines did not differ significantly from the media thickness measured on pathologic examination, leading the investigators to suggest that B mode imaging could present a useful approach to the measurement of IMT in vivo. Since then, calculation of carotid IMT (CIMT) is widely used as non-invasive measure of atherosclerosis currently employed by clinicians and clinical investigators to quantify the extent of subclinical disease; it could provide further benefit by quantifying atherosclerosis much earlier in its development in individual subjects with significant risk factors for cardiovascular disease, such as diabetics or patients with familial hypercholesterolemia. Employing B-mode ultrasound, the "double echo" pattern shown in the original paper to represent the combined width of the carotid artery intima and media, can be readily and reproducibly visualized in nearly all subjects (5). The space between the two hyperechoic lines is corresponding to the media; the carotid intima-media thickness (CIMT) represents the combined thickness of the hypoechoic space plus the hyperechoic line situated towards the interior of the vessel (Figure 1).
Figure 1. Two-dimensional longitudinal image of common carotid artery (CCA) showing double contour of anterior wall (AW) and posterior wall (PW) ("the double line" sign). The carotid intima- media thickness (CIMT) represents the combined thickness of the hypo-echoic space plus the hyper- echoic line situated towards the interior of the vessel. The measurement could be done at the level of anterior wall (near wall) or at the level of the posterior wall (far wall). See text.
Using B-mode imaging only, the common carotid artery (CCA) should be scanned along its length, in transverse and longitudinal sections, up to bifurcation, and along the internal carotid artery (ICA) and external carotid artery (ECA), as high up the neck can be seen (Figure 2). Typically, the carotid arteries are classified into three segments when undergoing ultrasound examination for measuring CIMT. The most proximal segment is the 1-cm straight segment of the extracranial carotid artery immediately prior to the bifurcation, the common carotid (CCA). Its distal boundary is identified by the divergence of the near and far walls as the artery begins to divide into its internal and external branches, ICA and ECA This focal widening of the bifurcation extends over approximately 1 cm and is labelled the carotid bulb (CB). Its distal margin is defined by the tip of the flow divider separating the diverging ICA and ECA. The final segment where the CIMT can be measured commonly is along the proximal 1 cm of the ICA (Figure 2). So, we can measure CIMT in the all three segments described above: along 1 cm of the distal CCA, at the level of CB and along the 1 cm of the proximal ICA.
Figure 2. 2D aspect of the common carotid artery bifurcation: internal carotid artery (ICA) and external carotid artery (ECA).

Although atherosclerosis and CIMT progress more rapidly in the bulb and internal carotid segments, limiting CIMT measurements to the far wall of the CCA is the preferred strategy for clinical testing, as stated in current guidelines for CIMT measurement. The term "near wall" is referring at the closest wall to the echo probe. In addition the "far wall" of the CCA is defined as the farthest carotid wall to the transducer. Because of superficial location, ease of accessibility and limited movement, the far wall of the CCA provides a convenient window to study arterial structure using B-mode ultrasound (6). In addition near wall CIMT is less accurate because the ultrasound beam is traveling from more echogenic to less echogenic layers at the adventitia-media and intima-lumen interfaces of the near wall.
In healthy middle-aged adult population, the CIMT at the level at CCA measures 0,6-0,7 mm. In the carotid bulb CIMT is generally higher, whereas values for the ICA resemble those of the CCA in healthy individuals. Thickness varies with age, gender, and ethnicity. It increases with age and is generally thicker in men than in women (6).
Normal values for CIMT are difficult to provide because the absolute value also depends on the location of the measurement (segments, near, or far wall), the ultrasound equipment used, and the off-line reading system employed (automated or manual tracings) (7) in addition to risk factors accumulation with older age. Generally, values of CIMT more than 0.9 mm are considered abnormal.
These latter aspects are some of the reasons why mean CIMT values may differ considerably between studies (Table 1). The design of CIMT trials has varied. Some studied only the CCA, while others included all three segments; some imaged only the far wall, others both the near and far wall. Even today, no single 'best practice' CIMT protocol has emerged, but for standardization purposes the far wall of the CCA should be used in clinical practice. The current guidelines recommend that ultrasound images of the distal 1 cm of the far wall of each CCA should be obtained and compared with values from a normative data set, averaging the values of CIMT over at least a 1-cm segment as is reported in observational studies.
Table 1.
Comparison of age range, CCA IMT and reproducibility from different populations (3)
| Intersonographer Reproducibility | |||||
|---|---|---|---|---|---|
| Population | Mean Age, y | Mean IMT, mm | Maximal IMT, mm | Intraclass Correlation Coefficient of CCA IMT | Average Absolute Difference of Replicate Measures, mm |
| ARIC*11,13 | 54 | 0.63±0.16 | ... | 0.48 | 0.17±0.17 |
| Rotterdam Study14 | 70 | 0.80±0.20 | 1.03±0.22 | 0.78-0.84 | 0.06±0.09 † |
| CHS12 | 73 | ... | 1.09±0.20 | ICC not given, r2 = 0.52 | 0.20±0.26 |
| Kitamura 200416 | 66 | ... | 1.03±0.43 | Not given ∫ | Not given ∫ |
| MDCS17 | 57 | 0.77±0.15 †,‡ | ... | ICC not given, r2 = 0.85 | 0.10±0.10 |
| CAPS19 | 50 | 0.73±0.16 | ... | 0.97 | 0.11±0.08 |
* Missing values if IMT sites were imputed; IMT values were adjusted according to reader differences. All statistics are the authors' own calculations on the ARIC dataset.
† Ascertained by contacting the corresponding author directly.
‡ Only right side was examined.
∫ Only 1 observer.
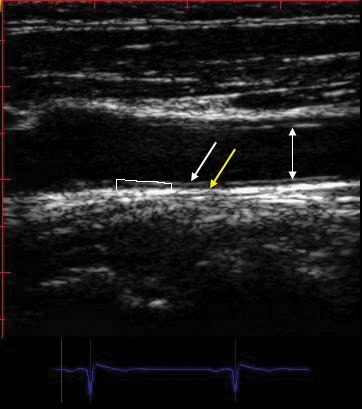
Figure 3. Example measurement of far wall common carotid artery (CCA) intima-media thickness (CIMT). The arrows from top to bottom show: the near wall, lumen and the far wall. The white arrowhead marks media – adventitia boundary. Measurement of CIMT involves tracing the blood-intima and media-adventitia interfaces of the far wall using a leading edge–to–leading edge technique along a 1 cm region. Mean CIMT should be reported. Simple point by point measurement are not accepted.
CIMT values greater than or equal to 75th percentile are considered high and indicative of increased CVD risk (Table 2A, 2B). Values in the 25th to 75th percentile are considered average and indicative of unchanged CVD risk. Values less than or equal to 25th percentile are considered lower CVD risk, but whether or not do they justify less aggressive preventive therapy than standard care is not known. These broad levels of risk should be reported (6). Mean CIMT values from the far walls of both right and left CCAs (mean-mean) should be reported. A thorough scan of the extracranial carotid arteries for the presence of plaques should be performed in order to increase sensitivity for identifying subclinical vascular disease.
Table 2A.
Comparison of age range, CCA IMT and reproducibility from different populations (3)
| Right common carotid artery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| Age, y/percentile | ≤30 | 31-40 | 41-50 | >50 | ≤30 | 31-40 | 41-50 | >50 |
| 25th | 0.39 | 0.42 | 0.46 | 0.46 | 0.39 | 0.42 | 0.44 | 0.50 |
| 50th | 0.43 | 0.46 | 0.50 | 0.52 | 0.40 | 0.45 | 0.48 | 0.54 |
| 75th | 0.48 | 0.50 | 0.57 | 0.62 | 0.43 | 0.49 | 0.53 | 0.59 |
| Left common carotid artery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| Age, y/percentile | ≤30 | 31-40 | 41-50 | >50 | ≤30 | 31-40 | 41-50 | >50 |
| 25th | 0.42 | 0.44 | 0.50 | 0.53 | 0.30 | 0.44 | 0.46 | 0.52 |
| 50th | 0.44 | 0.47 | 0.55 | 0.61 | 0.44 | 0.47 | 0.51 | 0.59 |
| 75th | 0.49 | 0.57 | 0.61 | 0.70 | 0.47 | 0.51 | 0.57 | 0.64 |
Table 2B.
Mean far wall common carotid artery carotid intima-media thickness values from the Carotid Atherosclerosis Progression Study (19)
| Male | |||||||
|---|---|---|---|---|---|---|---|
| Age, y/percentile | 25 | 35 | 45 | 55 | 65 | 75 | 85 |
| 25th | 0.515 | 0.585 | 0.634 | 0.68 | 0.745 | 0.814 | 0.63 |
| 50th | 0.567 | 0.633 | 0.686 | 0.746 | 0.83 | 0.914 | 0.937 |
| 75th | 0.633 | 0.682 | 0.756 | 0.637 | 0.921 | 1.028 | 1.208 |
| Female | |||||||
|---|---|---|---|---|---|---|---|
| Age, y/percentile | 25 | 35 | 45 | 55 | 65 | 75 | 85 |
| 25th | 0.524 | 0.575 | 0.619 | 0.665 | 0.718 | 0.771 | 0.807 |
| 50th | 0.567 | 0.615 | 0.665 | 0.719 | 0.778 | 0.837 | 0.880 |
| 75th | 0.612 | 0.66 | 0.713 | 0.776 | 0.852 | 0.921 | 0.935 |
Carotid plaque has been defined either as a focal structure that encroaches the arterial lumen at least 0.5 mm or 50% of the surrounding IMT value or a thickness >1.5 mm as measured from the media–adventia interface to the intima–lumen interface (8). Measurement of CIMT should be done in plaques free regions according with the guidelines recommendations (3). Nevertheless, if plaques are detected in the measured segment, they should be traced as part of the CIMT because they appear to have been included in CIMT measurements in most of the epidemiologic studies (3). ❑
CAROTID ULTRASOUND IMAGING PITFALLS AND POTENTIAL SOLUTIONS
The carotid arteries should be interrogated using an ultrasound system with a linear-array transducer operating at a fundamental frequency of at least 7 MHz. Use of nonfundamental frequencies can erroneously increase wall thickness.
Use of ultrasonic contrast is reserved for research and is not recommended for CIMT evaluation in current practice.
Using the zoom function of the echo machine is discouraged because most studies that correlated CIMT with cardiovascular events did not use zoomed images.
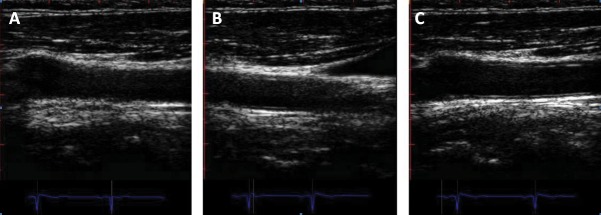
Several pitfalls and problems could be encountered in ultrasound examination of carotid arteries. Lack of "double-line" sign could be overcome by repeated attempts to obtain horizontal positioning of the vessel on the screen, moving the transducer perpendicular to the vessel, and adjusting focus and gain. In case of excessive vessel tortuosity, further extension and slightly neck rotation elongate the vessel segment. If the translation artifact from pulsatile jugular vein appears in the screen, have patient hold breath at mid-inspiration to stabilize image. This is one of the most important methodological issues to correctly assess CIMT (Figure 4).
Figure 4. Carotid ultrasound imaging pitfalls and potential solutions. 4A. Improper allignement of transducer with the vessel leads to lost of double lines sign at the level of common carotid artery. 4B. Image not horizontal. 4C. Correct alllignement of the transducer along with the common carotid artery segment.
Another important issue is related to the ultrasound system. Before starting the examination of carotid arteries one should ensure proper monitor settings, adjust time-gain compensators and overall gain in order to avoid under- or over-gained images that could erroneously thicken CIMT.
Using the proper depth is another important technical issue. Most patients can be scanned at a standard depth of 4 cm; however increased depth may be necessary in some patients with larger necks or deeper vessels. Resolution decreases by increasing imaging depth. The typical pixel size when imaging at a 4-cm depth is approximately 0.11 mm. Because CIMT measurements are extremely small, differences of one digital pixel can classify patients in different risk categories, so close attention to instrumentation and standardized imaging and reading protocols are critical (6).
Velazquez et al reported a very high (97.2%) interobserver correlation of the CIMT measurements using a standardized protocol. The result of their study show that CIMT measurement using THI (tissue harmonic imaging) sonography is accurate and reproducible (36).
In follow-up studies intrasonographer variability (expressed as SD of the mean absolute difference of paired replicate scans) of IMT measurements is around 0.04 mm in children (37), and 0.2 mm in older patients with peripheral vascular disease (38,39). In controlled clinical trials, measurement variability is decreasing (42). This is most likely due to the result of technical improvements, standardization and training. In studies carried out between 1985 and 1990, the measurement SD as calculated above was 0.2 mm in patients with coronary artery disease (37,39), whereas currently this figure is approximately 0.09 mm (40). Moreover, two decades ago intraclass correlations of 0.60–0.75 mm were reported, whereas currently they are often 0.90 mm or higher (41) (Table 1). ❑
CLINICAL SIGNIFICANCE OF CAROTID IMT
Many epidemiological studies established that CIMT is a marker of subclinical atherosclerosis associated with conventional CVD risk factors. An important condition to use CIMT to predict CVD is to demonstrate that it is associated with both prevalence and incidence of clinical vascular events. A number of longitudinal studies examined the relationship between CIMT and future events, most frequently the incidence of cardiac events (myocardial infarction [MI], angina pectoris and coronary revascularization) and cerebrovascular events (stroke or transient ischemic attacks [TIA]) (9-19).
In 2007 Lorenz et al published a systematic review and meta-analysis of eight relevant general population-based studies that previously reported on the ability of CIMT to predict future cardiovascular end points, involving a total of 37.197 subjects followed for a mean of 5.5 years (3). They reported that for an absolute CIMT difference of 0.1 mm, the future risk of MI increases by 10-15%, and the stroke risk increases by 13-18%.
Large follow-up studies such as the Rotterdam Study (10,14) and the Atherosclerosis Risk in Communities Study (ARIC) (11,13) have used B-mode ultrasonography to measure IMT to investigate the determinants of atherosclerotic disease in the general population. Rotterdam Study was a single-center, prospective, follow-up study of 7,983 individuals older than 55 years. The study also provided evidence for associations between carotid IMT and stroke, angina pectoris, myocardial infarction, intermittent claudication and essential hypertension (10,14). In ARIC, a US-based study that involved 15,800 adults, high-resolution B-mode ultrasonography was able to identify atherosclerotic lesions at all stages of development (11, 13). A seemingly small increase in mean carotid IMT of 0.2 mm was associated with an increase in relative risk for myocardial infarction and stroke of 33% and 28%, respectively – a link subsequently confirmed by many other studies (Table 3).
Table 3.
Comparison of age range and events rates from different trials (3)
| Population | Mean Age, y | Event Rate for MI (per 100 Person-Years) | Event Rate for Stroke (per 100 Person-Years) |
|---|---|---|---|
| ARIC11,13 | 54 | 4.4 | 2.4 |
| Rotterdam Study14 | 70 | ... | 11.3 |
| CHS12 | 73 | 9.6 | 10.2 |
| Kitamura 200416 | 66 | ... | 5.9 |
| MDCS17 | 57 | 3.2 | 2.4 |
| CAPS19 | 50 | 10.7 | 5.0 |
Currently over 20 cohort studies performed among subjects with or without previous vascular disease, and with and without CVD risk factors, showed consistently that increased CIMT relates to increased cardiovascular risk, independently of established vascular risk factors (5). ❑
INCREMENTAL VALUE FOR RISK STRATIFICATION
Although ultrasonically-derived carotid wall measurements provide a useful new tool in assessing CVD risk, it was less certain how much additional value CIMT measurement added to conventional risk-assessment methods such as the Framingham risk score or the Systematic Coronary Risk Evaluation (SCORE) system developed by the European Society of Cardiology in high and low risk regions of Europe (5). Framingham divides subjects into three risk categories based principally on age, gender, total cholesterol, systolic blood pressure and smoking: low (10% risk of a vascular event in 10 years), intermediate (10-20%) and high (20%).
A recent systematic review of the role of CIMT measurements in CVD screening concluded that the use of CIMT has limited value to help risk stratification on top of standard risk-derived functions such as Framingham risk score or SCORE (20).
Polack at al studied the value of intima–media thickness of the walls of the common carotid artery and internal carotid artery added to the Framingham risk score for predicting cardiovascular events (21). Their data clearly showed that addition of the intima–media thickness of the internal carotid artery, but not that of the common carotid artery, increases the net reclassification index for risk categories based on the Framingham risk factors. The maximum intima–media thickness of the internal carotid artery, as either a continuous measurement or a surrogate for the presence of plaque (above a threshold of 1.5 mm), contributed significantly but modestly to the predictive power of the risk factors used in calculating the Framingham risk score. The results of this study may have implications on how intima–media thickness should be used for the primary prevention of cardiovascular disease.
Data from the ARIC study suggest that carotid IMT data may enhance cardiovascular risk assessment, particularly among individuals classified as being at intermediate risk by use of conventional risk factors (22-23).
Nevertheless, the American College of Cardiology Foundation–American Heart Association guidelines published in 2010 establish a level IIa reccommendation for carotid intima–media thickness with the purpose of cardiovascular risk assessment (the same as the recommendation level for the ankle–brachial index or coronary-artery calcium scoring by multislice-CT) (24), with an emphasis of high risk if the common carotid artery intima–media thickness is above the 75th percentile (6). Besides that, the last American College of Cardiology Foundation–American Heart Association guidelines recently published, do not reccommend yet CIMT assessment as a screening method for atherothrombotic risk per se. There is no indication to measure IMT in patients with full-blown atherosclerotic disease expressed by the presence of carotid plaques or stenosis (25).
Surprisingly these guidelines lack quantitative criteria for the intima–media thickness of the internal carotid artery for risk classification in asymptomatic patients. ❑
RELATIONSHIP BETWEEN CIMT AND INFLAMMATORY MARKERS
A number of soluble markers of inflammation, endothelial damage and hemostasis have all been linked with the atherosclerotic process. Carotid IMT increases naturally with aging, but conventional and nonconventional atherosclerosis risk factors may accelerate the process (26). More than a hundred studies have been published so far associating CIMT to these soluble markers, with controversial results.
In a recently published trial, enrolling 5888 patients older than 65 years of age and who did not have proven CV disease on trial entry, with a 12 years follow up, a relationship between serum CRP and CIMT was noticed (27). CRP was not related to cardiovascular mortality when carotid atherosclerosis was lacking on inclusion as assessed by a normal IMT.
A systematic review of studies addressing the association between subclinical carotid atherosclerosis and soluble markers of inflammation, endothelial damage or hemostasis showed that plasma CRP and fibrinogen levels are the variables most consistently related to CIMT (28). No clear conclusions could be drawn for other soluble markers. Atherosclerotic burden didn't appear to account for the heterogeneity of the findings reported in the literature.
Among other patients characteristics, gender, presence of diabetes, and hypercholesterolemia were found to influence the association between CIMT and CRP, whereas blood pressure and hypercholesterolemia seemed to affect the association between CIMT and fibrinogen. For the other soluble markers investigated, the groups were too small for adequate statistical assessment. ❑
CIMT PROGRESSION IN INTERVENTION TRIALS
Because external ultrasound is non-invasive and has no known risk, early consideration was given to obtaining longitudinal CIMT measures in order to assess the impact of treatment on individuals.
Epidemiological studies showed that IMT progresses at an average rate of ≤0.03 mm per year (30-33). Progression can be delayed by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), the combination of colestipol and niacin and risk factor modifications (29-33).
However, there is no consistent data demonstrating a link between progression of CIMT and coronary and cerebral ischemic events (34). For these reasons, studies incorporating CIMT as a primary outcome to indicate cardiovascular risk of an intervention are inherently misleading.
The recently published simvastatin with or without ezetimibe Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial failed to show significant group differences in any of several end points with respect to CIMT, despite significant decreases in low-density lipoprotein cholesterol and C-reactive protein in patients with familial hypercholesterolemia (35). This study illustrates limitations in present technology in a very high risk patient subset, which does not necessarily imply the drugs were ineffective: there was a greater reduction in low-density lipoprotein cholesterol in the combined simvastatin plus ezitimibe group versus simvastatin monotherapy.
The eventual development of a necrotic core is considered a key indicator of significant plaque progression and a recognized feature of lesion vulnerability (34). Initiation of disease-related plaques begins as what is referred to as pathological intimal thickening, lesions characterized by the formation of lipid pools in the absence of a necrotic core. Necrotic cores are thought to arise from macrophage infiltration of lipid pools followed by secondary necrosis where defective clearance of debris, tissue disruption proteases, and intraplaque hemorrhage, likely contribute to its enlargement. The reliable detection of an enlarging necrotic core may identify a class of patients who stand to benefit from therapies targeted at plaque stabilization (34).
Moreover in most cases measures of plaque area or volume are generally considered better predictors of an inflammatory process consistent with atherosclerotic disease rather than intimal medial thickness (34).
Thus, the use of IMT measurements to guide treatment based on outcomes of specific interventions for patients has not been documented, as suggested in the recently published ACC/AHA Guidelines (25). ❑
CONCLUSION
Carotid ultrasound is likely to remain among the most widely employed imaging techniques for the tracking and quantification of subclinical atherosclerosis.
The major advantage of CIMT assessment is that it is completely non-invasive and can be repeated as often as required, it is relatively inexpensive to perform, the technology is widely available and its methodology is clearly standardized.
The clinician should take into account the global picture of the patient when CIMT assessment is used. When an increased CIMT is found in a young patient, otherwise healthy, the result could be interpreted with caution. But if this adds to other cardiovascular risk factors, the patient should be treated accordingly.
CIMT should not be use like screening method for atherosclerotic risk assessment per se.
We currently do not know whether a reduction in the progression or a regression of the carotid intima–media thickness after pharmacologic treatment is consistently associated with a risk reduction for atherothrombotic events. Thus, the measurement of CIMT in guide treatment on specific outcomes is not recommended.
The major limitations in the assessment of CIMT for prediction of cardiovascular events are: first, the association of CIMT and that of coronary heart events are modest and second, absence of data demonstrating a link between progression of CIMT and coronary and cerebral events.
Another aspect that should be bear in mind is that IMT of the internal carotid artery may contribute significantly to the predictive power of conventional risk factors used to calculate the Framingham risk score, but only modestly improve risk classification based on this score system. ❑
ACKNOWLEDGEMENTS
This work is supported by a CNCSIS – UEFISCSU Grant, project number PNII – IDEI, code 257/2008.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Plutzky J. Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am J Cardiol. 2001;88:10K–15K. doi: 10.1016/s0002-9149(01)01924-5. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz M. Prediction of clinical cardiovascular events with carotid intima media thickness. A systematic review and meta-analysis. Circulation. 2007;115:459–167. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 4.Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 5.O'Leary DH, Bots ML. Imaging of atherosclerosis: carotid intima-media thickness. Eur Heart J. 2010;31:1682–9. doi: 10.1093/eurheartj/ehq185. [DOI] [PubMed] [Google Scholar]
- 6.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Bots ML, Evans GW, Riley WA, et al. Carotid intima-media thickness measurements in interventional studies. Design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34:2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 8.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 9.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87(supplII):56–65. [PubMed] [Google Scholar]
- 10.Bots ML, Hoes AW, Koudstaal PJ, et al. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 11.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study (CHS) Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 13.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias del Sol A, Bots ML, Grobbee DE et al. Carotid intima-media thickness at different sites: relation to incident myocardial infarction; the Rotterdam Study. Eur Heart J. 2002;23:934–40. doi: 10.1053/euhj.2001.2965. [DOI] [PubMed] [Google Scholar]
- 15.Hollander M, Hak AE, Koudstaal PJ, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34:2367–72. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura A, Iso H, Imano H, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke. 2004;35:2788–94. doi: 10.1161/01.STR.0000147723.52033.9e. [DOI] [PubMed] [Google Scholar]
- 17.Rosvall M, Janzon L, Berglund G, et al. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med. 2005;257:430–7. doi: 10.1111/j.1365-2796.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosvall M, Janzon L, Berglund G, et al. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis. 2005;179:325–31. doi: 10.1016/j.atherosclerosis.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz MW, von Kegler S, Steinmetz H, et al. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 20.Plantinga Y, Dogan S, Grobbee DE, et al. Carotid intima-media thickness measurement in cardiovascular screening programmes. Eur J Cardiovasc Prev Rehabil. 2009;16:639–44. doi: 10.1097/HJR.0b013e3283312ece. [DOI] [PubMed] [Google Scholar]
- 21.Polak J, Pencina M, Pencina K, et al. Carotid-wall intima–media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–7. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein JH, Johnson HM. Carotid intima-media thickness, plaques, and cardiovascular disease risk: implications for preventive cardiology guidelines. J Am Coll Cardiol. 2010;55:1608–10. doi: 10.1016/j.jacc.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 24.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:2748–64. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 25.Brott T, Halperin JL, Abbara S, et al. Guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:e16–e94. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Simon A, Gariepy J, Chironi G, et al. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159–69. doi: 10.1097/00004872-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Cao J, Arnold A, Manolio T, et al. Association of carotid artery intima media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality The Cardiovascular Health Study. Circulation. 2007;116:32–8. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 28.Baldassarre D, de Jong A, Amato M, et al. Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med. 2008;40:21–44. doi: 10.1080/07853890701645399. [DOI] [PubMed] [Google Scholar]
- 29.Blankenhorn DH, Selzer RH, Crawford DW, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery: two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation. 1993;88:20–8. doi: 10.1161/01.cir.88.1.20. [DOI] [PubMed] [Google Scholar]
- 30.Furberg CD, Adams HP, Applegate WB, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation. 1994;90:1679–87. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 31.Hodis HN, Mack WJ, LaBree L, et al. Reduction in carotid arterial wall thickness using lovastatin and dietary therapy: a randomized controlled clinical trial. Ann Intern Med. 1996;124:548–56. doi: 10.7326/0003-4819-124-6-199603150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee D, Yadav JS. Carotid artery intimal-medial thickness: indicator of atherosclerotic burden and response to risk factor modification. Am Heart J. 2002;144:753–9. doi: 10.1067/mhj.2002.124865. [DOI] [PubMed] [Google Scholar]
- 33.Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses: Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1999;100:e14–7. doi: 10.1161/01.cir.100.3.e14. [DOI] [PubMed] [Google Scholar]
- 34.Finn A, Kolodgie F, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis. A point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–81. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 35.Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 36.Velazquez F, Berna JD, Abellan JL, et al. Reproducibility of sonographic measurement of carotid intima-media thickness. Acta Radiol. 2008;49:1162–6. doi: 10.1080/02841850802438520. [DOI] [PubMed] [Google Scholar]
- 37.de Groot E, Jukema JW, Montauban van Swijndregt AD, et al. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary angiographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS). J Am Coll Cardiol. 1998;31:1561–7. doi: 10.1016/s0735-1097(98)00170-3. [DOI] [PubMed] [Google Scholar]
- 38.Sramek A, Bosch JG, Reiber JH, et al. Ultrasound assessment of atherosclerotic vessel wall changes: reproducibility of intima–media thickness measurements in carotid and femoral arteries. Invest Radiol. 2000;35:699–706. doi: 10.1097/00004424-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomized, double-blind trial. Lancet. 2001;357:577–581. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 40.Meuwese MC, et al. Effect of ACAT inhibition on carotid atherosclerosis in familial hypercholesterolemia. J Clin Lipidol. 2007;1:382–382. [abstract] [Google Scholar]
- 41.Bots ML, Evans GW, Riley WA, et al. Carotid intima–media thickness measurements in intervention studies. Designs options, progression rates and sample size considerations: a point of view. Stroke. 2003;34:2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 42.de Groot E, van Leuven I, Duivenvoorden R, et al. Measurement of carotid intima–media thickness to assess progression and regression of atherosclerosis. Cardiovasc Med. 2008;8:280–88. doi: 10.1038/ncpcardio1163. [DOI] [PubMed] [Google Scholar]
- 43.Simon A, Gariepy J, Chironi G, et al. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159–69. doi: 10.1097/00004872-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Denarie N, Gariepy J, Chironi G, et al. Distribution of ultrasonographically-assessed dimensions of common carotid arteries in healthy adults of both sexes. Atherosclerosis. 2000;148:297–302. doi: 10.1016/s0021-9150(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 45.Allan PL, Mowbray PI, Lee AJ, et al. Relationship between carotid intima-media thickness and symptomatic and asymptomatic peripheral arterial disease: the Edinburgh artery study. Stroke. 1997;28:348–53. doi: 10.1161/01.str.28.2.348. [DOI] [PubMed] [Google Scholar]


