Abstract
The increasing incidence of type 2 diabetes mellitus is partially due to the rising obesity rates and the elevated levels of free fatty acids (FFAs). It is known that FFAs are putative mediators of beta-cell dysfunction, which is characterized with impaired glucose-stimulated insulin secretion and increased apoptosis, being defined as lipotoxicity. To date, many factors and their related signal pathways have been reported to be involved in FFA-induced beta-cell dysfunction. However, the entire blueprint is still not obtained. Some essential and newfound effectors, including the sterol regulatory element-binding protein (SREBP)-1c, farnesoid X receptor (FXR), forkhead box-containing protein O (FoxO) 1, ubiquitin C-terminal hydrolase L (UCHL) 1, N-myc downstream-regulated gene (NDRG) 2, perilipin family proteins, silent information regulator 2 protein 1 (Sirt1), pituitary adenylate cyclase-activating polypeptide (PACAP), and ghrelin are described in this review, which may help to further understand the molecular network for lipotoxicity.
In this review, the impact of genes influencing lipid metabolism on insulin-independent diabetes mellitus (type 2 diabetes) is discussed. These include sterol regulatory element-binding protein-1c, farnesoid X receptor, forkhead box-containing protein O, ubiquitin C-terminal hydrolase L, and others.
Introduction
To date, diabetes in all its forms afflicts at least 200 million people in the world and this number is substantially increasing, which is expected to double by the year 2025 (Meetoo et al., 2007). Type 2 diabetes mellitus (T2DM), as a common subtype of diabetes, involves two core defects, that is, insulin resistance and beta-cell dysfunction (Meetoo et al., 2007; Giacca et al., 2011; Zitkus, 2012). It is known that obesity is a major risk factor of T2DM, in part due to elevated circulating free fatty acids (FFAs) (Giacca et al., 2011). Chronic lipid accumulation plays an essential role in pancreatic beta-cell dysfunction characterized with impaired glucose-stimulated insulin secretion (GSIS) and increased levels of apoptosis, being recognized as lipotoxicity (Shao et al., 2010; Giacca et al., 2011). However, the mechanisms of FFA-induced GSIS impairment and lipoapoptosis are not fully understood and the entire blueprint is still under investigation. Some pertinent signaling molecules and their related signal pathways are identified, including sterol regulatory element-binding protein (SREBP)-1c, farnesoid X receptor (FXR), forkhead box-containing protein O (FoxO) 1, ubiquitin C-terminal hydrolase L (UCHL) 1, N-myc downstream-regulated gene (NDRG) 2, perilipin family proteins, silent information regulator 2 protein 1 (Sirt1), pituitary adenylate cyclase-activating polypeptide (PACAP), and ghrelin (Table 1). This review summarized these essential factors and analyzed their crosslinking in an integral view, thus providing a clearer understanding. This could contribute not only to identify the mechanism of beta-cell dysfunction under lipid stress, but also to develop the treatment strategies with new perspectives for T2DM.
Table 1.
Effectors Involved in Lipotoxicity and Their Established Functions
| Effectors involved in beta-cell function | Abbreviation | Function |
|---|---|---|
| Sterol regulatory element-binding protein-1c | SREBP-1c | Transcription factor regulating multiple pathways required for beta-cell function |
| Farnesoid X receptor | FXR | Transcription factor required for beta-cell function |
| Forkhead box-containing protein O1 | FoxO1 | Transcription factor involved in beta-cell survival and GSIS in PI3K/Akt pathway |
| Ubiquitin C-terminal hydrolaseL1 | UCHL1 | Deubiquitinating enzyme essential for beta-cell function and survival |
| N-myc downstream-regulated gene 2 | NDRG2 | Substrate of Akt involved in the Akt-mediated beta-cell survival |
| Perilipin Adipocyte differentiation-related protein | Perilipin ADFP | Protein associated to lipid storage |
| Silent information regulator 2 protein 1 | Sirt1 | Protein of sirtuin family involved in fat metabolism and GSIS |
| Pituitary adenylate cyclase-activating polypeptide | PACAP | Peptide of incretin family involved in beta-cell function |
| Ghrelin | Ghrelin | Peptide secreted from gastric fundus involved insulin secretion and survival |
GSIS, glucose stimulated insulin secretion; PI3K, phosphatidylinositol-3 kinase.
Sterol Regulatory Element-Binding Protein-1c
SREBP-1c, a member of the membrane-bound transcription factor basic helix-loop-helix leucine zipper family, is conventionally viewed as a nutritional regulator of lipogenic enzymes in the liver (Shao et al., 2009; Hong et al., 2012). It was upregulated by dietary intake of carbohydrates, sugars, and saturated fatty acids. This type of nutritional SREBP-1c regulation has recently been observed in both cultured beta cells and isolated islets of mice (Kato et al., 2008; Shao et al., 2009). Moreover, transgenic mice overexpressing the active form of SREBP-1c exhibited impaired glucose tolerance in vivo, indicating that activation of SREBP-1c could cause beta-cell dysfunction (Takahashi et al., 2005).
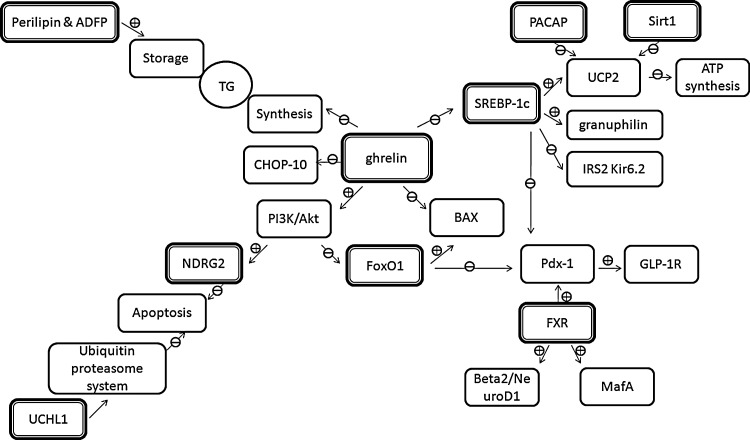
Insulin secretion in pancreatic islets was found to be impaired by the addition of palmitate, a typical saturated fatty acid, and this effect was abolished in SREBP-1c-null islets (Takahashi et al., 2005; Kato et al., 2008; Hong et al., 2012). These findings established an association between SREBP-1c and lipid-induced beta-cell dysfunction. Furthermore, palmitate was found to upregulate the expression level of SREBP-1c along with the downregulation of pancreatic and duodenal homeobox (Pdx)-1 and glucagon-like peptide-1 receptor (GLP-1R), two essential effectors for the beta-cell function, in INS-1 cells (Shao et al., 2009, 2010). Additionally, either SREBP-1c ablation or Pdx-1 overexpression could partially alleviate palmitate-induced GSIS impairment, suggesting that sequent SREBP-1c-Pdx-1-GLP-1R signal pathway was involved in the lipid caused GSIS impairment (Shao et al., 2009, 2010) (Fig. 1).
FIG. 1.
Schematic diagram of effector intercrossing network under physiological condition. Sterol regulatory element-binding protein (SREBP)-1c is involved in multiple gene regulation, including insulin receptor substrate (IRS)-2, pancreatic and duodenal homeobox (Pdx-1), granuphilin, uncoupling protein (UCP) 2, and ion channels. Pituitary adenylate cyclase-activating polypeptide (PACAP) and silent information regulator 2 protein 1 (Sirt1) could regulate the expression of UCP2 as well. Farnesoid X receptor (FXR) upregulates the expression of Pdx-1, NeuroD1, and MafA. Forkhead box-containing protein O1 (FoxO1) is the downstream mediator of phosphatidylinositol-3 kinase (PI3K)/Akt pathway, which could modulate the activity and/or expression of Pdx-1 and BAX. N-myc downstream-regulated gene (NDRG2) (substrate of Akt) and deubiquitinating enzyme ubiquitin C-terminal hydrolase L (UCHL1) are both involved in beta-cell survival. Perilipin and adipocyte differentiation-related protein (ADFP) can regulate the triglycerides (TG) storage in beta cells. The function of ghrelin includes the activation of PI3K/Akt pathway, the nuclear exclusion of FoxO1, the reduction in cytoplasmic TG synthesis, and the downregulation of BAX, SREBP-1c, and CHOP-10. Frame in bold line, direct target of lipid stress; ⊕, enhance; ⊖, inhibit.
Besides Pdx-1, SREBP-1c, as a transcription factor, was also reported to regulate the expression of genes involved in the beta-cell function, including uncoupling protein (UCP) 2, a protein mediating the energy dissipation in mitochondria instead of ATP synthesis, insulin receptor substrate (IRS)-2, which was involved in the insulin/insulin-like growth factor-1 pathway, GTPase granuphilin related to insulin granules transport for exocytosis, and electrophysiological function-related factors (Kir6.2, Kv1.2, Syntaxin-1a, and Munc18-1) (Kato et al., 2008; Shao et al., 2009, 2010). However, it was unclear whether these effectors are associated with SREBP-1c-involved lipotoxicity (Fig. 2).
FIG. 2.
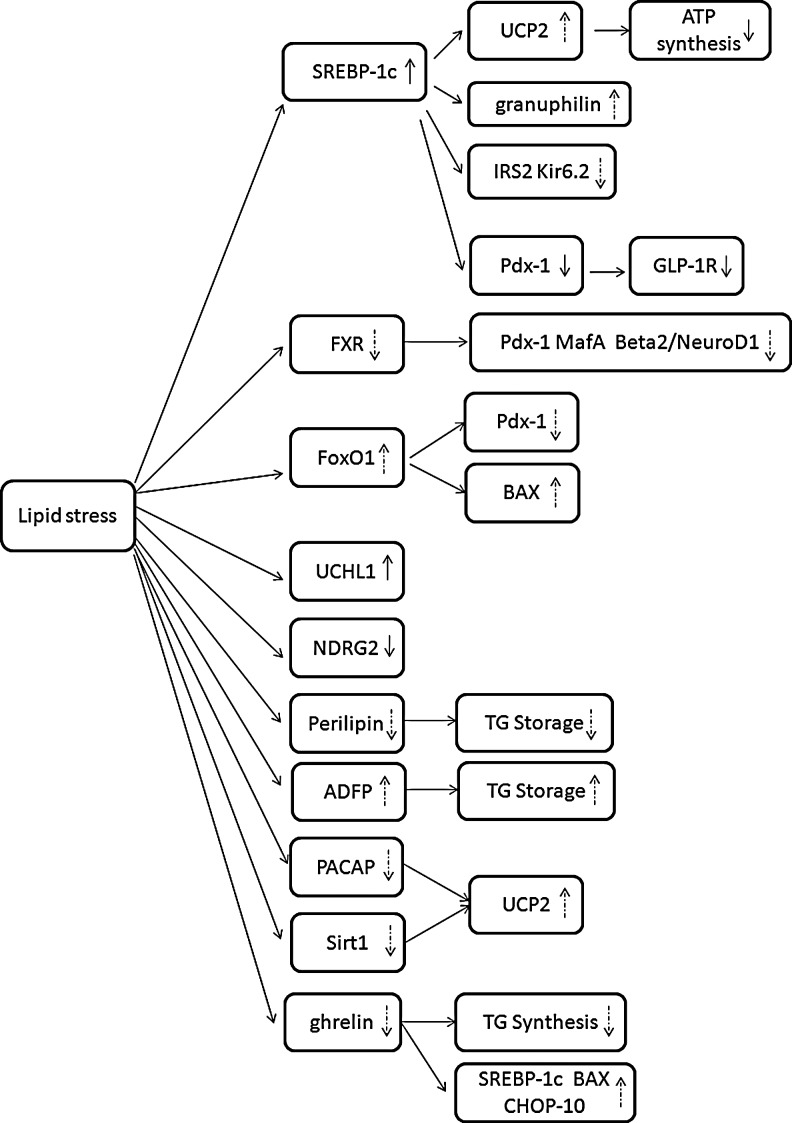
Expression/activity of effectors under lipotoxic conditions. Lipid stress elevates the expression/activity of SREBP-1c and UCHL1, but downregulates the activity of NDRG2. The regulation of FXR, FoxO1, perilipin, ADFP, PACAP, Sirt1, and ghrelin under lipid stress needs to be further verified. Solid arrows stand for events that have been confirmed and dashed arrows for the events requiring substantiation.
Together, the findings reveal that lipid stress could increase the expression level of SREBP-1c. SREBP-1c-Pdx-1-GLP-1R may be one of the signal pathways involved in the lipid-caused GSIS impairment. Various beta-cell function-related molecules, including UCP2, IRS-2, granuphilin, and Kir6.2 are regulated by SREBP-1c. Nevertheless, whether they are the downstream mediators in the high FFA-SREBP-1c pathway remains to be further identified.
Farnesoid X Receptor
The FXR, as a transcription factor, was highly expressed in liver, intestine, and adrenal glands, where it controlled bile acids, lipid, and glucose homeostasis (Makishima et al., 1999; Popescu et al., 2010; Dufer et al., 2012). It has been found that FXR gene expression was induced by glucose in rat hepatocytes, whereas insulin reversed this effect (Lefebvre et al., 2009). Moreover, FXR mRNA levels were increased in livers of diabetic db/db mice (Zhang et al., 2006). In addition, FXR−/− mice displayed impaired glucose and insulin tolerance due to blunted insulin signaling pathways in the skeletal muscle and white adipose tissue (Ma et al., 2006; Popescu et al., 2010).
Recent data demonstrated that FXR was also expressed and functional in pancreatic islets and beta-cell lines, both in rodents and humans (Popescu et al., 2010). Interestingly, FXR was predominantly cytosolic localized in the islets of lean mice, but nuclear in obese mice. Therefore, it is speculated that the lipid stress could lead to the translocation of FXR from nuclear to cytoplasm, resulting in the inactivation of FXR. Further investigation is required to identify this (Fig. 2).
Furthermore, Popescu et al. (2010) found that treatment of human islets with FXR agonists protected islets from palmitate-induced triglycerides (TG) accumulation, indicating the protective role of FXR on human islets under lipid-induced metabolic stress. In addition, GSIS was found to be impaired in islets isolated from FXR−/− mice (Popescu et al., 2010). V-maf musculoaponeurotic fibrosarcoma oncogene homolog (Maf ) A, a master transcription factor regulating the insulin gene and GSIS in the mature beta cell (Shao et al., 2009), was decreased in FXR−/− pancreas. Besides, Beta2/NeuroD1, a key transcription factor of islet cells (Shao et al., 2009), in addition to Pdx-1 were also reduced in FXR−/− pancreas (Popescu et al., 2010) (Fig. 1). Whether these targets are involved in the lipid stress–FXR signal pathway requires further exploration.
In conclusion, the identification of FXR in the control of beta-cell function opens promising new notions for the prevention of lipotoxicity. Nevertheless, the function of FXR under physiological conditions is still not thoroughly identified although its protective effect on the beta-cell function is found in FXR−/− mice. In addition, the downstream mediators in the high FFAs-FXR pathway are not completely obvious. Further research is demanded.
Forkhead Box-Containing Protein O1
Forkhead box-containing O (FoxO) proteins (FoxO1, FoxO3, FoxO4, and FoxO6) are a subclass of the large family of forkhead proteins featured by the presence of a winged helix DNA-binding domain called the forkhead box (Kaestner et al., 2000). They are important for cellular differentiation, proliferation, apoptosis, and stress resistance. FoxO1, the most abundant isoform in liver, adipose tissue, and beta cells, is conventionally viewed as a regulator in glucose and lipid production in liver (Kitamura et al., 2002). In addition, FoxO1 was found to suppress the expression of genes related to lipogenesis, including SREBP-1c in liver (Zhang et al., 2006) and stimulate fatty acid uptake and utilization in muscle (Bastie et al., 2005).
It is known that a high FFA load, when exceeding beta-cell esterification capacity, might impair endoplasmic reticulum (ER) functions and trigger an ER stress response, contributing to beta-cell toxicity (Martinez et al., 2008; Giacca et al., 2011). Inhibition of FoxO1 decreased the expression of the ER stress marker gene and consequently promoted beta-cell survival under FFA stress, suggesting the effect of FoxO1 on lipid-induced beta-cell apoptosis (Martinez et al., 2008). Further investigation demonstrated that, as a prominent downstream target of FoxO1, the proapoptotic BCL-2 family member BAX mediated the effect of FoxO1 on lipoapoptosis (Kim et al., 2005) (Fig. 1). More recently, transgenic mice with constitutively active FoxO1 in the pancreas presented impaired glucose tolerance in addition to the reduction of beta-cell mass (Kikuchi et al., 2012). On the contrary, FoxO1 ablation in beta cells resulted in enhanced insulin secretion at high glucose concentrations, indicating the role of FoxO1 on GSIS (Miyazaki et al., 2012). Whether such negative effects of FoxO1 on insulin secretion act under lipid stress requires further exploration.
Additionally, Pdx-1 was found to be regulated by another forkhead transcription factor FoxA2 (Lee et al., 2002). FoxO1 and FoxA2 shared common DNA-binding sites in the Pdx-1 promoter. Thus, FoxO1 may inhibit the transcription of Pdx-1 through competing the binding sites with FoxA2 (Fig. 1). Furthermore, transgenic mice with FoxO1 overexpression in the pancreas developed diabetes with the decreased expression of Pdx1 in islets, which further demonstrated the inhibitory role of FoxO1 on Pdx-1expression (Kikuchi et al., 2012).
Besides, evidence has indicated that activation of the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (PKB, also known as Akt) pathway prevented palmitate-induced toxicity of pancreatic beta cells (Granata et al., 2007). Recent studies further identified that FoxO1 was an important mediator in the PI3K/Akt pathway, which could be phosphorylated on Thr24, Ser256, and Ser316 by Akt, resulting in transport of FoxO1 from the nucleus to the cytoplasm (Kim et al., 2005; Fang et al., 2012) (Fig. 1). When FoxO1 was inhibited, pancreatic beta cells were protected against FFA-induced apoptosis (Martinez et al., 2008). These findings indicated the essential role of the PI3K/Akt/FoxO1 pathway in beta-cell lipoapoptosis.
Taken together, the PI3K/Akt/FoxO1 pathway may be of importance in beta-cell lipoapoptosis and GSIS impairment. Stronger clues are required for the direct correlation between lipid stress and FoxO1 activation (Fig. 2). Moreover, negative regulation of Pdx-1 by FoxO1 may also contribute to the development of lipotoxicity.
Ubiquitin C-Terminal Hydrolase L 1
Ubiquitination, an important reversible post-translational modification, can target proteins for degradation or modify their activity (Hershko et al., 2000). An emerging clue implies essential roles for ubiquitination in both beta-cell survival and insulin secretion.
UCHL1 was first identified as a deubiquitinating enzyme that hydrolyzed the peptide bond at the C terminus of ubiquitin and was involved in the processing of ubiquitin precursors and the polyubiquitin chains (Nijman et al., 2005). Additionally, UCHL1 could stabilize free ubiquitin and prevent its degradation (Nijman et al., 2005). The expression of UCHL1 was enriched in the brain, testis/ovary, and pancreatic islets (Lopez-Avalos et al., 2006). Mutations and modifications of UCHL1 were associated with human neurodegenerative diseases, such as Parkinson's and Alzheimer's diseases (Choi et al., 2004).
Recently, emerging evidence pointed to the importance of UCHL1 in pancreatic beta cells (Lopez-Avalos et al., 2006). UCHL1 was identified in a proteomic screen as the most upregulated protein in MIN6 beta cells treated with palmitate (Chu et al., 2012). In this study, Chu et al. used a genetic loss-of-function model to test the hypothesis that UCHL1 was required for normal beta-cell function and fate under lipotoxic conditions and it was found that a 4-week high-fat (HFa) diet caused glucose intolerance and impaired insulin secretion in UCHL1−/− mice. In addition, increased ER stress and beta-cell apoptosis were observed in UCHL1−/− mice as well (Chu et al., 2012).
These data suggest that UCHL1 has essential functional and antiapoptotic roles in beta cells under lipid stress, highlighting a novel understanding of the importance of UCHL1 and the ubiquitin proteasome system in the pathobiology of lipotoxicity and T2DM.
N-myc Downstream-Regulated Gene 2
Human NDRG2 was first cloned from a human brain cDNA library by subtractive hybridization (Lachat et al., 2002). NDRG2, as a member of the NDRG gene family, played a variety of roles in cell proliferation and differentiation, stress response, and p53- and HIF-1-mediated apoptosis (Lachat et al., 2002). Several reports have provided insight into the physiological roles of NDRG2 in the central nervous system (Shen et al., 2008). In addition to its known functions in the brain, NDRG2 acted as a novel regulator for myoblast proliferation and was regarded as a putative tumor suppressor in human cancer (Foletta et al., 2009; Shen et al., 2010). Absence of NDRG2 may influent cell apoptosis under specific stress conditions (Foletta et al., 2009).
However, the function of NDRG2 in the pancreas remains to be established. Hu et al. (2006) demonstrated NDRG2 immunoreactivity in mouse islets. Further investigation revealed the strong NDRG2 expression in pancreatic beta cells, suggesting a potential role of NDRG2 in beta-cell function (Shen et al., 2010). To expand earlier observations, Shen and colleague investigated the biological functions of NDRG2 and found that NDRG2 was a potential substrate of protein kinase Akt (Shen et al., 2010). Akt, an important molecule in the insulin signaling pathway, could promote the survival of pancreatic islets and prevent beta-cell apoptosis induced by FFAs (Granata et al., 2007). In Shen's study, when clonal beta-cell line b-TC3 cells were exposed chronically to high levels of FFAs, cell viability was impaired along with the reduced phosphorylation of Akt and NDRG2 (Shen et al., 2010). In addition, the overexpression of constitutively active Akt enhanced NDRG2 phosphorylation and abolished the apoptosis induced by FFAs in b-TC3 cells, whereas NDRG2 knockdown attenuated Akt-mediated protection of beta cells against lipoapoptosis (Shen et al., 2010).
Collectively, these data indicate that NDRG2 is involved in the Akt-mediated protection of beta cells against lipotoxicity and functions as a key molecule in beta-cell survival. The findings may represent a novel area for therapeutic intervention in lipid stress. However, the downstream pathway of Akt-NDRG2 remains to be identified as well.
Perilipin Family
Lipid droplets (LDs), as dynamic functional organelles, is of importance in cellular energy balance, the structure of which contains a core of neutral lipid (TGs and cholesterol ester) coated by an interface composed of a monolayer of phospholipids, free cholesterol, and proteins (Ducharme et al., 2008). The storage droplets may help transport the neutral lipids to specific cellular destinations or direct them to specific metabolic or signaling pathways. Such coordination of lipid metabolism was likely controlled by the LD coat proteins of the perilipin family, including the founding member perilipin, as well as the adipocyte differentiation-related protein (ADFP), tail-interacting protein of 47 kilodaltons (TIP47), S3–12, and oxidative tissue-enriched PAT protein (OXPAT) (Miura et al., 2002). These perilipin family proteins shared sequence similarity and localized to LDs, either constitutively (perilipin, ADFP) or in response to lipogenic stimuli (TIP47, S3–12, and OXPAT) (Brasaemle et al., 2007).
Perilipin, the most highly studied member of the perilipin family, played an important role in the regulation of basal and hormonally stimulated lipolysis (Forcheron et al., 2005; Mishra et al., 2005; Borg et al., 2009). It has been known that the excess accumulation of lipids in islets contribute to the development of T2DM in obesity by the impairment of beta-cell function. Furthermore, an emerging perspective indicates that sequestration of cytosolic FFAs in cellular TG stores represents a cytoprotective mechanism for lipotoxicity in beta cells. Borg et al. (2009) provided evidences for expression of perilipin in rat, mouse, and human islets as well as the rat clonal beta-cell line INS-1 at low levels and showed that overexpression of perilipin increased the capability of lipid storage in beta cells (Fig. 1). To examine whether the development of lipotoxicity could be prevented by manipulating the conditions for lipid storage, INS-1 cells with perilipin overexpression were exposed to lipotoxic conditions and the GSIS was retained, indicating that overexpression of perilipin appeared to confer a protective effect against FFA load. This may be attributed to the expansion of lipid storage in beta cells (Borg et al., 2009) (Fig. 2). However, it was still not clear whether the high level of FFAs would affect the expression of perilipin. Identifying this should be of significance.
Besides perilipin, ADFP is also localized on the surface of LDs in a wide range of cells with islets included, and serves crucial functions in intracellular lipid metabolism. The upregulation of ADFP was found at the site of increased lipid accumulation, such as fatty liver (Heid et al., 1998). Moreover, Imai et al. reported that the reduction of ADFP via antisense oligonucleotide (ASO) could reverse fatty liver and alter lipid metabolism (Imai et al., 2007). More recently, it was found that the HFa diet could markedly increase the expression level of ADFP in murine islets along with the incremental expression of ADFP in human islets by addition of FFAs (Faleck et al., 2010). Effect of long-term lipotoxicity on the expression/activity of ADFP in beta cells remains to be identified (Fig. 2). Furthermore, the downregulation of ADFP in MIN6 cells by ASO resulted in the suppression of the TG accumulation upon FFAs loading, indicating that ADFP may participate in the regulation of intracellular lipid metabolism in islet beta cells (Faleck et al., 2010) (Fig. 1). However, few studies focused on the association of ADFP and beta-cell function under lipid stress. Future investigations should address whether ADFP has a unique role in the prevention of lipotoxicity in islets, and it is plausible that ADFP has a distinct position in the development of lipotoxic-induced beta-cell dysfunction as a LD protein.
Taken together, these results indicate a new concept to abolish the lipotoxic effects of chronic exposure to FFAs by increasing the capacity of beta cells for efficiently storing excess FFAs. Further studies of perilipin and ADFP will increase our understanding of lipid metabolism in pancreatic islets, which is critical in targeting lipotoxicity. Roles of other members of the perilipin family in lipotoxic-induced beta-cell dysfunction should be further explored.
Silent Information Regulator 2 Protein 1
Sirtuins (Sirt) belong to a highly conserved family of protein deacetylases and ADP-ribosyltransferases with seven members (Sirt1–7) in mammals (Haigis and Guarente, 2006). Sirt1, the best studied sirtuin, is expressed throughout the body, including the brain, heart, liver, pancreas, skeletal muscle, spleen, and adipose tissues. It is known that Sirt1 plays an essential role in multiple biological processes encompassing metabolism, oxidative stress, cellular proliferation, cellular aging, endothelial functions, and genomic stability (Haigis and Guarente, 2006; Chong et al., 2012).
Sirt1 is now considered to be closely connected to the development of T2DM in virtue of its activity on insulin sensitivity. In insulin-resistant cells, the Sirt1 protein was markedly decreased and the reduction of Sirt1 levels in the gastrocnemius muscle in mice resulted in glucose intolerance (Sun et al., 2007). In contrast, overexpression of Sirt1 in the liver served to attenuate hepatic steatosis and improved insulin sensitivity, resulting in ameliorative glucose homeostasis (Li et al., 2011).
Moreover, Sirt1 not only had a role in insulin sensitivity, but also could affect fat metabolism and obesity. In obese mice, Sirt1 expression was low in adipose tissue and loss of Sirt1 in white adipose cells resulted in the impairment of fatty acid mobilization (Picard et al., 2004). Recent reports found that Sirt1 levels were increased in fat tissues in response to fasting and calorie restriction (CR) in rodents (Cohen et al., 2004; Al-Regaiey et al., 2005). In addition, Chen et al. (2010) found that treatment with the CR diet in rats increased the expression of Sirt1 in the pancreatic beta cells, while treatment with the HFa diet caused a decrease. Therefore, it is speculated that lipid stress may regulate the expression/activity of Sirt1 in beta cells (Fig. 2).
Besides, recent evidence demonstrated that the improvement of GSIS in INS-1 cells and human islets by the Sirt1 activator resveratrol was dependent on active Sirt1 (Vetterli et al., 2011). Furthermore, it was found that Sirt1 could promote insulin secretion in pancreatic beta cells in response to glucose, partly, through the repression of UCP2 (Moynihan et al., 2005; Bordone et al., 2006; Ramsey et al., 2008) (Fig. 1). Pancreatic beta cell-specific Sirt1-overexpressing (BESTO) transgenic mice exhibited enhanced GSIS and improved glucose tolerance (Moynihan et al., 2005). Additionally, BESTO islets showed reduced levels of UCP2 and correspondingly increased levels of ATP. Consistent with the results from BESTO mice, insulin secretion was impaired along with the increased level of UCP2 in whole-body Sirt1−/− mice, where Sirt1 was knocked down by RNA interference (Bordone et al., 2006). Evidence from BESTO and Sirt1−/− mice suggests that Sirt1 promotes insulin secretion by repressing the expression of UCP2.
Taken together, Sirt1 has been implicated in numerous metabolic pathways, including adipogenesis and insulin secretion. In particular, CR/HFa diets could regulate the expression of Sirt1 in beta cells, suggesting that Sirt1 activation may have some positive actions in lipotoxicity. Direct evidence is required to uncover it and ultimately provide a clearer perspective. Furthermore, it has been identified that the beneficial effect of Sirt1 on beta-cell function is attributed to the repression of UCP2. However, it is unclear whether this effect could play a part under the lipotoxic condition in T2DM. In addition, further studies are needed to identify other target molecules regulated by Sirt1 in addition to UCP2. Such challenges must be met to provide novel and urgently needed therapeutic strategies for the treatment of T2DM, and to prevent the associated lipid stress.
Pituitary Adenylate Cyclase-Activating Polypeptide
PACAP, a peptide of incretin family, is known to be located in the central and peripheral nervous systems. Recent data demonstrated that it located in pancreatic islets as well and could potentiate GSIS as an effective insulinotropin in an autocrine and/or paracrine manner (Filipsson et al., 1997; Sakurai et al., 2011). Further studies found that PACAP could be synthesized by islets and stored in the secretory granules of both beta cells and alpha cells (Nakata and Yada, 2007). The insulinotropic action involved the binding of PACAP to its specific receptors and the successive coupling to both cyclic AMP and Ca2+ signaling, the pathways known to be employed also by other incretin hormones, such as GLP-1 (Nakata and Yada, 2007; Sakurai et al., 2011). In addition, transgenic mice with beta-cell-specific overexpression of PACAP were resistant to streptozotocin-induced beta-cell destruction, suggesting the protective effect of PACAP for islet beta cells (Yamamoto et al., 2003).
In the study from Nakata, short-term treatment of islets with palmitate was performed and it was found that the treatment markedly impaired both [Ca2+]i and GSIS in islets of PACAP-null, but not wild-type mice (Nakata et al., 2010). These results indicated that endogenous PACAP in islets played an important role in protecting beta cells against lipotoxicity. Moreover, treatment with palmitate also obviously increased the expression of UCP2 mRNA in islets of PACAP-null compared with wild-type mice, suggesting that PACAP exerted its protective effect, at least partly, via counteracting the elevation of UCP2 expression (Fig. 1). Nevertheless, all these findings were under the experimental manipulation. Whether the synthesis and activity of PACAP are affected by lipotoxic condition remain to be researched. In addition, the downstream effectors in the PACAP pathway need to be expanded.
Collectively, islet-produced PACAP could protect beta cells from lipotoxicity, indicating a potential antidiabetic role for PACAP to prevent and/or treat lipid-induced beta-cell dysfunction.
Ghrelin
Ghrelin, a 28-amino-acid peptide, is secreted predominantly from X/A-like cells of the gastric fundus. Through the orphan growth hormone secretagogue receptor type 1a (GHSR1a), ghrelin acted as a growth hormone-releasing peptide to affect and/or modulate energy and glucose homeostasis; gastrointestinal, cardiovascular, pulmonary, and immune functions; and cell proliferation and differentiation (Soares and Leite-Moreira, 2008).
Recently, the effect of ghrelin on pancreatic beta-cell function attracted much attention. Emerging studies have identified ghrelin-secreting cells (epsilon-cells) in the verges of pancreatic islets, suggesting that ghrelin affected beta cells through both the endocrine and paracrine pathways (Prado et al., 2004; Vignjević et al., 2012). Further study reported that ghrelin could stimulate insulin secretion (Granata et al., 2007), although inhibitory effects were also reported (Dezaki et al., 2004; Sun et al., 2006; Tong et al., 2010). In addition, Bando et al. (2012) developed a transgenic mouse model in which the ghrelin genes were overexpressed; however, such overexpression of intraislet ghrelin did not affect insulin secretion in vivo. This controversy may be, at least in part, attributed to physiologic or pharmacologic doses of ghrelin, which necessitates further investigation.
Despite the inconsistent effects regarding the effect of ghrelin on insulin secretion, accumulating evidence supports a role of ghrelin in the regulation of proliferation and differentiation of pancreatic beta cells. It was reported that ghrelin could inhibit beta-cell apoptosis induced by the interferon-r/tumor necrosis factor-a (IFN-r/TNF-a), doxorubicin, and lipid stress (Granata et al., 2007; Zhang et al., 2007; Wang et al., 2010). Furthermore, it was reported that the protective effect of ghrelin against lipid stress may via, at least in part, the PI3K/Akt signaling pathway by increasing beta-cell proliferation and survival (Granata et al., 2007). Additionally, ghrelin also inhibited the FFA-induced ER stress pathway of apoptosis in MIN6 cells, decreased expression of cytoplasmic TG, and downregulated gene expression of Bcl-2-associated X (BAX), SREBP-1c, and C/EBP homologous protein (CHOP-10) (Wang et al., 2010). Furthermore, Wang et al. (2010) found that ghrelin inhibited the nuclear translocation of FoxO1 in pancreatic beta cells under lipotoxic pressure, suggesting that the inhibition of FoxO1 may play a key role in the antilipotoxic effect of ghrelin in beta cells.
In summary, the protective action of ghrelin on beta-cell function under lipid stress involves the activation of the PI3K/Akt signaling pathway, the nuclear exclusion of FoxO1, a decrease in cytoplasmic TG synthesis, and the downregulation of BAX, SREBP-1c, and CHOP-10 (Fig. 1).
Conclusion
Lipotoxicity-induced beta-cell dysfunction includes impaired GSIS and increased lipoapoptosis. To date, many effectors have been found to be involved in lipotoxicity (Table 1). It is identified that the lipotoxic condition can elevate the expression/activity of SREBP-1c and UCHL1, but downregulate the activity of NDRG2. Whereas, stronger evidences for the expression/activity of other factors, including FXR, FoxO1, perilipin, ADFP, PACAP, Sirt1, and ghrelin, under lipid stress are necessitated (Fig. 2).
SREBP-1c, FXR, and FoxO1 are the most studied transcription factors for lipotoxicity and now attracting much attention from researchers in this field. Various beta-cell function-related molecules, including Pdx-1, UCP2, IRS-2, granuphilin, and Kir6.2, are known to be regulated by SREBP-1c (Fig. 1). Among these effectors, the Pdx-1 and SREBP-1c-Pdx-1-GLP-1R pathway may be one of essential signal pathways involved in the lipid-induced beta-cell dysfunction. However, whether other effectors mentioned above are involved in the lipotoxicity-SREBP-1c pathway is not quite clear (Fig. 2). FXR can enhance the expression of Pdx-1, NeuroD1, and MafA as shown in Figure 1 under physiological conditions. In addition, the regulation of Pdx-1 and BAX by FoxO1 is identified as well. However, the function of FXR and FoxO1 and their related signaling pathways under lipid stress need further investigation.
The PI3K/Akt pathway is essential for lipid-induced toxicity in pancreatic beta cells. The most studied downstream effector in this pathway is FoxO1. Recent evidences indicate that NDRG2, as a potential substrate of Akt, is involved in the Akt-mediated protection of beta cells against lipoapoptosis as well. However, the downstream pathway of Akt/NDRG2 is still not completely uncovered. It would be of significance to find out other effectors in the PI3K/Akt signal pathway under lipid stress.
Ghrelin is found to be an important peptide, which is secreted predominantly from the gastric fundus. As shown in Figure 1, it stands in the center of the molecular network related to lipotoxicity. It regulates the activity and/or expression of transcription factors SREBP-1c and FoxO1, the synthesis of cytoplasmic TG, the activity of PI3K/Akt, and the expression of the BCL-2 family member BAX. Other possible targets are required to be identified.
Furthermore, both PACAP and Sirt1 could repress the expression of UCP2, resulting in the increase of ATP synthesis. Moreover, emerging understanding of the importance of UCHL1 and the ubiquitin proteasome system in addition to lipid storage-related proteins (perilipin and ADFP) opens new perspectives in the molecular mechanisms for lipotoxicity. It would be much meaningful to explore other new and possible mechanisms in this field.
To summarize, although substantial work has been done, the entire blueprint related to FFA-induced beta-cell dysfunction is still not obtained. A consistent line connecting all these various molecules remains to be explored. Furthermore, application of these involved effectors into the clinical therapy for T2DM is still in the initial phase. To thoroughly understand the molecular network for lipotoxicity may help to postpone and/or terminate the development of T2DM under lipid stress.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (81100581 to S. Shao) and by a grant from the China International Medical Foundation (CIMF) - novo nordisk China Diabetes Young Scientific Talent Research Funding (20110059 to S. Shao).
Disclosure Statement
No competing financial interests exist.
References
- Al-Regaiey K.A. Masternak M.M. Bonkowski M. Sun L. Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Bando M. Iwakura H. Ariyasu H. Hosoda H. Yamada G. Hosoda K. Adachi S. Nakao K. Kangawa K. Akamizu T. Transgenic overexpression of intraislet ghrelin does not affect insulin secretion or glucose metabolism in vivo. Am J Physiol Endocrinol Metab. 2012;302:E403–E408. doi: 10.1152/ajpendo.00341.2011. [DOI] [PubMed] [Google Scholar]
- Bastie C.C. Nahle Z. McLoughlin T. Esser K. Zhang W. Unterman T. Abumrad N.A. FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem. 2005;280:14222–14449. doi: 10.1074/jbc.M413625200. [DOI] [PubMed] [Google Scholar]
- Bordone L. Motta M.C. Picard F. Robinson A. Jhala U.S. Apfeld J. McDonagh T. Lemieux M. McBurney M. Szilvasi A. Easlon E.J. Lin S.J. Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. Klint C. Wierup N. Strom K. Larsson S. Sundler F. Lupi R. Marchetti P. Xu G. Kimmel A. Londos C. Holm C. Perilipin is present in islets of Langerhans and protects against lipotoxicity when overexpressed in the beta-cell line INS-1. Endocrinology. 2009;150:3049–3057. doi: 10.1210/en.2008-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle D.L. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- Chen Y.R. Fang S.R. Fu Y.C. Zhou X.H. Xu M.Y. Xu W.C. Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem Cell Biol. 2010;88:715–722. doi: 10.1139/O10-010. [DOI] [PubMed] [Google Scholar]
- Choi J. Levey A.I. Weintraub S.T. Rees H.D. Gearing M. Chin L.S. Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- Chong Z.Z. Shang Y.C. Wang S. Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K.Y. Li H. Wada K. Johnson J.D. Ubiquitin C-terminal hydrolase L1 is required for pancreatic beta cell survival and function in lipotoxic conditions. Diabetologia. 2012;55:128–140. doi: 10.1007/s00125-011-2323-1. [DOI] [PubMed] [Google Scholar]
- Cohen H.Y. Miller C. Bitterman K.J. Wall N.R. Hekking B. Kessler B. Howitz K.T. Gorospe M. de Cabo R. Sinclair D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Dezaki K. Hosoda H. Kakei M. Hashiguchi S. Watanabe M. Kangawa K. Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- Ducharme N.A. Bickel P.E. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- Dufer M. Horth K. Krippeit-Drews P. Drews G. The significance of the nuclear farnesoid X receptor (FXR) in beta cell function. Islets. 2012;4:333–338. doi: 10.4161/isl.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faleck D.M. Ali K. Roat R. Graham M.J. Crooke R.M. Battisti R. Garcia E. Ahima R.S. Imai Y. Adipose differentiation-related protein regulates lipids and insulin in pancreatic islets. Am J Physiol Endocrinol Metab. 2010;299:249–257. doi: 10.1152/ajpendo.00646.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D. Huang Z. Guan H. Liu J. Yao B. Xiao H. Li Y. The Akt/FoxO1/p27 pathway mediates the proliferative action of liraglutide in β cells. Mol Med Report. 2012;5:233–238. doi: 10.3892/mmr.2011.607. [DOI] [PubMed] [Google Scholar]
- Filipsson K. Tornoe K. Holst J. Ahren B. Pituitary adenylate cyclase-activating polypeptide stimulates insulin and glucagon secretion in humans. J Clin Endocrinol Metab. 1997;82:3093–3098. doi: 10.1210/jcem.82.9.4230. [DOI] [PubMed] [Google Scholar]
- Foletta V.C. Prior M.J. Stupka N. Carey K. Segal D.H. Jones S. Swinton C. Martin S. Cameron-Smith D. Walder K.R. NDRG2, a novel regulator of myoblast proliferation, is regulated by anabolic and catabolic factors. J Physiol. 2009;587:1619–1634. doi: 10.1113/jphysiol.2008.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcheron F. Legedz L. Chinetti G. Feugier P. Letexier D. Bricca G. Beylot M. Genes of cholesterol metabolism in human atheroma: overexpression of perilipin and genes promoting cholesterol storage and repression of ABCA1 expression. Arterioscler Thromb Vasc Biol. 2005;25:1711–1717. doi: 10.1161/01.ATV.0000174123.19103.52. [DOI] [PubMed] [Google Scholar]
- Giacca A. Xiao C. Oprescu A.I. Carpentier A.C. Lewis G.F. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–E262. doi: 10.1152/ajpendo.00416.2010. [DOI] [PubMed] [Google Scholar]
- Granata R. Settanni F. Biancone L. Trovato L. Nano R. Bertuzzi F. Destefanis S. Annunziata M. Martinetti M. Catapano F. Ghe C. Isgaard J. Papotti M. Ghigo E. Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3',5'-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology. 2007;148:512–529. doi: 10.1210/en.2006-0266. [DOI] [PubMed] [Google Scholar]
- Haigis M.C. Guarente L.P. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Heid H.W. Moll R. Schwetlick I. Rackwitz H.R. Keenan T.W. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- Hershko A. Ciechanover A. Varshavsky A. Basic Medical Research Award. The ubiquitin system. Nat Med. 2000;6:1073–1081. doi: 10.1038/80384. [DOI] [PubMed] [Google Scholar]
- Hong S.W. Lee J. Park S.E. Rhee E.J. Park C.Y. Oh K.W. Park S.W. Lee W.Y. Repression of sterol regulatory element-binding protein 1-c is involved in the protective effects of exendin-4 in pancreatic beta-cell line. Mol Cell Endocrinol. 2012;362:242–252. doi: 10.1016/j.mce.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Hu X.L. Liu X.P. Deng Y.C. Lin S.X. Wu L. Zhang J. Wang L.F. Wang X.B. Li X. Shen L. Zhang Y.Q. Yao L.B. Expression analysis of the NDRG2 gene in mouse embryonic and adult tissues. Cell Tissue Res. 2006;325:67–76. doi: 10.1007/s00441-005-0137-5. [DOI] [PubMed] [Google Scholar]
- Imai Y. Varela G.M. Jackson M.B. Graham M.J. Crooke R.M. Ahima R.S. Reduction of hepato-steatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–1954. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Kaestner K.H. Knochel W. Martinez D.E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- Kato T. Shimano H. Yamamoto T. Ishikawa M. Kumadaki S. Matsuzaka T. Nakagawa Y. Yahagi N. Nakakuki M. Hasty A.H. Takeuchi Y. Kobayashi K. Takahashi A. Yatoh S. Suzuki H. Sone H. Yamada N. Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes. 2008;57:2382–2392. doi: 10.2337/db06-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi O. Kobayashi M. Amano K. Sasaki T. Kitazumi T. Kim H.J. Lee Y.S. Yokota-Hashimoto H. Kitamura Y.I. Kitamura T. FoxO1 gain of function in the pancreas causes glucose intolerance, polycystic pancreas, and islet hypervascularization. PLoS One. 2012;7:e32249. doi: 10.1371/journal.pone.0032249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J. Winter K. Nian C. Tsuneoka M. Koda Y. McIntosh C.H. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem. 2005;280:22297–22307. doi: 10.1074/jbc.M500540200. [DOI] [PubMed] [Google Scholar]
- Kitamura T. Nakae J. Kitamura Y. Kido Y. Biggs W.H., 3rd Wright C.V. White M.F. Arden K.C. Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachat P. Shaw P. Gebhard S. van, Belzen N. Chaubert P. Bosman F.T. Expression of NDRG1, a differentiation-related gene, in human tissues. Histochem Cell Biol. 2002;118:399–408. doi: 10.1007/s00418-002-0460-9. [DOI] [PubMed] [Google Scholar]
- Lee C.S. Sund N.J. Vatamaniuk M.Z. Matschinsky F.M. Stoffers D.A. Kaestner K.H. Foxa2 controls Pdx1 gene expression in pancreatic beta-cells in vivo. Diabetes. 2002;51:2546–2551. doi: 10.2337/diabetes.51.8.2546. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. Cariou B. Lien F. Kuipers F. Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li Y. Xu S. Giles A. Nakamura K. Lee J.W. Hou X. Donmez G. Li J. Luo Z. Walsh K. Guarente L. Zang M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Avalos M.D. Duvivier-Kali V.F. Xu G. Bonner-Weir S. Sharma A. Weir G.C. Evidence for a role of the ubiquitin-proteasome pathway in pancreatic islets. Diabetes. 2006;55:1223–1231. doi: 10.2337/db05-0450. [DOI] [PubMed] [Google Scholar]
- Ma K. Saha P.K. Chan L. Moore D.D. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M. Okamoto A.Y. Repa J.J. Tu H. Learned R.M. Luk A. Hull M.V. Lustig K.D. Mangelsdorf D.J. Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Martinez S.C. Tanabe K. Cras-Meneur C. Abumrad N.A. Bernal-Mizrachi E. Permutt M.A. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–859. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- Meetoo D. McGovern P. Safadi R. An epidemiological overview of diabetes across the world. Br J Nurs. 2007;16:1002–1007. doi: 10.12968/bjon.2007.16.16.27079. [DOI] [PubMed] [Google Scholar]
- Mishra R. Simonson M.S. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptoticsignaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol. 2005;10:2. doi: 10.1186/1475-2840-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S. Gan J.W. Brzostowski J. Parisi M.J. Schultz C.J. Londos C. Oliver B. Kimmel A.R. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;30:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. Minamida R. Furuyama T. Tashiro F. Yamato E. Inagaki S. Miyazaki J. Analysis of Foxo1-regulated genes using Foxo1-deficient pancreatic beta cells. Genes Cells. 2012;17:758–767. doi: 10.1111/j.1365-2443.2012.01625.x. [DOI] [PubMed] [Google Scholar]
- Moynihan K.A. Grimm A.A. Plueger M.M. Bernal-Mizrachi E. Ford E. Cras-Meneur C. Permutt M.A. Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nakata M. Shintani N. Hashimoto H. Baba A. Yada T. Intra-islet PACAP protects pancreatic beta-cells against glucotoxicity and lipotoxicity. J Mol Neurosci. 2010;42:404–410. doi: 10.1007/s12031-010-9383-4. [DOI] [PubMed] [Google Scholar]
- Nakata M. Yada T. PACAP in the glucose and energy homeostasis: physiological role and therapeutic potential. Curr Pharm Des. 2007;13:1105–1112. doi: 10.2174/138161207780618948. [DOI] [PubMed] [Google Scholar]
- Nijman S.M. Luna-Vargas M.P. Velds A. Brummelkamp T.R. Dirac A.M. Sixma T.K. Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Popescu I.R. Helleboid-Chapman A. Lucas A. Vandewalle B. Dumont J. Bouchaert E. Derudas B. Kerr-Conte J. Caron S. Pattou F. Staels B. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010;584:2845–2851. doi: 10.1016/j.febslet.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Picard F. Kurtev M. Chung N. Topark-Ngarm A. Senawong T. Machado De Oliveira R. Leid M. McBurney M.W. Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado C.L. Pugh-Bernard A.E. Elghazi L. Sosa-Pineda B. Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K.M. Mills K.F. Satoh A. Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y. Shintani N. Hayata A. Hashimoto H. Baba A. Trophic effects of PACAP on pancreatic islets: a mini-review. J Mol Neurosci. 2011;43:3–7. doi: 10.1007/s12031-010-9424-z. [DOI] [PubMed] [Google Scholar]
- Shao S. Fang Z. Yu X. Zhang M. Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Commun. 2009;384:401–404. doi: 10.1016/j.bbrc.2009.04.135. [DOI] [PubMed] [Google Scholar]
- Shao S. Liu Z. Yang Y. Zhang M. Yu X. SREBP-1c, Pdx-1, and GLP-1R involved in palmitate-EPA regulated glucose-stimulated insulin secretion in INS-1 cells. J Cell Biochem. 2010;111:634–642. doi: 10.1002/jcb.22750. [DOI] [PubMed] [Google Scholar]
- Shen L. Zhao Z.Y. Wang Y.Z. Ji S.P. Liu X.P. Liu X.W. Che H.L. Lin W. Li X. Zhang J. Yao L.B. Immunohistochemical detection of Ndrg2 in the mouse nervous system. Neuroreport. 2008;19:927–931. doi: 10.1097/WNR.0b013e32830163d0. [DOI] [PubMed] [Google Scholar]
- Shen L. Liu X. Hou W. Yang G. Wu Y. Zhang R. Li X. Che H. Lu Z. Zhang Y. Yao L. NDRG2 is highly expressed in pancreatic beta cells and involved in protection against lipotoxicity. Cell Mol Life Sci. 2010;67:1371–1381. doi: 10.1007/s00018-010-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.B. Leite-Moreira A.F. Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides. 2008;29:1255–1270. doi: 10.1016/j.peptides.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Soares J.B. Leite-Moreira A.F. Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides. 2008;29:1255–1270. doi: 10.1016/j.peptides.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Sun Y. Asnicar M. Saha P.K. Chan L. Smith R.G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sun C. Zhang F. Ge X. Yan T. Chen X. Shi X. Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Takahashi A. Motomura K. Kato T. Yoshikawa T. Nakagawa Y. Yahagi N. Sone H. Suzuki H. Toyoshima H. Yamada N. Shimano H. Transgenic mice overexpressing nuclear SREBP-1c in pancreatic beta-cells. Diabetes. 2005;54:492–499. doi: 10.2337/diabetes.54.2.492. [DOI] [PubMed] [Google Scholar]
- Tong J. Prigeon R.L. Davis H.W. Bidlingmaier M. Kahn S.E. Cummings D.E. Tschöp M.H. D'Alessio D. Ghrelin Suppresses Glucose-stimulated Insulin Secretion and Deteriorates Glucose Tolerance in Healthy Humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetterli L. Brun T. Giovannoni L. Bosco D. Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem. 2011;286:6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjević S. Todorović V. Damjanović S. Budeč M. Mitrović O. Djikić D. Drndarević N. Mićić M. Mišković-Krivokapić J. Djuričić S. Nikolić I. Similar Developmental Patterns of Ghrelin- and Glucagon-Expressing Cells in the Human Pancreas. Cells Tissues Organs. 2012;196:362–373. doi: 10.1159/000335469. [DOI] [PubMed] [Google Scholar]
- Wang W. Liu Y. Chen Y. Cao C. Xiang Y. Zhang D. Han L. Zhao H. Liu G. Inhibition of Foxo1 mediates protective effects of ghrelin against lipotoxicity in MIN6 pancreatic beta-cells. Peptides. 2010;31:307–314. doi: 10.1016/j.peptides.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. Hashimoto H. Tomimoto S. Shintani N. Miyazaki J. Tashiro F. Aihara H. Nammo T. Li M. Yamagata K. Miyagawa J. Matsuzawa Y. Kawabata Y. Fukuyama Y. Koga K. Mori W. Tanaka K. Matsuda T. Baba A. Overexpression of PACAP in transgenic mouse pancreatic beta-cells enhances insulin secretion and ameliorates streptozotocin-induced diabetes. Diabetes. 2003;52:1155–1162. doi: 10.2337/diabetes.52.5.1155. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Lee F.Y. Barrera G. Lee H. Vales C. Gonzalez F.J. Willson T.M. Edwards P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Ying B. Shi L. Fan H. Yang D. Xu D. Wei Y. Hu X. Zhang Y. Zhang X. Wang T. Liu D. Dou L. Chen G. Jiang F. Wen F. Ghrelin inhibit cell apoptosis in pancreatic beta cell line HIT-T15 via mitogen-activated protein kinase/phosphoinositide 3-kinase pathways. Toxicology. 2007;237:194–202. doi: 10.1016/j.tox.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Zitkus B.S. Type 2 diabetes mellitus: an evidence-based update. Nurse Pract. 2012;37:28–37. doi: 10.1097/01.NPR.0000415243.66858.27. [DOI] [PubMed] [Google Scholar]