Abstract
Background
The prevalence of cardiometabolic disease in Africa now rivals that of Western nations. Therefore, screening programs that lead to effective prevention of cardiometabolic disease in Africans is imperative. Most screening tests for cardiometabolic disease use triglyceride (TG) levels as a criterion. However, the failure rate of TG-based screening tests in African Americans is high. In Africans, the efficacy of TG-based screening tests is unknown. Our goal was to determine the association between hypertriglyceridemia (TG ≥150 mg/dL) and cardiometabolic disease in African and African-American men.
Research Design and Methods
This was a cross-sectional study of 155 men (80 African immigrants, 75 African Americans) [age, 35±9 years, mean±standard deviation (SD), body mass index (BMI) 28.5±5.2 kg/m2] who self-identified as healthy. Lipid profiles were performed. Glucose tolerance and insulin resistance was determined by oral glucose tolerance tests (OGTT) and the insulin sensitivity index (SI), respectively. Cardiometabolic disease was defined by four possible subtypes—prediabetes, diabetes, insulin resistance, or metabolic triad [hyperinsulinemia, hyperapolipoprotein B, small low-density lipoprotein (LDL) particles].
Results
TG levels were higher in men with cardiometabolic disease than without (88±43 versus 61±26 mg/dL, P<0.01). However, <10% of men with cardiometabolic disease had TG ≥150 mg/dL. Even within each cardiometabolic disease subtype, the prevalence of TG ≥150 mg/dL was <10%. Furthermore, TG levels in the 5% of men identified by OGTT as diabetic were ≤100 mg/dL (mean 71±24, range 45–100 mg/dL).
Conclusions
Hypertriglyceridemia is a poor marker of cardiometabolic disease in men of African descent. Therefore TG-based screening tests fail to identify both African immigrants and African-American men with cardiometabolic disease. As a consequence, the opportunity for early intervention and prevention is lost.
Background
As the prevalence of cardiometabolic disease reaches epidemic proportions in Africa, effective screening and treatment is an African health-care imperative.1 Most screening tests for cardiometabolic disease use triglyceride (TG) levels as a criterion.2–5 However, in contrast to whites, Hispanics, and Asians, TG levels in African Americans with cardiometabolic disease are typically normal.6–15 Therefore, TG-based tests usually fail to recognize African Americans at risk.16 It is possible that the African-American experience with cardiometabolic disease screening will forecast the African experience. Prior to investing scarce health-care resources, it is critical to determine the effectiveness of TG-based screening tests in Africans.
Studies in whites, Hispanics, and Asians show a powerful association between hypertriglyceridemia and cardiovascular disease.7–9 As a result, TG levels have been incorporated into many screening paradigms, including the metabolic syndrome and the hypertriglyceridemic waist, which is better known in Canada and Europe than in the United States.2,17–21 In whites, Hispanics, and Asians, the link between cardiometabolic disease and hypertriglyceridemia is insulin resistance.2,7 However, the association between hypertriglyceridemia and insulin resistance is not universal. Insulin-resistant African Americans are much more likely than whites or Hispanics to have normal TG levels.12–14,22 Therefore, cardiometabolic disease in African Americans develops due to TG-independent pathways such as inflammation, endothelial dysfunction, and hypertension.23,24
To determine the efficacy of TG-based screening tests in men of African descent, we studied the association between hypertriglyceridemia (TG ≥150 mg/dL) and cardiometabolic disease in both African immigrants to the United States and in African Americans. Cardiometabolic disease was defined by four subtypes, each of which is highly associated with coronary artery disease—prediabetes, diabetes, insulin resistance, or metabolic triad.2,25,26 The metabolic triad consists of fasting hyperinsulinemia, hyperapolipoprotein B, and small low-density lipoprotein (LDL) particles.26
Methods
The participants were 155 men (52% African, 48% African American) who self-identified as healthy and were enrolled in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) protocol Diabetes and Heart Disease in blacks. Sixty percent of the men had participated in a previous study of the metabolic syndrome.27 African Americans were born in the United States with both parents self-identifying as African Americans born in the United States. Africans were born in Africa (55% west, 30% central, 15% east) and immigrated to the Washington, DC, area [age at immigration 26±8 years (mean±standard deviation (SD), years in United States 10±10 years]. No subjects were taking hypoglycemics or hypolipidemics. Recruitment was achieved with newspaper advertisements, flyers, and websites. The study was approved by the NIDDK Institutional Review Board. Subjects gave informed consent.
The study consisted of three outpatient visits to the Clinical Research Center. The first visit was a screening and was performed without regard to time of day. The second and third visits were performed at 7 AM after a 12-h fast. The screening visit was done to confirm that the subject felt well and had a normal physical exam and no clinically significant evidence of liver, kidney, or thyroid dysfunction. At the second visit fasting lipids were obtained and a 75-gram oral glucose tolerance test (OGTT; Trutol75, Fisher Diagnostics, Middletown, VA) was performed with glucose and insulin measured at 0 and 2 h. On the basis of elevated 2-h glucose levels, 5 of the 155 participants met the American Diabetes Association (ADA) criteria for diabetes28 and did not proceed to visit 3. Of the remaining 150 participants, 137 underwent an insulin-modified frequently sampled intravenous glucose tolerance test (IM-FSIGT).29 Scheduling conflicts were the most common reason for not undergoing the IM-FSIGT.
For the IM-FSIGT, an intravenous catheter was placed in each antecubital vein. At time 0, dextrose (0.3 g/kg) was intravenously administered over 1 min. Starting at the 20-min time point, insulin (4 mU/kg−1·min−1) was infused for 5 min. Blood samples for the measurement of glucose and insulin levels were obtained at −10, −5, −1, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 min. IM-FSIGT data were used to calculate the insulin sensitivity index (SI) and acute insulin response to glucose (AIRg). SI was determined using minimal model analyses (MinMod Millenium V6.02; Los Angeles, CA).30 AIRg, a measure of β-cell function, was calculated as the area under the insulin curve from 0 to 10 min for the insulin concentration above basal level.30
Cardiometabolic disease
Cardiometabolic disease was defined by the presence of prediabetes, diabetes, insulin resistance, or metabolic triad. Prediabetes was diagnosed if fasting glucose was between 100 mg/dL and 125 mg/dL or 2-h glucose was between 140 mg/dL and 199 mg/dL.28 Diabetes was defined as fasting glucose ≥126 mg/dL or 2-h glucose ≥200 mg/dL.28 To identify insulin resistance, the population was divided into quartiles of SI. Insulin resistance was defined as the lowest quartile of SI (<2.37 liter/mU−1·min−1). As described by Lemieux et al., metabolic triad was defined by the presence of fasting hyperinsulinemia (≥2.3 μU/mL), hyperapolipoprotein B (≥70 mg/dL), and small LDL particles (<21.4 nm), with thresholds determined by the median values in the normal weight men (BMI <25 kg/m2).3
Abdominal adipose tissue
Visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAT) measurements were obtained with a density mask inclusive of pixels with attenuation values from −150 to −50 Hounsfield units using a HiSpeed Advantage CT/I scanner (GE Medical Systems, Milwaukee, WI). Measurements were analyzed on a SUN workstation using the MEDx image analysis software package (Sensor System, Inc., Sterling, VA).31
Assays
Cholesterol, TG, high-density lipoprotein cholesterol (HDL-C), and apolipoprotein B (ApoB) levels were determined with a Siemens Dimension Vista analyzer (Newark, DE). LDL-C was calculated with the Friedewald equation.32 Lipid particle size was determined by nuclear magnetic resonance (NMR) spectroscopy (LipoScience, Raleigh, NC). Glucose was measured by the glucose oxidase method (Siemens Dimension Vista analyzer) and insulin by the electrochemiluminescence immunoassay (Roche Cobas e601 analyzer, Indianapolis, IN).
Statistics
Comparisons were by the Student t-test or chi-squared test. P value ≤0.05 was considered significant. Analyses were performed with STATA, v12.0.
Results
Participant characteristics are provided in Table 1. Although age did not differ between the groups, BMI was lower in Africans than African Americans. Waist circumference (WC), adjusted for BMI, did not differ by ethnicity. VAT was greater and SAT was lower in Africans than African Americans. Systolic and diastolic blood pressures were higher in Africans than African Americans. Africans were more likely to be married and less likely to smoke (Table 1). Exercise frequency and alcohol intake did not differ by ethnicity (Table 1). In Africans, average weight gain after immigration was 3±7 kg/year.
Table 1.
Subject Characteristics
| Parameter | African n=80 | African American n=75 | P valuea |
|---|---|---|---|
| Age (years) | 36±9 | 35±8 | 0.33 |
| BMI (kg/m2) | 27.3±3.8 | 29.8±6.2 | <0.01 |
| WC (cm)b | 93±6 | 95±6 | 0.11 |
| VAT (cm2)b,c | 117±45 | 93±45 | <0.01 |
| SAT (cm2)b,c | 142±66 | 165±64 | 0.04 |
| Systolic blood pressure (mmHg) | 126±13 | 121±12 | 0.01 |
| Diastolic blood pressure (mmHg) | 76±9 | 71±10 | <0.01 |
| Married | 46% | 28% | 0.02 |
| Smoke | 6% | 21% | <0.01 |
| Exercise frequency (≥3×/week) | 27% | 35% | 0.25 |
| Alcohol intake (≤1/week) | 73% | 73% | 0.96 |
| Cardiometabolic disease | 63% | 65% | 0.71 |
| Insulin resistance | 23% | 27% | 0.66 |
| Prediabetes | 40% | 23% | 0.02 |
| Diabetes | 6% | 0% | 0.02 |
| Metabolic triad | 33% | 52% | 0.01 |
| Fasting glucose (mg/dL) | 92±8 | 88±8 | <0.01 |
| 2-h glucose (mg/dL) | 130±35 | 118±25 | 0.01 |
| Fasting insulin (μU/mL) | 4.0±3.4 | 7.6±5.2 | <0.01 |
| SI (liter/mU−1·min−1)c | 4.86±3.25 | 4.17±2.66 | 0.18 |
| AIRg (mU/liter−1·min)c | 604±436 | 874±758 | 0.01 |
| Cholesterol (mg/dL) | 158±31 | 174±41 | 0.01 |
| TG (mg/dL) | 74±40 | 83±39 | 0.18 |
| HDL-C (mg/dL) | 46±11 | 46±10 | 0.73 |
| HDL size (nm) | 9.17±0.41 | 8.84±0.44 | <0.01 |
| LDL-C (mg/dL) | 97±28 | 111±39 | 0.01 |
| LDL size (nm) | 21.0±0.7 | 20.9±0.6 | 0.40 |
| ApoB (mg/dL) | 80±21 | 87±30 | 0.07 |
Continuous variables compared by the Student t-test and categorical variables by chi-squared test.
Adjusted for BMI.
Data in 69 Africans, 68 African Americans.
BMI, body mass index; WC, waist circumference; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; SI, insulin sensitivity index; AIRg, acute insulin response to glucose; TG, triglycerides; HDL-C, high-density lipoprotein choesterol; LDL-C, low-density lipoprotein cholesterol; ApoB, apolipoprotein B.
Our key finding is that even though men with cardiomtabolic disease had higher TG levels than men without (88±43 vs. 61±26 mg/dL, P<0.01), less than 10% of men with cardiometabolic disease had TG ≥150 mg/dL (Fig. 1). This low prevalence of hypertriglyceridemia was true for each cardiometabolic subtype. Even when prediabetes, diabetes, metabolic triad, and insulin resistance were tested separately, the prevalence of TG ≥150 mg/dL was <10% in each. Furthermore, 5 Africans were newly diagnosed as diabetic and had mean TG levels of 71±24, range 45–100 mg/dL (Fig. 1).
FIG. 1.
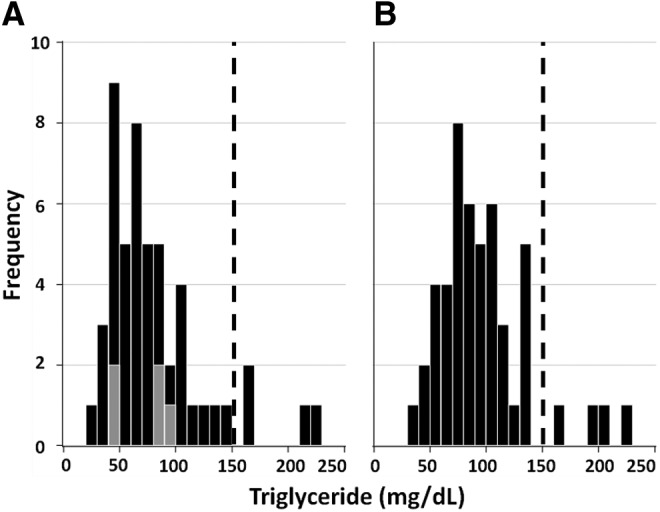
Frequency distribution of triglycerides (TG) levels in men with cardiometabolic disease. (A) African men; (B) African-American men. Gray boxes in A represent Africans with newly diagnosed diabetes.
The prevalence of cardiometabolic disease (63% vs. 65%, P=0.71) and insulin resistance (23% vs. 27%, P=0.66) did not differ in Africans and African Americans (Table 1). Yet Africans were more likely to have prediabetes or diabetes, whereas the metabolic triad was more common in African Americans.
The differences by ethnicity in the presentation of cardiometabolic disease can be explained by examining the glycemic and lipid profiles of each group. Both fasting and 2-h glucose levels were higher in Africans than African Americans. Hence the prevalence of prediabetes was almost twice as high in Africans (Table 1). In addition, after OGTT testing, 5 Africans, but no African Americans, were found to have diabetes. Because SI values were similar in the two groups (4.86±3.25 vs. 4.17±2.66 liter/mU−1·min−1, P=0.18), the prevalence of insulin resistance did not differ by ethnicity. However, AIRg was lower in Africans than in African Americans (604±436 vs. 874±758 mU/liter−1·min, P=0.01). This degree of β-cell failure superimposed on a similar degree of insulin resistance could account for the higher prevalence of prediabetes and diabetes in Africans.
In contrast, metabolic triad and dyslipidemia were more prominent in African Americans than Africans. Specifically, total cholesterol and LDL-C were higher and HDL particle size was lower in African Americans than in Africans (Table 1).
Discussion
In this investigation, we extend the finding that hypertriglyceridemia is a poor marker of cardiometabolic disease in African Americans to Africans. We report that hypertriglyceridemia, defined as TG ≥150 mg/dL, occurred in less than 10% of African and African-American men with cardiometabolic disease. Therefore, prior to the widespread institution of TG-based screening tests in Africans, caution is warranted. Our data clearly raise the possibility that TG-based screening tests led to the categorization of African and African-American men as at low risk for cardiometabolic disease when these men are, in fact, at high risk.
In the United States, African Americans have worse cardiometabolic health than whites, even after adjusting for socioeconomic status and health insurance.33,34 We have long postulated that failure of screening tests must be considered.5,13–15,35 Recently, Carnethon et al. determined that diabetics of normal weight had higher overall mortality and higher cardiovascular mortality than their more overweight and obese counterparts.36 To account for this unexpected finding, the investigators suggested that normal-weight diabetics were diagnosed late in the course of their disease because their health-care providers assumed they were low risk.36 We echo the concern of Carnethon et al. that late diagnosis leads to poor outcomes.
Of the many examples of TG-based screening tests, we discuss three—metabolic syndrome, abbreviated metabolic syndrome, and hypertriglyceridemic waist.5 The diagnosis of metabolic syndrome requires the presence of three of five parameters (hypertriglyceridemia (TG ≥150 mg/dL), low HDL-C, central obesity, hypertension, and fasting hyperglycemia). Therefore, hypertriglyceridemia is a possible, but not a required, criterion for the diagnosis of metabolic syndrome. However, the metabolic syndrome is based on the principle that all of its parameters are highly correlated with insulin resistance.2 When the metabolic syndrome was formulated, it was not appreciated that the association between hypertriglycerdemia and insulin resistance is not universal. In people of African descent, insulin resistance is not routinely associated with hypertriglyceridemia.12–14 Therefore, metabolic syndrome underdiagnoses risk in African Americans.11–13
In growing recognition of the failure of the metabolic syndrome to detect risk for cardiometabolic disease in African Americans, the 2012 Endocrine Society Position Statement on Health Disparities suggests moving away from the metabolic syndrome and focusing instead on blood pressure and degree of glycemia.16 Due to the similarities that we have demonstrated between African Americans and African immigrants, the same approach should be applied to Africans. In an earlier investigation, we found that metabolic syndrome performed poorly in predicting cardiometabolic risk in Africans.27 Specifically, the prevalence of hypertension and hyperglycemia was twice as high in Africans than African Americans, but the prevalence of metabolic syndrome was the same. Therefore, the metabolic syndrome did not detect Africans with increased metabolic risk. Our data suggest that the underperformance of the metabolic syndrome in men of African descent may be secondary to an absence of an association between insulin resistance and TG >150 mg/dL.
In contrast to the metabolic syndrome, hypertriglyceridemia is a mandatory criterion in the abbreviated metabolic syndrome. In fact, the abbreviated metabolic syndrome has only three parameters, and all three must be present—hypertriglyceridemia (TG ≥150 mg/dL), fasting hyperglycemia, and central obesity. Based on a combined analysis of four studies, Meigs et al. concluded that the abbreviated metabolic syndrome should be required as a cost-effective prerequisite for proceeding to an OGTT.37 However, only 3% of the participants in their study were African Americans. In our study, if hypertriglyceridemia dictated who should have an OGTT, all of the Africans with diabetes and >90% of the men with prediabetes would have not have been detected.
Although the abbreviated metabolic syndrome has three criteria, the hypertriglyceridemic waist has just two, hypertriglyceridemia (TG ≥177 mg/dL) and central obesity (>90 cm).3 According to Lemieux et al., if hypertriglyceridemia and central obesity occur together, the highly atherogenic metabolic triad is highly likely to be present.3 Overall, the hypertriglyceridemic waist, which is both inexpensive and easy to obtain, and widely used in Europe and Canada, is achieving increasing acclaim in the United States.17,18,20,21,38
However, there are no data on whether the hypertriglyceridemic waist predicts metabolic triad in blacks. Our investigation is the first. We found that 86% of the men with metabolic triad met the waist circumference threshold but less than 5% had TG ≥177 mg/dL. Therefore, in men of African descent, the hypertriglyceridemic waist fails as an alternative to the metabolic triad.
During this investigation, we made an observation that warrants further study. Specifically, the prevalence of cardiometabolic disease in Africans and American Americans was similar, but the path to cardiometabolic disease differed. Africans were more likely to be hyperglycemic, whereas African Americans were more likely to be dyslipidemic. The etiology of this ethnic difference in cardiometabolic disease subtype is unclear. However, VAT was higher in Africans than African Americans. VAT adversely affects glucose tolerance status and is associated with rapid weight gain.39 Therefore, the rapid weight gain that Africans experience after immigration may account, at least in part, for their worse glucose tolerance status. In addition, while the degree of insulin resistance was similar in Africans and African Americans, AIRg, a marker of β-cell function was lower in Africans. β-cell failure in the Africans may also contribute their higher rate of diabetes and prediabetes.
When comparing Africans and African Americans, it is also important to consider social and cultural factors. Although neither exercise intensity nor alcohol intake differed by ethnicity, African Americans were three times more likely than Africans to be smokers. The adverse impact of smoking on the lipid profile, particularly the smaller HDL particles observed in African Americans, must be considered.40 In this study, we did not have dietary intake information. It is likely that there is variation in the nutrient intake of Africans and African Americans, and this might also account for some of the differences observed in the glucose and lipid profile, including HDL size.41
This study had limitations. First, the study design is cross-sectional, so cardiometabolic events cannot be predicted with certainty. To overcome this deficit, we used proven markers of cardiometabolic risk, specifically insulin resistance, prediabetes, diabetes, and metabolic triad.23–26,42 Second, only men were enrolled. Because TG levels are even lower in black women than men, it is likely that the inability of TG levels to predict cardiometabolic disease would be magnified in women. But, this remains to be determined.13,35 Third, we are studying Africans living in the United States rather than Africa. However, when analyses were restricted to Africans who had lived in the United States for less than 5 years, the results did not change. Fourth, the determinants of normal TG levels in the presence of cardiometabolic disease were not explored. However, it is known that the activity of lipoprotein lipase (LPL), the enzyme that clears TG from the circulation, is higher in blacks than whites.43 In addition, even though insulin resistance should impair LPL activity,44 we have previously shown that LPL activity does not decline in insulin-resistant African Americans.45
Conclusion
TG-based screening tests for the detection of cardiometabolic disease have been applied globally. However, cardiometabolic disease usually occurs in African and American-American men when hypertriglyceridemia is absent. Therefore, in men of African descent, these tests routinely fail to detect those at highest risk. Therefore, even though TG-based screening tests are inexpensive and effective in other populations, reliance on TG-based screening to detect cardiometabolic risk in blacks risks late diagnosis and a lost opportunity for early intervention.
Acknowledgments
Anne E. Sumner, the senior author, is the guarantor of the manuscript. The authors thank Michelle Y. O'Connor and Amber B. Courville for their thoughtful reviews of the manuscript. N.L.M.R., D.C.C., M.R., and A.E.S. were supported by the Intramural Research Program of NIDDK, NIH. S.S.K.Y. was supported through the Clinical Research Training Program, a public–private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc). The funding bodies had no role in the design, collection, analyses, or interpretation of the data. S.S.K.Y., N.L.M.R., D.C.C., M.R., and A.E.S. collected the data. S.S.K.Y., N.L.M.R., M.R., and A.E.S. analyzed the data. S.S.K.Y., D.C.C., and A.E.S. wrote the manuscript. S.S.K.Y., N.L.M.R., D.C.C., M.R., and A.E.S. provided critical rewrites.
Author Disclosure Statement
All authors declare they have no competing interests.
References
- 1.Levitt NS. Steyn K. Dave J, et al. Chronic noncommunicable diseases and HIV-AIDS on a collision course: relevance for health care delivery, particularly in low-resource settings—insights from South Africa. Am J Clin Nutr. 2011;94:1690S–1696S. doi: 10.3945/ajcn.111.019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti KG. Eckel RH. Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Lemieux I. Pascot A. Couillard C, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin T. Abbasi F. Cheal K, et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Sumner AE. “Half the dsylipidemia of insulin resistance” is the dsylipidemia of insulin-resistant Blacks. Ethn Dis. 2009;19:462–465. [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SX. Carnethon M. Szklo M, et al. Racial/ethnic differences in the association of triglycerides with other metabolic syndrome components: The Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2011;9:35–40. doi: 10.1089/met.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Larrad MT. Lorenzo C. Gonzalez-Villalpando C, et al. Associations between surrogate measures of insulin resistance and waist circumference, cardiovascular risk and the metabolic syndrome across Hispanic and non-Hispanic white populations. Diabet Med. 2012;29:1390–1394. doi: 10.1111/j.1464-5491.2012.03723.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel A. Barzi F. Jamrozik K, et al. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110:2678–2686. doi: 10.1161/01.CIR.0000145615.33955.83. [DOI] [PubMed] [Google Scholar]
- 9.Sarwar N. Danesh J. Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 10.Anuurad E. Chiem A. Pearson TA, et al. Metabolic syndrome components in african-americans and European-american patients and its relation to coronary artery disease. Am J Cardiol. 2007;100:830–834. doi: 10.1016/j.amjcard.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Giannini E. Testa R. The metabolic syndrome: all criteria are equal, but some criteria are more equal than others. Arch Intern Med. 2003;163:2787–2788. doi: 10.1001/archinte.163.22.2787. author reply 88. [DOI] [PubMed] [Google Scholar]
- 12.Osei K. Metabolic syndrome in blacks: Are the criteria right? Curr Diab Rep. 2010;10:199–208. doi: 10.1007/s11892-010-0116-4. [DOI] [PubMed] [Google Scholar]
- 13.Sumner AE. Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Sumner AE. Finley KB. Genovese DJ, et al. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165:1395–1400. doi: 10.1001/archinte.165.12.1395. [DOI] [PubMed] [Google Scholar]
- 15.Yu SS. Castillo DC. Courville AB, et al. The triglyceride paradox in people of African descent. Metab Syndr Relat Disord. 2012;10:77–82. doi: 10.1089/met.2011.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golden SH. Brown A. Cauley JA, et al. Health disparities in endocrine disorders: Biological, clinical, and nonclinical factors—an endocrine society scientific statement. J Clin Endocrinol Metab. 2012;97:E1579–E1639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arsenault BJ. Lemieux I. Despres JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182:1427–1432. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn P. Lemieux I. Almeras N, et al. The hypertriglyceridemic waist phenotype versus the National Cholesterol Education Program-Adult Treatment Panel III and International Diabetes Federation clinical criteria to identify high-risk men with an altered cardiometabolic risk profile. Metabolism. 2009;58:1123–1130. doi: 10.1016/j.metabol.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn P. Lemieux I. Lamarche B, et al. Type 2 diabetes without the atherogenic metabolic triad does not predict angiographically assessed coronary artery disease in women. Diabetes Care. 2008;31:170–172. doi: 10.2337/dc07-0272. [DOI] [PubMed] [Google Scholar]
- 20.Egeland GM. Cao Z. Young TK. Hypertriglyceridemic-waist phenotype and glucose intolerance among Canadian Inuit: The International Polar Year Inuit Health Survey for Adults 2007–2008. CMAJ. 2011;183:E553–E558. doi: 10.1503/cmaj.101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Huelgas R. Bernal-Lopez MR. Villalobos A, et al. Hypertriglyceridemic waist: An alternative to the metabolic syndrome? Results of the IMAP Study (multidisciplinary intervention in primary care) Int J Obes (Lond) 2011;35:292–299. doi: 10.1038/ijo.2010.127. [DOI] [PubMed] [Google Scholar]
- 22.Kim-Dorner SJ. Deuster PA. Zeno SA, et al. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism. 2010;59:299–304. doi: 10.1016/j.metabol.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 23.de Luca C. Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manrique C. Lastra G. Gardner M, et al. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93:569–582. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haffner SM. Stern MP. Hazuda HP, et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 26.Lamarche B. Tchernof A. Mauriege P, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–1961. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 27.Ukegbu UJ. Castillo DC. Knight MG, et al. Metabolic syndrome does not detect metabolic risk in African men living in the U.S. Diabetes Care. 2011;34:2297–2299. doi: 10.2337/dc11-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumner AE. Luercio MF. Frempong BA, et al. Validity of the reduced-sample insulin modified frequently-sampled intravenous glucose tolerance test using the nonlinear regression approach. Metabolism. 2009;58:220–225. doi: 10.1016/j.metabol.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boston RC. Stefanovski D. Moate PJ, et al. MINMOD Millennium: A computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 31.Sumner AE. Sen S. Ricks M, et al. Determining the waist circumference in african americans which best predicts insulin resistance. Obesity (Silver Spring) 2008;16:841–846. doi: 10.1038/oby.2008.11. [DOI] [PubMed] [Google Scholar]
- 32.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 33.Groman R. Ginsburg J. Racial and ethnic disparities in health care: A position paper of the American College of Physicians. Ann Intern Med. 2004;141:226–232. doi: 10.7326/0003-4819-141-3-200408030-00015. [DOI] [PubMed] [Google Scholar]
- 34.Spertus JA. Jones PG. Masoudi FA, et al. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–324. doi: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumner AE. Harman JL. Buxbaum SG, et al. The triglyceride/high-density lipoprotein cholesterol ratio fails to predict insulin resistance in African-American women: An analysis of Jackson Heart Study. Metab Syndr Relat Disord. 2010;8:511–514. doi: 10.1089/met.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carnethon MR. De Chavez PJ. Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meigs JB. Williams K. Sullivan LM, et al. Using metabolic syndrome traits for efficient detection of impaired glucose tolerance. Diabetes Care. 2004;27:1417–1426. doi: 10.2337/diacare.27.6.1417. [DOI] [PubMed] [Google Scholar]
- 38.Eckel RH. The complex metabolic mechanisms relating obesity to hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2011;31:1946–1948. doi: 10.1161/ATVBAHA.111.233049. [DOI] [PubMed] [Google Scholar]
- 39.Ionut V. Liu H. Mooradian V, et al. Novel canine models of obese prediabetes and mild type 2 diabetes. Am J Physiol Endocrinol Metab. 2010;298:E38–E48. doi: 10.1152/ajpendo.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gossett LK. Johnson HM. Piper ME, et al. Smoking intensity and lipoprotein abnormalities in active smokers. J Clin Lipidol. 2009;3:372–378. doi: 10.1016/j.jacl.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mietus-Snyder ML. Shigenaga MK. Suh JH, et al. A nutrient-dense, high-fiber, fruit-based supplement bar increases HDL cholesterol, particularly large HDL, lowers homocysteine, and raises glutathione in a 2-wk trial. FASEB J. 2012;26:3515–3527. doi: 10.1096/fj.11-201558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutinho M. Gerstein HC. Wang Y, et al. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 43.Despres JP. Couillard C. Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: The Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 44.Maheux P. Azhar S. Kern PA, et al. Relationship between insulin-mediated glucose disposal and regulation of plasma and adipose tissue lipoprotein lipase. Diabetologia. 1997;40:850–858. doi: 10.1007/s001250050759. [DOI] [PubMed] [Google Scholar]
- 45.Sumner AE. Vega GL. Genovese DJ, et al. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism. 2005;54:902–909. doi: 10.1016/j.metabol.2005.03.001. [DOI] [PubMed] [Google Scholar]


