Abstract
Host cell-derived danger-associated molecular patterns (DAMPs), such as the hemoglobin (Hb) can interact with the innate immune system either directly or through binding to pathogen-associated molecular patterns (PAMPs). Hemolysis occurs under various pathological conditions, leading to hemoglobinemia. In the extracellular environment, the Hb becomes a redox-reactive DAMP molecule. In severe hemolysis, the massive level of extracellular pro-oxidative Hb generates reactive oxygen species (ROS), which perturbs the innate immune homeostasis. The Hb also binds to PAMPs and triggers Toll-like receptor-mediated signal transduction. In this perspective, we review the roles of cell-free Hb in the innate immune system, focusing on the plausible interactions among Hb, pathogens, host cell components, and innate immune cells, all of which remain to be explored with experiential detail.
Of the two alarm systems hosts use to detect infection or danger, the danger-associated molecular pattern (DAMP) recognition pathways have gotten less attention than the pathogen-associated molecular pattern (PAMP) recognition pathways. In this Bit, the induction of oxidative stress following binding of hemoglobin to its DAMP receptor is discussed.
The Roles of Reactive Oxygen and Nitrogen Species in Innate Immunity
The importance of reactive oxygen (ROS) and nitrogen (RNS) species in innate immune defense has been well recognized (Nathan and Ding, 2010; Wink et al., 2011). Although ROS exerts deleterious effects, such as mutagenesis and aging, it is indispensible for the maintenance of the innate immune system. The positive effects of ROS and RNS are displayed in chronic granulomatous disease (CGD), which is a rare inherited genetic disorder attributable to the lack of NADPH oxidase. The CGD individual is defective in producing superoxide anions and is prone to infection by peroxidase-positive pathogens (Hampton et al., 1998; Song et al., 2011). Under severe infection and chronic inflammation, CGD patients suffer enlarged lymph nodes, pulmonary fibrosis, and widespread tissue granulomas and Crohn-like colitis. IFNγ treatment-mediated nitric oxide (NO) production was reported to rescue the impaired phagocytosis of apoptotic cells in CGD patients (Fernandez-Boyanapalli et al., 2010; Naderi Beni et al., 2012); hence, the positive attribute of NO to innate immune defense.
In wild-type mice, lipopolysaccharide (LPS) induces p47phox-dependent ROS production and IL-10 secretion from the macrophage (Deng et al., 2012). On the other hand, when challenged with LPS, mice deficient in p47phox (a component of the NADPH oxidase), suffer severe lung inflammation due to the decreased anti-inflammatory cytokine, IL-10. NADPH oxidase-deficient mice also show increased susceptibility to infection by Listeria monocytogenes (Dinauer et al., 1997). The NO controls Th1 immune response by regulating inflammatory-like DC subset activation (Giordano et al., 2011). Mice lacking inducible nitric oxide synthase (iNOS, which produces NO), are more susceptible to Leishmania major infection (Wei et al., 1995) and experimental autoimmune encephalomyelitis (Sahrbacher et al., 1998). Besides playing a crucial role in infection and immunity, other biologically beneficial functions of NO include neurotransmission, intracellular signaling, and regulation of tumorigenesis.
Hemoglobin-Mediated Oxidative Stress
Under tightly regulated conditions, ROS and RNS are key players in maintaining normal physiology. However, excessive levels of these pro-oxidative free radicals may overwhelm and impair our antioxidants, causing protein oxidation, lipid peroxidation, and nucleic acid oxidation, and leading to cellular dysfunction and cell death (Auten and Davis, 2009).
Hemoglobin (Hb) possesses pseudoperoxidase (POX) activity, which is triggered synergistically by microbial proteases and pathogen-associated molecular patterns (PAMPs), such as LPS and LTA, to produce superoxide anion (Jiang et al., 2007). Hb is an iron-containing metalloprotein normally sequestered in the erythrocytes to transport oxygen. However, under certain pathophysiological conditions, hemolysis occurs, releasing massive amounts of Hb into the blood stream. For instance, infection by bacteria, such as Staphylococcus aureus (Grenny and Stevens, 1935), parasites, such as Plasmodium falciparum (Rudzinska et al., 1965), and influenza virus (Maeda et al., 1981), may induce hemolysis. Hemolysis is also a common symptom in many inherited and acquired hematologic disorders, such as sickle cell anemia, glucose-6-phosphate dehydrogenase deficiency, and paroxysmal nocturnal hemoglobinuria. Transfusion with improperly stored red blood cells (Hod et al., 2010) and treatment with anti-infective anti-inflammatory drugs, such as cotrimoxazole, ciprofloxacin, fludarabine, lorazepam, and diclofenac, may also induce hemolysis (Garbe et al., 2011).
The released cell-free Hb scavenges NO, which normally plays an important beneficial role in vascular homeostasis, without which, smooth muscle dystonia, vasculopathy, thrombosis, endothelial dysfunction, and platelet aggregation may ensue (Reiter et al., 2002; Olson et al., 2004; Rother et al., 2005). Peroxynitrite produced by the reaction between Hb and NO generates reactive hydroxyl radicals, which can initiate membrane lipid peroxidation and cellular damage (Auten and Davis, 2009). The plasma level of heme, which is increased by elevated proteolytic digestion of cell-free Hb in pathological conditions, such as cystic fibrosis, is also known to induce inflammation, coagulation, and tissue damage. In other instances, the highly expressed proteases in the lung of cystic fibrosis patient, cleave Hb to release heme and this increases the level of iron in the lung, which is exploited by pathogenic bacteria as nutrient. Generally, free heme is highly redox-reactive and induces oxidative stress. Thus, hemolysis unleashes Hb as a double-edged sword intraerythrocytic Hb, which normally transports oxygen for our survival, transforms into a redox-reactive danger-associated molecular pattern (DAMP) when it becomes extracellular and poses oxidative stress, turning the tide against our survival.
Toll-Like Receptors in Innate Immune System–Implications in ROS and RNS Sensing
ROS and RNS are also known to regulate Toll-like receptor (TLR)-mediated signaling (Asehnoune et al., 2004; Ryan et al., 2004; Nicholas and Sumbayev, 2010). Heme is reported to activate a TLR adaptor, MyD88, to induce IL-8 expression in human bronchial epithelial cells (Cosgrove et al., 2011). TLRs play a central role in the initiation of innate immune responses. Until now, 10 human TLRs and 13 mouse TLRs have been discovered. Each TLR recognizes its specific ligand, such as Pam3CSK4 by TLR1/TLR2, double-strand RNA by TLR3, LPS by TLR4, flagellin by TLR5, peptidoglycan by TLR2/6, imiquimod TLR7, R848 by TLR7/TLR8 and bacterial CpG DNA by TLR9. Upon binding of their ligands, the TLRs signal through adaptors and mediators, such as MyD88, TRIF, and IRAK to activate transcription factors, such as NF-κB and AP-1, which result in the release of cytokines and chemokines and the expression of iNOS and antimicrobial peptides (Park et al., 2004). Activation of TLR1, TLR2, and TLR4 induces translocation of TRAF6 leading to ubiquitination of evolutionarily conserved signaling intermediate in Toll (ECSIT), in the macrophage. However, when macrophages are depleted of TRAF6 and ESCIT, the generation of mitochondrial ROS is impaired, thus reducing the bactericidal (West et al., 2011). IL-8 production induced by the TLR4 ligand, LPS, is reduced by treatment with antioxidants, implying that the cellular redox state exerts a potent effect on TLR-dependent cytokine release (Ryan et al., 2004). Bone marrow-derived dendritic cells isolated from iNOS-deficient mouse respond to TLR2, TLR4, and TLR9 stimulation more strongly than wild-type mouse cells (Giordano et al., 2011).
PAMPs are well known to transduce signals for the expression of immune-responsive genes through TLRs. Host cell-derived DAMPs are also recognized by TLRs. Thus, while TLR-mediated signaling plays a pivotal role in the frontline of innate immune defense against pathogen invasion, uncontrolled TLR signaling may also cause autoimmune diseases (Akira and Takeda, 2004; Mills, 2011).
Hb and TLR Ligand Interactions
Recently, the interrelationship between ROS and the innate immune system in acute lung injury (Xiang and Fan, 2010), CGD (Hartl et al., 2008), hemorrhagic shock, and ischemia (Gill et al., 2010) has been reported. Furthermore, the interactive role of ROS in the cellular response against TLR ligands, such as LPS and flagellin has been elucidated (Qin et al., 2005; Ivison et al., 2010). The direct interaction between Hb and TLR ligands or PAMPs appears to be widespread in occurrence (Yang et al., 2002; Cox et al., 2007; Lin et al., 2012). Since Hb readily forms complexes with PAMPs and DAMPs, it is important to understand the roles of the Hb-PAMP and Hb-DAMP complexes in an innate immune response.
By surface plasmon resonance analysis, we found that both the α and β subunits of Hb possess LPS-binding sites with KD in the nM range (Bahl et al., 2011). Computational analysis has further shown that interactions between Hb and LPS or Hb and LTA result in a conformational change in the Hb molecule, causing it to convert to the oxidized form, metHb. Consequently, the relaxation of the structural rigidity between Hbα1-β2 and Hbα2-β1 interfaces increases the latent POX activity of Hb (Du et al., 2010). Other studies have shown that coincubation of Hb with multiple TLR ligands or PAMPs synergistically induces cytokines, such as IL-1β, IL-6, IL-8, and TNFα in macrophages (Bodet et al., 2007; Lin et al., 2010). LTA, a TLR2 ligand, induces IL-6 secretion and this is significantly enhanced in the presence of Hb. On the other hand, the TLR4-deficient macrophage shows a lack of production of IL-6 when exposed to the TLR2 ligand. Because LTA is known to bind exclusively to TLR2, the abolished response of TLR4-deficient cells to LTA may be due to a possible link between TLR2 and TLR4 in the cell's response to the LTA-Hb complex (Cox et al., 2007). These results suggest that Hb binds certain PAMPs with specificity to function in nonself recognition and plausibly, to protect the host by producing ROS (Jiang et al., 2007).
Perspectives
Severe hemorrhagic shock and hepatic ischemia-reperfusion injury, which accompany oxidative stress, induce TLR4 expression in the lung and liver (Powers et al., 2006). However, tissue injury and inflammation caused by Hb-mediated oxidative stress was found to be ameliorated by knocking out TLR4 (Chen et al., 2009). In hypoxia inducible factor-1-deficient mice, the TLR2 and TLR6 induction by hypoxia is abolished (Gill et al., 2010). Furthermore, TLR4 is upregulated by the oxidized low-density lipoprotein (LDL) in the macrophage in atherosclerotic plaques (Xu et al., 2001). It was found that colocalization of oxidized LDL with TLR2 and TLR4 in human coronary artery endothelial cells corresponds to BMP-2 expression, which is an osteogenic factor expressed in calcified human atherosclerotic plaques (Su et al., 2011). All of the above-mentioned observations suggest a close relationship between TLR signaling and oxidative stress. Conceivably, such oxidative stress-related dysfunctions can be triggered by cell-free Hb, which is redox reactive.
Severe oxidative stress-induced cellular apoptosis and necrosis are known to release intracellular DAMPs, such as the heat shock protein, high-mobility group box1 (HMGB1), S100A8, and serum amyloid A, which alert the host innate immune system (Carta et al., 2009). Some DAMPs are known to bind to and activate TLR2 and TLR4 signaling pathways under oxidative stress conditions, for example, the Hb-DAMP complex, such as Hb-HMGB1, was reported to synergistically increase cytokine secretion (Lin et al., 2012). TLR9 also recognizes the non-DNA ligand, namely, hemozoin, which is a hydrophobic heme polymer generated by a malaria parasite, P. falciparum. Hemozoin increases the expression of CD40 and CD86 as well as the production of TNFα, IL-12p40, MCP-1, and IL-6 in murine dendritic cells (Coban et al., 2005). In addition to TLR9, it has also been reported that TLR2 and TLR4 respond to the P. falciparum glycosylphosphatidylinositols, which induces proinflammatory responses (Coban et al., 2005; Krishnegowda et al., 2005).
The Haptoglobin-Hb (Hp-Hb) complex in the plasma is cleared by CD163, an Hb scavenger receptor on monocytes, whose expression is altered by specific TLR activation (Weaver et al., 2006; Lin et al., 2010). Our recent study also revealed that Apolipoprotein A-1, a major component of high-density lipoprotein, associates with Hb, and the complex is internalized by the recognition of the cell surface scavenger receptor class B type 1 in macrophages and hepatocytes, resulting in the clearance of Hb from the plasma (Du et al., 2012). Although LPS is well known as a TLR4 ligand, it also binds to RP105/MD1 (a class B scavenger receptor), CD36, and DC-SIGN, which are expressed on the lymphocytes, neutrophils, and dendritic cells (Yazawa et al., 2003; Zhang et al., 2006; Baranova et al., 2012). Thus, the human system is well endowed with many nonredundant Hb- or Hb-PAMP- specific binding proteins and cell surface receptors, all of which simultaneously recognize, endocytose, and clear the redox-reactive plasma Hb. Indeed, as a highly potent producer of harmful ROS, it is crucial for blood cells to harbor highly efficient pathways of sensing, suppressing, and rapidly detoxifying the cell-free Hb.
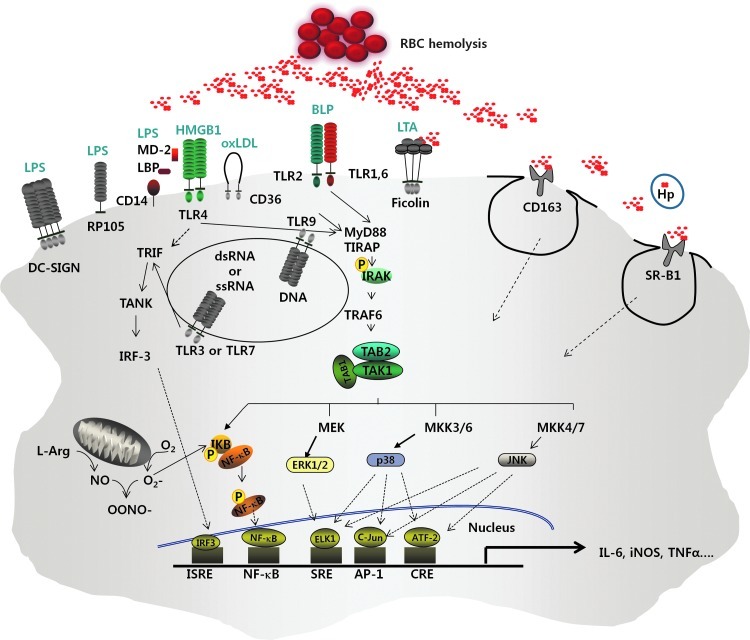
Taken together, all the findings reviewed here corroborate a high likelihood that Hb and other blood components bind to certain PAMPs and DAMPs, and the resulting Hb-PAMP and Hb-DAMP complexes modulate the functions of ligands on the pattern recognition receptors, such as the TLRs, to stimulate different types of immune cells and distinct signaling pathways (Fig. 1). However, current findings on the role of Hb in immune responses have been obtained from studies limited to an in vitro cell culture system. These findings restrict a full insight on the biological implications in vivo. A thorough systemic investigation is mandatory to better understand the role of Hb in pathogen infection and autoimmune diseases and to provide insights into inflammation mechanisms with a view to the future development of anti-inflammatory therapeutics.
FIG. 1.
A model depicting the involvement of extracellular hemoglobin in host cell innate immune responses via Toll-like receptors (TLRs) and other membrane-bound receptors. Extracellular hemoglobin (Hb) released by hemolysis is normally endocytosed by Hb scavengers, such as haptoglobin, scavenger receptor class B type 1 (SR-B1), and CD163 for intracellular degradation. However, excessive levels of extracellular hemoglobin produced under abnormal conditions, overwhelms the Hb scavengers and membrane receptors. The cell-free Hb binds to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). The Hb-PAMP and the Hb-DAMP complexes are recognized by pattern recognition receptors, such as TLRs in various immune cells, triggering proinflammatory responses.
Acknowledgments
This work is supported by grants from The Ministry of Education (T208B03109) and BMRC A*STAR (10/1/21/19/658). Jeak Ling Ding is affiliated with the “Singapore-MIT Alliance” and the “NUS Graduate School for Integrative Sciences and Engineering Program.”
Disclosure Statement
No competing financial interests exist.
References
- Akira S. Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Asehnoune K. Strassheim D. Mitra S. Kim J.Y. Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- Auten R.L. Davis J.M. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66:121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- Bahl N. Du R. Winarsih I. Ho B. Tucker-Kellogg L. Tidor B. Ding J.L. Delineation of lipopolysaccharide (LPS)-binding sites on hemoglobin: from in silico predictions to biophysical characterization. J Biol Chem. 2011;286:37793–37803. doi: 10.1074/jbc.M111.245472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova I.N. Vishnyakova T.G. Bocharov A.V. Leelahavanichkul A. Kurlander R. Chen Z. Souza A.C. Yuen P.S. Star R.A. Csako G., et al. Class B Scavenger Receptor Types I and II and CD36 Mediate Bacterial Recognition and Proinflammatory Signaling Induced by Escherichia coli, Lipopolysaccharide, and Cytosolic Chaperonin 60. J Immunol. 2012;188:1371–1380. doi: 10.4049/jimmunol.1100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodet C. Chandad F. Grenier D. Hemoglobin and LPS act in synergy to amplify the inflammatory response. J Dent Res. 2007;86:878–882. doi: 10.1177/154405910708600914. [DOI] [PubMed] [Google Scholar]
- Carta S. Castellani P. Delfino L. Tassi S. Vene R. Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- Chen C. Wang Y. Zhang Z. Wang C. Peng M. Toll-like receptor 4 regulates heme oxygenase-1 expression after hemorrhagic shock induced acute lung injury in mice: requirement of p38 mitogen-activated protein kinase activation. Shock. 2009;31:486–492. doi: 10.1097/SHK.0b013e318188f7e1. [DOI] [PubMed] [Google Scholar]
- Coban C. Ishii K.J. Kawai T. Hemmi H. Sato S. Uematsu S. Yamamoto M. Takeuchi O. Itagaki S. Kumar N., et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove S. Chotirmall S.H. Greene C.M. McElvaney N.G. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J Biol Chem. 2011;286:7692–7704. doi: 10.1074/jbc.M110.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K.H. Ofek I. Hasty D.L. Enhancement of macrophage stimulation by lipoteichoic acid and the costimulant hemoglobin is dependent on Toll-like receptors 2 and 4. Infect Immun. 2007;75:2638–2641. doi: 10.1128/IAI.01320-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J. Wang X. Qian F. Vogel S. Xiao L. Ranjan R. Park H. Karpurapu M. Ye R.D. Park G.Y., et al. Protective role of reactive oxygen species in endotoxin-induced lung inflammation through modulation of IL-10 expression. J Immunol. 2012;188:5734–5740. doi: 10.4049/jimmunol.1101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M.C. Deck M.B. Unanue E.R. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J Immunol. 1997;158:5581–5583. [PubMed] [Google Scholar]
- Du R. Ho B. Ding J.L. Rapid reprogramming of haemoglobin structure-function exposes multiple dual-antimicrobial potencies. EMBO J. 2010;29:632–642. doi: 10.1038/emboj.2009.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R. Winarsih I. Ho B. Ding J.L. Lipid-free apolipoprotein A-I exerts an antioxidative role against cell-free hemoglobin. Am J Clin Exp Immunol. 2012;1:33–48. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Boyanapalli R. McPhillips K.A. Frasch S.C. Janssen W.J. Dinauer M.C. Riches D.W. Henson P.M. Byrne A. Bratton D.L. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe E. Andersohn F. Bronder E. Klimpel A. Thomae M. Schrezenmeier H. Hildebrandt M. Spath-Schwalbe E. Gruneisen A. Mayer B., et al. Drug induced immune haemolytic anaemia in the berlin case-control surveillance study. Br J Haematol. 2011;154:644–653. doi: 10.1111/j.1365-2141.2011.08784.x. [DOI] [PubMed] [Google Scholar]
- Gill R. Tsung A. Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano D. Li C. Suthar M.S. Draves K.E. Ma D.Y. Gale M., Jr. Clark E.A. Nitric oxide controls an inflammatory-like Ly6C(hi)PDCA1 + DC subset that regulates Th1 immune responses. J Leukoc Biol. 2011;89:443–455. doi: 10.1189/jlb.0610329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenny A.T. Stevens M.F. Staphylococcus toxins and antitoxins. J Pathol Bacteriol. 1935;40:201–210. [Google Scholar]
- Hampton M.B. Kettle A.J. Winterbourn G.G. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Hartl D. Lehmann N. Hoffmann F. Jansson A. Hector A. Notheis G. Roos D. Belohradsky B.H. Wintergerst U. Dysregulation of innate immune receptors on neutrophils in chronic granulomatous disease. J Allergy Clin Immunol. 2008;121:375–382. doi: 10.1016/j.jaci.2007.10.037. e379. [DOI] [PubMed] [Google Scholar]
- Hod E.A. Zhang N. Sokol S.A. Wojczyk B.S. Francis R.O. Ansaldi D. Francis K.P. Della-Latta P. Whittier S. Sheth S., et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivison S.M. Wang C. Himmel M.E. Sheridan J. Delano J. Mayer M.L. Yao Y. Kifayet A. Steiner T.S. Oxidative stress enhances IL-8 and inhibits CCL20 production from intestinal epithelial cells in response to bacterial flagellin. Am J Physiol Gastrointest Liver Physiol. 2010;299:G733–G741. doi: 10.1152/ajpgi.00089.2010. [DOI] [PubMed] [Google Scholar]
- Jiang N. Tan N.S. Ho B. Ding J.L. Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat Immunol. 2007;8:1114–1122. doi: 10.1038/ni1501. [DOI] [PubMed] [Google Scholar]
- Krishnegowda G. Hajjar A.M. Zhu J. Douglass E.J. Uematsu S. Akira S. Woods A.S. Gowda D.C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Kwak Y.H. Sammy F. He P. Thundivalappil S. Sun G. Chao W. Warren H.S. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J Infect Dis. 2010;202:624–632. doi: 10.1086/654929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Sammy F. Yang H. Thundivalappil S. Hellman J. Tracey K.J. Warren H.S. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol. 2012;189:2017–2022. doi: 10.4049/jimmunol.1103623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T. Kawasaki K. Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc Natl Acad Sci U S A. 1981;78:4133–4137. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.H. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Naderi Beni F. Fattahi F. Mirshafiey A. Ansari M. Mohsenzadegan M. Movahedi M. Pourpak Z. Moin M. Increased production of nitric oxide by neutrophils from patients with chronic granulomatous disease on interferon-gamma treatment. Int Immunopharmacol. 2012;12:689–693. doi: 10.1016/j.intimp.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Nathan C. Ding A. SnapShot: reactive oxygen intermediates (ROI) Cell. 2010;140:951–951. doi: 10.1016/j.cell.2010.03.008. e952. [DOI] [PubMed] [Google Scholar]
- Nicholas S.A. Sumbayev V.V. The role of redox-dependent mechanisms in the downregulation of ligand-induced Toll-like receptors 7, 8 and 4-mediated HIF-1 alpha prolyl hydroxylation. Immunol Cell Biol. 2010;88:180–186. doi: 10.1038/icb.2009.76. [DOI] [PubMed] [Google Scholar]
- Olson J.S. Foley E.W. Rogge C. Tsai A.L. Doyle M.P. Lemon D.D. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Park H.S. Jung H.Y. Park E.Y. Kim J. Lee W.J. Bae Y.S. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- Powers K.A. Szaszi K. Khadaroo R.G. Tawadros P.S. Marshall J.C. Kapus A. Rotstein O.D. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–1961. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L. Li G. Qian X. Liu Y. Wu X. Liu B. Hong J.S. Block M.L. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- Reiter C.D. Wang X. Tanus-Santos J.E. Hogg N. Cannon R.O., 3rd Schechter A.N. Gladwin M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Rother R.P. Bell L. Hillmen P. Gladwin M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- Rudzinska M.A. Trager W. Bray R.S. Pinocytotic uptake and the digestion of hemoglobin in malaria parasites. J Protozool. 1965;12:563–576. doi: 10.1111/j.1550-7408.1965.tb03256.x. [DOI] [PubMed] [Google Scholar]
- Ryan K.A. Smith M.F., Jr. Sanders M.K. Ernst P.B. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun. 2004;72:2123–2130. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahrbacher U.C. Lechner F. Eugster H.P. Frei K. Lassmann H. Fontana A. Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1332–1338. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Song E. Jaishankar G.B. Saleh H. Jithpratuck W. Sahni R. Krishnaswamy G. Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin Mol Allergy. 2011;9:10. doi: 10.1186/1476-7961-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X. Ao L. Shi Y. Johnson T.R. Fullerton D.A. Meng X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem. 2011;286:12213–12220. doi: 10.1074/jbc.M110.214619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L.K. Hintz-Goldstein K.A. Pioli P.A. Wardwell K. Qureshi N. Vogel S.N. Guyre P.M. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol. 2006;80:26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- Wei X.Q. Charles I.G. Smith A. Ure J. Feng G.J. Huang F.P. Xu D. Muller W. Moncada S. Liew F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- West A.P. Brodsky I.E. Rahner C. Woo D.K. Erdjument-Bromage H. Tempst P. Walsh M.C. Choi Y. Shadel G.S. Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D.A. Hines H.B. Cheng R.Y. Switzer C.H. Flores-Santana W. Vitek M.P. Ridnour L.A. Colton C.A. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M. Fan J. Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury. Mediators Inflamm. 2010:2010. doi: 10.1155/2010/916425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.H. Shah P.K. Faure E. Equils O. Thomas L. Fishbein M.C. Luthringer D. Xu X.P. Rajavashisth T.B. Yano J., et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- Yang H. Wang H. Bernik T.R. Ivanova S. Ulloa L. Roth J. Eaton J.W. Tracey K.J. Globin attenuates the innate immune response to endotoxin. Shock. 2002;17:485–490. doi: 10.1097/00024382-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Yazawa N. Fujimoto M. Sato S. Miyake K. Asano N. Nagai Y. Takeuchi O. Takeda K. Okochi H. Akira S., et al. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood. 2003;102:1374–1380. doi: 10.1182/blood-2002-11-3573. [DOI] [PubMed] [Google Scholar]
- Zhang P. Snyder S. Feng P. Azadi P. Zhang S. Bulgheresi S. Sanderson K.E. He J. Klena J. Chen T. Role of N-acetylglucosamine within core lipopolysaccharide of several species of gram-negative bacteria in targeting the DC-SIGN (CD209) J Immunol. 2006;177:4002–4011. doi: 10.4049/jimmunol.177.6.4002. [DOI] [PubMed] [Google Scholar]