Abstract
Introduction
A bioartificial liver comprising alginate-encapsulated liver cell spheroids (ELS) could bridge the gap to transplant or spontaneous recovery in acute liver failure, but will be required for emergency use, necessitating cryopreservation. A cryopreservation protocol has been developed, but beyond this, the feasibility of cold-chain storage is considered here. Cryopreservation will be increasingly required for timely delivery of tissue and bioengineered products, and significant, but often, over-looked factors that impact on cost and ease of clinical application are the storage temperature and useful preservation time. Storage in the vapor phase of liquid nitrogen (∼−170°C) is the gold standard, but for safety and economic purposes, storing ELS in electric freezers at −80°C may be preferable.
Methods
ELS were cryopreserved using an optimized protocol and stored at either −80°C or at −170°C for up to 1 year. ELS were removed from storage after 1, 2, 3, 6, 9, or 12 months, and recovery was assessed 24 h postwarming. Cell recovery was assessed using viability (fluorescent staining with image analysis), cell number (nuclei count), and functional (hepatospecific protein enzyme-linked immunosorbent assay) assays.
Results
Viability, the viable cell number, and function of ELS stored at −170°C were maintained at similar values throughout the year. In contrast, ELS stored at −80°C exhibited decreased viability, viable cell numbers, and function by as early as 1 month. Progressive deterioration was subsequently observed. After 12 months of storage at −80°C, viable cell recovery of ELS was ∼15% that of ELS stored at −170°C.
Conclusions
While convenience and cost might support the use of −80°C for storage of multicellular bioengineered products such as ELS, results indicate rapid deterioration in functional recoveries after only a few weeks. This study demonstrates that storage temperature is an important consideration in regenerative medicine and caution should be applied by limiting storage at −80°C to only a few weeks.
Introduction
Future applications in regenerative medicine will increasingly require a robust cryobanking step to be able to deliver cell products, produced under regulatory-compliant conditions to be delivered as and when required by health services. Our group has focused on development of encapsulated liver cell spheroids (ELS) as a part of a bioartificial liver (BAL) to bridge the gap either to transplant or to spontaneous recovery in patients suffering from acute liver failure (ALF).1 The system requires conditioning culture for up to 10 days to allow formation of 3D ELS that are sufficiently functional to treat ALF. ALF, however, can develop within 1–2 days and requires immediate support.2 To meet this demand with sufficient quality-assured, batch-tested ELS, a scale-up process with cryobanking is a necessary step in the overall production plan. Cryopreservation of ELS with high functional recoveries in postwarming cultures itself is a challenge, but we have recently developed an improved protocol that has centered around control of the ice nucleation step to reduce random super cooling (to below the melting point of the mixture) and possible subsequent intracellular ice formation, by including an ice-nucleating agent, cholesterol.3 Samples of ELS were routinely stored in the vapor-phase nitrogen (below −170°C) before rewarming.
To move to scale-up and eventual clinical trials, there are several factors to consider in developing a suitable cold-chain for delivery, which include not only scientific optimization of the cryopreservation step but also costs, logistics, and ease of end-user application. One of these factors is end temperature for cryopreservation and storage of the cryopreserved products of ELS (which link through to the often-overlooked logistics required for transport of cryobanked materials between production facilities and end-user sites). Vapor-phase nitrogen storage is the gold standard in many cell cryobanking facilities,4 but requires the logistical resources for delivery, handling, and maintenance of liquid nitrogen supplies. Moreover, the use of nitrogen requires personnel to be trained in its use and specific storage facilities (which must be well ventilated, etc.) to meet safety requirements. Storage in electrical freezers at −80°C may simplify and reduce costs in the cryobanking stage, and can be readily interfaced with automated sample management.5
There have been previous reports from the tissue-banking literature, particularly for cryobanking of heart valves that −80°C storage is inferior to that in vapor-phase nitrogen.6 On the other hand, in different cell types (peripheral blood mononuclear cells), storage at −80°C was suggested to yield acceptable recoveries for up to 1.5 years (although recovery did decline beyond this storage time).7
Despite these reports, little data are available regarding appropriate storage temperature for cell therapy products such as cell organoids. In particular, we could find no literature describing the rate of attrition (if any) on functional recoveries of organoid systems such as ELS. If, however, storage at −80°C did result in acceptable functional recoveries even for a few months, this information could be used to formulate a cold-chain strategy, and a conventional −80°C storage freezer could be utilized. The aim of this study was to investigate the effects of 2 storage temperatures (−170°C and −80°C) on functional recovery of ELS for storage periods up to and, including, 1 year.
Materials and Methods
Chemicals
Unless otherwise stated, all chemicals were sourced from Sigma (Poole, United Kingdom).
HepG2 monolayer cell culture for production of ELS
HepG2 cells were obtained from the ECACC (Wiltshire, United Kingdom) and maintained in a modified MEM-alpha medium (Gibco, Paisley, United Kingdom) supplemented with 10% FBS (Hyclone, Loughborough, United Kingdom), 100 IU/mL penicillin, and 0.1 mg/mL streptomycin. The medium was changed every 2–3 days. Cells were counted using a hemocytometer, and the viability was assessed using trypan blue dye exclusion.
Alginate encapsulation and culture of ELS
HepG2 cells were encapsulated into alginate as described previously. Briefly, 80% confluent monolayer cultures were trypsinized and encapsulated into 1% alginate (alginic acid sodium salt Macrocystis pyrifera kelp).8 Alginate beads containing cells were resuspended in the culture medium at a ratio of 1:32. The medium was changed every 2–3 days unless otherwise stated.
Cryopreservation and storage of ELS over extended periods
About 250 μL beads of ELS were exposed to 1 mL cryopreservation medium containing 15% w/v dimethyl sulfoxide (DMSO) in the University of Wisconsin solution (Belzer, Madison, WI) to achieve a final DMSO concentration of 12% with an additional 1.1 mg/mL cholesterol powder as an ice-nucleating agent, for 10 min at 4°C, and transferred to 1.8-mL cryovials (Nunc, Loughborough, United Kingdom) in 1.25-mL volumes. A preprogrammed slow-cooling protocol9 was applied using a Planer Kryo 10 Freezer (Planer, Sunbury-on-Thames, United Kingdom) with the main cooling ramp planned to control the sample temperature change at ∼−2°C/min. However, there were additional brief nonlinear steps, and the full protocol was as follows: 8-min hold at 0°C to ensure all samples had reached equilibrium at this temperature; −2°C/min to −8°C; −35°C/min to −28°C; −2.5°C to −33°C; +2.5°C/min to −28°C; −2°C/min to −60°C; −10°C/min to −100°C; and −20°C/min to −160°C. All cryovials for these studies were loaded with the same cell batch on the same day for cryopreservation and storage.
ELS destined for storage in the vapor phase of liquid nitrogen (Group A) were removed once the cycle was complete and transferred to the vapor phase of liquid nitrogen. However, ELS destined for storage at −80°C (Group B) were removed once the chamber temperature of the Kryo10 Freezer had reached −80°C and transferred to an electrical freezer set at −80°C (Revco, Langensebald, Germany). When samples were removed for −80°C storage, the Kryo10 automatically paused the cooling program until the chamber lid was replaced before continuing cooling at 10°C/min to −100°C. The −80°C freezer was fitted with alarms, and no incidents of power failure or temperature drift were observed over the whole 12-month period. Similarly, nitrogen vapor storage was routinely checked three times per week, and liquid nitrogen levels were maintained by filling three times per week.
Groups A and B were stored for up to 1 year, but were thawed at incremental time points (1, 2, 3, 6, 9, and 12 months). ELS were thawed rapidly in a 37°C water bath until all visible ice disappeared. ELS were washed twice in a complete culture medium [first, a chilled medium was added slowly over 12 min, and then replaced with a warm (37°C) medium] and returned to culture. Recovery of cryopreserved ELS was assessed at 24 h postwarming, as we have previously shown this time point to be when the damage of ELS from cryopreservation is most apparent.3 For comparison, unfrozen ELS were assessed for viability and viable cell number at the equivalent time point (i.e., 24 h after ELS were removed for cryopreservation).
Viability assay
ELS (250 μL) were washed twice with 500 μL PBS/Ca2+/Mg2+ (Lonza, Slough, United Kingdom) before dual staining with 10 μL of 1 mg/mL fluorescein diacetate (FDA/DMSO) and 20 μL of 1 mg/mL propidium iodide (PI/water). ELS were incubated for 90 s at room temperature before 2 further washes with 500 μL PBS/Ca2+/Mg2+. Images were captured using Lucia Imaging Software with a DX1200 camera at exposures of 64 (FDA) and 250 ms (PI).
Quantification of viability
For quantitative measurement, the integral densities of each stain were assessed using Lucia imaging software, and the viability was calculated as the percentage of live cells within the total cell field.
Quantification of cell numbers from ELS
Beads were removed from culture, washed twice with HBSS, dissolved in 16 mM EDTA/0.15 M NaCl for 10 min, and centrifuged at 13,000 rcf for 5 min. The supernatant was removed, pellet vortexed, and resuspended in phosphate-buffered saline. The cell concentration was assessed using the Nucleoview System (Sartorius Stedim, Epsom, United Kingdom) according to the manufacturer's instructions. Viable cell numbers were calculated by multiplying the cell number (determined using the Nucleoview system) by the viability (determined using fluorescent staining with image analysis) for each sample.
Functional assay by albumin secretion
Albumin secretion over 24 h was quantified by sandwich enzyme-linked immunosorbent assay (ELISA). A fresh medium (5 mL) was harvested after 24 h and stored at −20°C. A standard curve (25–200 ng/mL) using a known human protein serum calibrator (Dako, Ely, United Kingdom) was produced. Species-specific primary (Dako) and secondary horseradish peroxidise-linked (Abcam, Cambridge, United Kingdom) antibodies were used. Nonspecific binding was reduced using 5% nonfat milk. Optical density (492 nm) was measured (Anthos HTIII plate reader; Labtech, Salzburg, Austria), and data were analyzed using Biolise software (Labtech).
Differential scanning calorimetry
The glass-transition temperature of the cryopreservation medium was determined using modulated differential scanning calorimetry (DSC) with a Q2000 calorimeter (TA Instruments, Elstree, United Kingdom). Briefly, aliquots of ∼80 μL were sealed inside high-volume stainless-steel pans and compared with an empty reference pan. Samples were cooled at 20°C/min to −150°C on 1 run. Analysis during warming was at 3°C/min with modulation of 1°C/min to 25°C. Data were analyzed using Universal Analysis software (TA Instruments).
Statistics
Statistical analyses were performed by Student's t-Test using Excel software.
Results
Viability of thawed ELS after storage at either −170 °C (Group A) or at −80 °C (Group B)
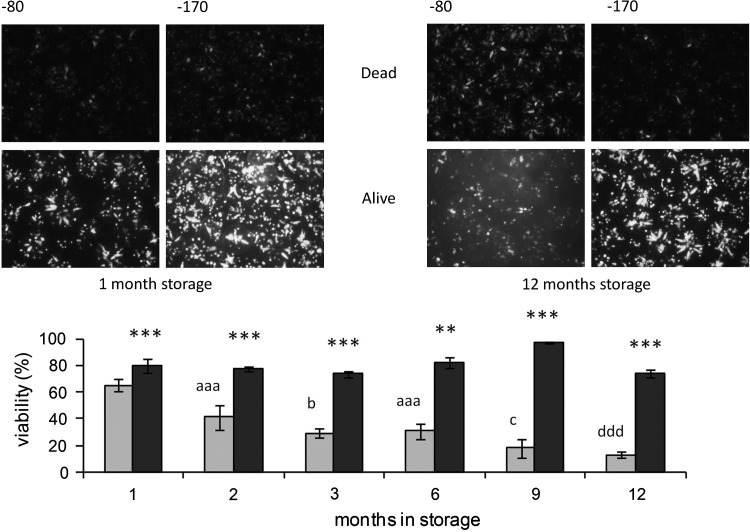
Viability of control, unfrozen ELS remained high at 99.8%±0.3%. After storage for 1 month, viabilities of the 2 cryopreserved cohorts showed a relatively small, yet significant, difference (group A-1m: 80%±5% vs. group B-1m: 65%±5%; p<0.005). At all other time periods for storage at −170°C (groups A-2m, A-3m, A-6m, A-9m, and A-12m), the recovered viability remained in the region of 75%, with no statistical deterioration over time (see Fig. 1). However, after storage at the higher subzero temperature (−80°C), viability decreased progressively, such that in B-3m, it had fallen below 30%, and in B-12m, it was lower again at ∼15%. Viability in group B-12m was also statistically lower than B-1m (p<0.005) (see Fig. 1).
FIG. 1.
Effect of storage temperature for up to 1 year on viability of encapsulated liver cell spheroids (ELS) at 24 h in postwarming cultures. ELS were thawed, and viability was assessed at 24 h postwarming. Micrographs (top) show fluorescein diacetate (FDA) (alive) and propidium iodide (PI) staining (dead) after storage time indicated. Images captured: ×4 original magnification, 64 ms exposure FDA, and 250 ms exposure PI. Viability of ELS stored either at −170°C (dark bars) or at −80°C (light bars) was quantified using image analysis. For the −80°C stored samples, a clear deterioration over time was observed, to a minimum after 12 months. **p<0.01, ***p<0.005–170°C compared to −80°C at the same time point; aaa p<0.005 cf. 1-month storage at −80°C, b p<0.05 cf. 2-month storage at −80°C and p<0.005 cf. 1-month storage at −80°C, c p<0.05 cf. 6-month storage at −80°C and p<0.005 cf. 1-,2-, and 3-month storage at −80°C, ddd p<0.005 cf. 1-,2-,3-, and 6-month storage at −80°C.
Recoveries of viable cell numbers from ELS after storage at either −170 °C (Group A) or −80 °C (Group B)
Viable cell numbers of control unfrozen ELS were 4.4±0.2 million viable nuclei per mL alginate. Viable cell numbers were maintained for the entire 12 months in ELS stored at −170°C (Group A) at ∼3.5 million viable nuclei per mL alginate. Conversely, viable cell numbers of ELS stored at −80°C (Group B) showed a progressive decline in number to a minimum after 12 months by which time, only ∼0.3 million viable nuclei per mL alginate remained (see Fig. 2) (p<0.005). At all time points, viable cell numbers were significantly improved by storage at −170°C (Group A) compared with −80°C storage (Group B) (p<0.005) (see Fig. 2).
FIG. 2.
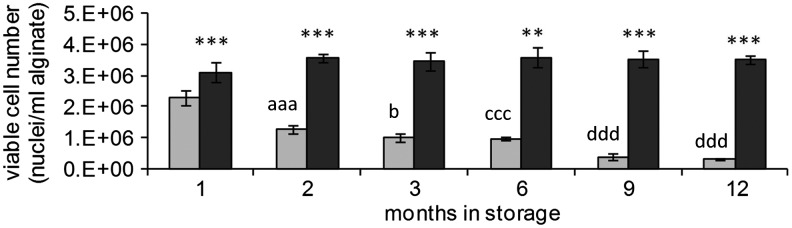
Effect of storage temperature for up to 1 year on viable cell numbers of ELS at 24 h in postwarming cultures. ELS were thawed and viable cell numbers measured at 24 h postwarming. Viable cell numbers of ELS stored either at −170°C (dark bars) or at −80°C (light bars) were quantified using nuclei count. For the −80°C stored samples, there was a clear progression in the loss of viability with time, with each later time point presenting a lower viable cell number than the previous times points (except at 9 and 12 months, where the viable cell numbers were very low, and no statistical difference could be measured between the two values). **p<0.01, ***p<0.005–170°C compared to −80°C at the same time point; aaa p<0.005 cf. 1-month storage at −80°C, b p<0.05 cf. 2 months of storage at −80°C, and p<0.005 cf. 1-month storage at −80°C, ccc p<0.005 cf. 1 and 2 months of storage at −80°C, ddd p<0.005 cf. 1,2,3, and 6 months of storage at −80°C.
Functional recovery (albumin synthesis and secretion) of ELS after storage at either −170 °C (Group A) or −80 °C (Group B)
Similarly, when functional activities of ELS were investigated by measuring albumin secretion, storage at −170°C was consistently superior (see Fig. 3)—at all time points, Group A had significantly higher albumin secretion (p<0.01) versus Group B. For this assessment, already by 1 month, albumin secretion had fallen by more than 60% after storage at −80°C (Group B), whereas by 12 months storage at this temperature, it was almost undetectable, and these losses were significantly greater than in ELS stored for 1 month (p<0.005).
FIG. 3.
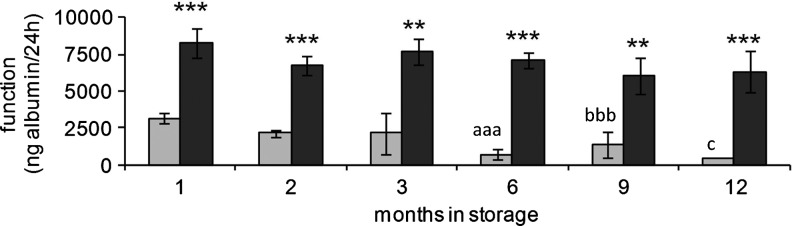
Effect of storage temperature for up to 1 year on function of ELS at 24 h in postwarming cultures. ELS were thawed and viable cell numbers measured at 24 h postwarming. Total function of ELS stored either at −170°C (dark bars) or at −80°C (light bars) were quantified using enzyme-linked immunosorbent assay. There was again a progressive loss in function as assessed by protein synthesis in samples stored at–80°C, although this was not so clear as the loss in viability and viable cell numbers at the early time points (1–3 months); however, for albumin synthesis, there was a statistical deterioration now evident between 9 and 12 months of storage. **p<0.01, ***p<0.005–170°C compared to −80°C at the same time point; aaa p<0.005 cf. 1-month storage at −80°C and p<0.05 cf. 2 months of storage at −80°C, bbb p<0.005 cf. 1-month storage at −80°C, c p<0.005 cf. 1-month storage at −80°C, and p<0.05 cf. 2,3 and 9 months of storage at −80°C.
Differential scanning calorimetry
During rewarming, alterations in reverse heat flow were observed between −120.2°C and −119.6°C, which are characteristic of glass-transition temperatures seen in aqueous mixtures such as those used here.10
Discussion
The outcomes from this study clearly show that viability, viable cell number, and integrated metabolic function were lost in ELS stored at −80°C compared to those stored at −170°C. ELS stored in the vapor phase of liquid nitrogen were maintained over the year, with no change in recovery of ELS. These findings are in agreement with other studies investigating the effect of long-term storage at or close to −170°C of various cell and tissue types, including sperm11 and cardiac valves,12 where no loss of viability or function was observed during long-term storage, even for as long as 28 years.13
Conversely, ELS stored at −80°C deteriorated over time, resulting in significant losses in viability and function. These data confirm other findings in either intact tissues (heart valves)14,6 or single-cell suspensions of bone marrow progenitor cells 15. In studies on the fibroblast populations of heart valves (found unsuitable for transplantation), Brockbank et al. found that those stored for up to 2 years below −135°C maintained activity (glycine incorporation). However, fibroblast populations stored at −80°C showed a general decline in activity for periods out to 24 weeks, although numbers were small.14 Similarly, Feng and colleagues6 investigated prostacyclin release as indicators of endothelial cell function in porcine heart valve leaflets, and reported that while function was maintained for 2 weeks at −80°C, after 8 weeks of storage at this temperature, no prostacyclin synthesis could be detected. Interestingly, when valves were stored at −170°C, a fall in prostacyclin synthesis was detected after 6 months, but this remained constant at 12 months. These differences are not easily explained, because in theory, living cells that have survived during the cooling to temperatures below −170°C should be stable for many years.
Conversely, in a study on cryopreservation of human peripheral blood progenitor cells, Sputtek and colleagues15 stored samples for extended periods (5–52 months) in a paired fashion at either −80°C or −170°C. In this case, recoveries of cell numbers after thawing did not differ between the paired units, whereas colony-forming cultures (proliferation) were reduced by more than 90% in cells recovered from −80°C storage with storage times close to 2 years (range 5–52 months).
In our present work, this was the first study undertaken on the effect of extended storage temperatures in encapsulated cells (multicellular ELS) that we could find. The study also benefitted from the prospective storage of sufficient samples from one initial cell production to allow direct comparisons to be made at the different storage times, so avoiding intersample variation, and also a robust ability to assess outcomes against those measured after shorter storage periods. Once again, storage at −170°C was seen to be superior in both recoveries of viable cells, and in functional recovery estimated from albumin synthesis. Even by 1 month, there was a small, but significant, attrition in recovery of viable cell numbers after storage at −80°C (reduced by ∼15%), but a much larger drop in albumin secretion (by about 60%) compared to those stored at −170°C. It is generally recognized that while activities such as urea production or drug metabolism can be maintained to relatively high levels even after cryopreservation, albumin synthesis is much more sensitive to cryopreservation-induced hepatocyte injury,16,17 and this concurs with our previous observations.3 For these reasons and the importance of albumin synthesis in BAL function, it was the marker of choice to investigate effects of different storage temperatures.
These assessments were made at 24 h post-thawing, as this is the time at which damage from cryopreservation is most apparent. We have previously shown that ELS undergo cryopreservation-induced delayed-onset cell-death (CIDOCD), which is expressed over the first 24 h following rewarming, and that recovery may be overestimated if measured too soon after thawing, as CIDOCD is not yet fully apparent.3 Rather, these differences in outcome after 1 month at −80°C suggest that there may be subtle levels of latent cryoinjury (at the subcellular level of organization, which can interfere with protein synthesis, but which is not reflected by the penetration and activation of fluorescent stains). More importantly, in terms of scale-up production of ELS, even short-term storage at −80°C would appear to be unwise from a scientific perspective. In contrast, in ELS stored at −170°C, the indices of cell recoveries and function remained unchanged over the entire 12-month period.
Encapsulation has been suggested as one strategy to minimize the mechanical injuries imposed on cells during cryopreservation and low temperature banking, an injury that is probably of smaller significance than those attributed to extreme dehydration or to intracellular ice formation, but nevertheless, a potentially avoidable stress.18,19,20 However, our current work provided no evidence for this. Indeed, ELS stored at −80°C showed progressive injury over the 12 months, which argues against any evidence for a major physical protection by encapsulation during storage at temperatures such as −80°C.
It is clear that end-temperature cryobanking and storage of ELS at −80°C is not sufficiently low to prevent losses in functional recovery. It is now generally accepted that for cells to be successfully stored in the long-term, cells and tissues need to achieve ultra-low temperatures below the glass transition of the cell–cryprotectant mixture, so that no mobile water fraction persists in the stored sample. These glass-transition temperatures are typically quoted to be close to −120°C in the types of aqueous media containing DMSO that were used here for cryopreservation of ELS,14,21 in agreement with our own DSC findings in this study.
Thus, at −80°C, the temperature can be high enough for slow, but significant, alterations in the physical state of water, including recrystallization of ice and/or devitrification of freeze-concentrated fractions, which can cause injury. The logistical benefits of using electrical freezers may seem attractive in some cell therapy applications (for example, short-term storage of adipose tissue samples to isolate adipocytes or tissue stem cells for reconstructive surgery down to −70°C), but even after 1 or 2 days, poor recoveries of isolated cells were reported with losses of more than 90%.22,23
Such evidence also cautions against the absolute assumption that cells stored at −170°C can safely enter a cold chain for distribution in a transporter at −80°C if maximum cell numbers are required immediately on thawing, although this was not a part of the current work and will need careful evaluation in the future. The debate on transport temperatures has been recently highlighted by work in other systems (in this case, cryobanked embryos) where even transition to vapor-phase nitrogen for transport may have coincided with a loss of viability.24 Much is likely to depend on the time that cryobanked materials are exposed to variable cryogenic temperatures during transit. For ELS, specifically, future studies will need to be carried out to define the safest conditions (e.g., rates of warming,21 and requirements for annealing steps), as samples pass through temperatures in the glass-transition range before safe transport at −80°C can be assured. This may be different if cryopreserved cells are being shipped to establish new growing cultures at end use, where significant cell losses can be tolerated as long as some cells recover after thawing and enter the growth phase in culture, and cellular debris from dead/dying cells can be removed from post-thaw cultures so as not to damage the surviving cell population. For a BAL to be successful, though immediate post-thaw recovery is required, and so −80°C storage, even for brief periods, appears to be inappropriate for this application. Not only is there a risk of insufficient functional biomass being available following storage at −80°C, cellular debris (including DNA) from dead ELS may be inadvertently returned to the BAL recipient with potential inflammatory responses.
However, some groups have described that storage at −80°C is suitable, and differing (i.e., higher) glass-transition temperatures (resulting from the use different cryoprotectants) may offer an explanation for this. Alternatively, different cell types may possess a greater capability to cope with recrystallization and/or devitrification. Therefore, although this study is conclusive for storage of ELS here, the same does not necessarily apply to all cell and tissue types using all cryopreservation protocols.
In a recent report, Seki and Mazur examined the relationship between mouse oocytes stored at −80°C or −196°C.25 In this study, vitrification techniques with high CPA concentrations were applied, which differ from the slow-cooling protocol used for the ELS. Nevertheless, these authors showed a significant fall-off in viability by 3 months when the oocytes were stored at −80°C, and this was more pronounced at slower warming rates. The authors also suggested that this was indicative of devitrification or recrystallization of ice in oocytes held at −80°C, which is a temperature higher than the glass-transition range for their CPA mixture (∼ −130°C). Interestingly, in oocytes exposed to a vitrification solution EAFS10/10 (with a total solute concentration of ∼60% w/w), but only cooled to −80°C and then stored at this temperature, ice nucleation events could be detected, suggesting at least a small amount of extracellular ice. If those partially nucleated −80°C stored oocytes were warmed extremely rapidly (+3000°C/min), only a small viability loss by 28 days (from ∼90% to ∼60%) was experienced, although further attrition took place by 3 months to 40% viability. These positive outcomes for oocytes stored at −80°C, dependent on the ultrafast warming, are difficult to translate to ELS where large-volume cryopreservation will be required, but they do add support to the ideas about changes in the water state in temperatures in the region of −80°C.
In summary, cold-chain delivery of cell products in regenerative medicine is only one aspect of the overall process, but it can have significant bearing on the clinical or economic outcomes in the production process. Maintenance of appropriate ultra-low temperatures appears to be crucial. New cryotechnologies are being developed to address these issues, and these need to be fully evaluated in the next few years.26,27
Acknowledgments
We thank the Liver Group Charity for funding this study. We also thank the Wellcome Trust for fluorescent microscope facilities (Wellcome grant number 066327). Finally, we also thank Dr. Paul Matejtschuk, Chinwe Duru, and Kiran Malik (NIBSC, a Centre of the Health Protection Agency) for enabling DSC analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Coward S.M. Legallais C. David B. Thomas M. Foo Y. Mavri-Damelin D. Hodgson H.J. Selden C. Alginate-encapsulated HepG2 cells in a fluidized bed bioreactor maintain function in human liver failure plasma. Artif Organs. 2009;33:1117. doi: 10.1111/j.1525-1594.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 2.Polson J. Lee W.M. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 3.Massie I. Selden C. Hodgson H. Fuller B. Cryopreservation of encapsulated liver spheroids for a bioartificial liver: reducing latent cryoinjury using an ice nucleating agent. Tissue Eng Part C-Methods. 2011;17:765. doi: 10.1089/ten.TEC.2010.0394. [DOI] [PubMed] [Google Scholar]
- 4.Stacey G.N. Masters J.R. Cryopreservation and banking of mammalian cell lines. Nat Protoc. 2008;3:1981. doi: 10.1038/nprot.2008.190. [DOI] [PubMed] [Google Scholar]
- 5.Owen J.M. Woods P. Designing and implementing a large-scale automated-80 degrees C archive. Int J Epidemiol. 2008;37:56. doi: 10.1093/ije/dym293. [DOI] [PubMed] [Google Scholar]
- 6.Feng X.J. Van Hove C.E. Walter P.J. Herman A.G. Effects of storage temperature and fetal calf serum on the endothelium of porcine aortic valves. J Thorac Cardiovasc Surg. 1996;111:218. doi: 10.1016/S0022-5223(96)70419-1. [DOI] [PubMed] [Google Scholar]
- 7.Valeri C.R. Pivacek L.E. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996;36:303. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 8.Coward S.M. Selden C. Mantalaris A. Hodgson H.J. Proliferation rates of HepG2 cells encapsulated in alginate are increased in a microgravity environment compared with static cultures. Artif Organs. 2005;29:152. doi: 10.1111/j.1525-1594.2005.29026.x. [DOI] [PubMed] [Google Scholar]
- 9.Diener B. Utesch D. Beer N. Durk H. Oesch F. A method for the cryopreservation of liver parenchymal cells for studies of xenobiotics. Cryobiology. 1993;30:116. doi: 10.1006/cryo.1993.1011. [DOI] [PubMed] [Google Scholar]
- 10.MacFarlane D.R. Devitrification in glass-forming aqueous solutions. Cryobiology. 1986;23:230. [Google Scholar]
- 11.Yogev L. Kleiman S.E. Shabtai E. Botchan A. Paz G. Hauser R. Lehavi O. Yavetz H. Gamzu R. Long-term cryostorage of sperm in a human sperm bank does not damage progressive motility concentration. Hum Reprod. 2010;25:1097. doi: 10.1093/humrep/deq041. [DOI] [PubMed] [Google Scholar]
- 12.Mirabet V. Carda C. Solves P. Novella-Maestre E. Carbonell-Uberos F. Caffarena J.M. Hornero F. Montero J.A. Roig R.J. Long-term storage in liquid nitrogen does not affect cell viability in cardiac valve allografts. Cryobiology. 2008;57:113. doi: 10.1016/j.cryobiol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Clarke G.N. Liu D.Y. Baker H.W.G. Recovery of human sperm motility and ability to interact with the human zona pellucida after more than 28 years of storage in liquid nitrogen. Fertil Steril. 2006;86:721. doi: 10.1016/j.fertnstert.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 14.Brockbank K.G.M. Carpenter J.F. Dawson P.E. Effects of storage-temperature on viable bioprosthetic heart-valves. Cryobiology. 1992;29:537. doi: 10.1016/0011-2240(92)90058-a. [DOI] [PubMed] [Google Scholar]
- 15.Sputtek A. Nowicki B. Rowe A. Kuehnl P. Long-term cryopreservation of human peripheral blood progenitor cells: influence of storage temperature (-80 C vs<-170 C) on cell recovery, membrane integrity and clonogenicity. Abstract presented at the Cryobiology Meeting, 2004. Abstract no. 61. Cryobiology; 2004. p. 314. [Google Scholar]
- 16.Dixit V. Darvasi R. Arthur M. Lewin K. Gitnick G. Cryopreserved microencapsulated hepatocytes—transplantation studies in Gunn rats. Transplantation. 1993;55:616. doi: 10.1097/00007890-199303000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Sosef M.N. Baust J.M. Sugimachi K. Fowler A. Tompkins R.G. Toner M. Cryopreservation of isolated primary rat hepatocytes - Enhanced survival and long-term hepatospecific function. Ann Surg. 2005;241:125. doi: 10.1097/01.sla.0000149303.48692.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusano T. Aoki T. Yasuda D. Matsumoto S. Jin Z. Nishino N. Hayashi K. Odaira M. Yamada K. Koizumi T. Izumida Y. Mitamura K. Enami Y. Niiya T. Murai N. Kato H. Shimizu Y. Kou K. Furukawa Y. Matsusita M. Todo S. Shioda S. Kusano M. Microencapsule technique protects hepatocytes from cryoinjury. Hepatol Res. 2008;38:593. doi: 10.1111/j.1872-034X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Malpique R. Osorio L.M. Ferreira D.S. Ehrhart F. Brito C. Zimmermann H. Alves P.M. Alginate encapsulation as a novel strategy for the cryopreservation of neurospheres. Tissue Eng Part C Methods. 2010;16:965. doi: 10.1089/ten.TEC.2009.0660. [DOI] [PubMed] [Google Scholar]
- 20.Rialland L. Guyomard C. Scotte M. Chesne C. Guillouzo A. Viability and drug metabolism capacity of alginate-entrapped hepatocytes after cryopreservation. Cell Biol Toxicol. 2000;16:105. doi: 10.1023/a:1007690009927. [DOI] [PubMed] [Google Scholar]
- 21.Pegg D.E. Wusteman M.C. Boylan S. Fractures in cryopreserved elastic arteries. Cryobiology. 1997;34:183. doi: 10.1006/cryo.1996.1997. [DOI] [PubMed] [Google Scholar]
- 22.Son D. Oh J. Choi T. Kim J. Han K. Ha S. Lee K. Viability of fat cells over time after syringe suction lipectomy the effects of cryopreservation. Ann Plast Surg. 2010;65:354. doi: 10.1097/SAP.0b013e3181bb49b8. [DOI] [PubMed] [Google Scholar]
- 23.Wolter T.P. von Heimburg D. Stoffels I. Groeger A. Pallua N. Cryopreservation of mature human adipocytes - In vitro measurement of viability. Ann Plast Surg. 2005;55:408. doi: 10.1097/01.sap.0000181345.56084.7d. [DOI] [PubMed] [Google Scholar]
- 24.McDonald C. Valluzo L. Chuang L. Poleshchuk F. Copperman A. Barritt J. Nitrogen vapor shipment of vitrified oocytes: time for caution. Fertil Steril. 2011;95:2628. doi: 10.1016/j.fertnstert.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 25.Seki S. Mazur P. Stability of mouse oocytes at −80?°C: the role of the recrystallization of intracellular ice. Reproduction. 2011;141:407. doi: 10.1530/REP-10-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massie I. Selden C. Hodgson H. Fuller B. Cholesterol reduces supercooling and improves post-warming survival of 3d liver cell spheroids for a bioartificial liver. Int J Artif Organs. 2009;32:413. [Google Scholar]
- 27.Wang L. Pegg D.E. Lorrison J. Vaughan D. Rooney P. Further work on the cryopreservation of articular cartilage with particular reference to the liquidus tracking (LT) method. Cryobiology. 2007;55:138. doi: 10.1016/j.cryobiol.2007.06.005. [DOI] [PubMed] [Google Scholar]