Abstract
Background
Although autologous fat grafting has revolutionized the field of soft tissue reconstruction and augmentation, long-term maintenance of fat grafts is unpredictable. Recent studies have reported survival rates of fat grafts to vary anywhere between 10% and 80% over time. The present study evaluated the long-term viability of human fat grafts in a murine model using a novel imaging technique allowing for in vivo volumetric analysis.
Methods
Human fat grafts were prepared from lipoaspirate samples using the Coleman technique. Fat was injected subcutaneously into the scalp of 10 adult Crl:NU-Foxn1nu CD-1 male mice. Micro-computed tomography (CT) was performed immediately following injection and then weekly thereafter. Fat volume was rendered by reconstructing a three-dimensional (3D) surface through cubic-spline interpolation. Specimens were also harvested at various time points and sections were prepared and stained with hematoxylin and eosin (H&E), for macrophages using CD68 and for the cannabinoid receptor 1 (CB1). Finally, samples were explanted at 8- and 12-week time points to validate calculated micro-CT volumes.
Results
Weekly CT scanning demonstrated progressive volume loss over the time course. However, volumetric analysis at the 8- and 12-week time points stabilized, showing an average of 62.2% and 60.9% survival, respectively. Gross analysis showed the fat graft to be healthy and vascularized. H&E analysis and staining for CD68 showed minimal inflammatory reaction with viable adipocytes. Immunohistochemical staining with anti-human CB1 antibodies confirmed human origin of the adipocytes.
Conclusions
Studies assessing the fate of autologous fat grafts in animals have focused on nonimaging modalities, including histological and biochemical analyses, which require euthanasia of the animals. In this study, we have demonstrated the ability to employ micro-CT for 3D reconstruction and volumetric analysis of human fat grafts in a mouse model. Importantly, this model provides a platform for subsequent study of fat manipulation and soft tissue engineering.
Introduction
The history of modern soft tissue augmentation dates to 1893 when Neuber performed the first fat transplantation to fill depressed facial scars.1 Since then, autologous fat grafting has been used extensively for the augmentation of a wide range of facial soft tissue defects, including those from congenital or acquired anomalies, such as Romberg's disease, depressed scars, wrinkles, senile atrophy, and pitting acne.2–4 And while safety and efficacy remain to be fully determined, recent studies have also evaluated the utility of fat transfer for breast augmentation.5–8
Although fat grafting has revolutionized the field of soft tissue reconstruction, long-term maintenance of transplanted fat remains unpredictable. In 1910, Lexer described the technique of using single, large block grafts to treat a malar depression and a receding chin and reported excellent short- and long-term results. However, others have failed to duplicate his long-term results and, instead, have seen high resorption rates over time. In the late 1950s, Peer postulated that the ultimate degree of fat graft survival correlates with the number of viable adipocytes at the time of transplantation. The most widely accepted explanation for resorption has been based on this “cell survival theory.”9 Many surgeons have refined their techniques of harvesting, processing, and injection in search of improved fat transfer viability. Among several novel techniques suggested for achieving improved fat graft viability are the creation of subcutaneous tunnels in the recipient region, low-pressure harvesting, repeated washing, centrifugation, and the addition of growth factors (e.g., bFGF, IGF-1, VEGF, and PDGF) and pharmacologic agents, such as insulin, steroids, and vitamin E.10–12 In some respects, it seems that there has been little improvement since the 1950s, when Peer reported that 50% loss of volume could be expected in the transplanted fat.
In the 1990s, Sydney Coleman introduced a technique for fat grafting that minimized trauma to the adipocytes.13 Although Coleman has reported good long-term retention of fat grafts using his “structural fat grafting” technique, recent studies have reported survival rates that vary widely from 10% to 80% over time.10 This high level of variability reported for rates of fat graft survival may be a reflection of the lack of objective measurements for fat graft viability. Most of the published results on fat grafting in terms of survivability and long-term outcomes are based on subjective analysis of photographs or anecdotal reports. In humans, only a few objective studies have been attempted using magnetic resonance imaging, ultrasonography, computed tomography (CT), and three-dimensional (3D) imaging.14–16
Contrasting these clinical studies, the use of laboratory animal models has facilitated more rigorous quantitative and qualitative evaluations of fat graft survival and take. But, while there are numerous studies reporting on histologic measures of fat graft viability in animals, the full histological process is laborious and time consuming and not amenable to examining large tissue volumes. Further, it may require sacrifice of the animal so that tissues can be harvested for processing, embedding, sectioning, and staining with subsequent analytical evaluation. The aim of the present study was to thus evaluate fat graft survival in a well-established nude mouse animal model using an objective in vivo method of measurement without sacrifice of the animal. To our knowledge, such a prospective comparative quantification of fat graft viability in vivo with long-term follow-up using micro-CT has not been previously performed. It is therefore important to establish the validity of such a modality for in vivo volume analysis of fat survival.
Materials and Methods
Fat harvesting
Liposuction aspirates were obtained from two healthy female donors (age: 43 and 45 years) after acquiring informed consent from patients, in accordance with Stanford University Institutional Review Board guidelines. Fat was harvested using the Coleman technique. Briefly, the aspirated fat was processed under sterile conditions and centrifuged for 5 min at 500 g in an Eppendorf 5810 centrifuge (Eppendorf) to separate fat cells from fluid and cell debris.17–19 The fat was then immediately transferred to a Luer-lock syringe with an 18-gauge needle for injection.
Animal model
Ten adult, 60-day-old Crl:NU-Foxn1nu male mice (Charles River Laboratories International) were used for experiments in this study. The scalp of the mice was chosen as the recipient site for fat injection because of the absence of subcutaneous fat in this area, a fact that would facilitate delineation of injected fat. A subcutaneous tunnel was first created by passing the needle antegrade beneath the scalp and fat was then injected in retrograde fashion while pulling the needle out (Fig. 1).
FIG. 1.
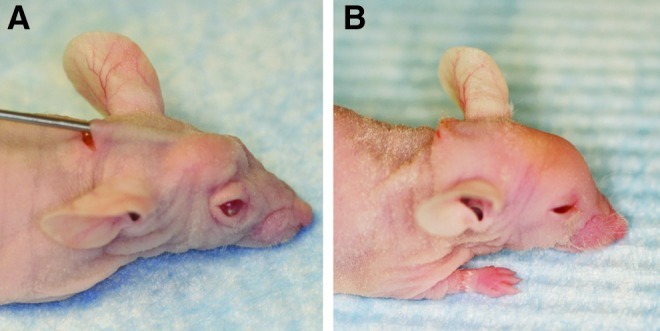
Photographs of mouse (A) during and (B) immediately after fat injection. Following brief centrifugation, fat was injected subcutaneously into the scalp of the nude mouse. Color images available online at www.liebertpub.com/tec
CT analysis
To determine accurate physical densities from the tissue linear attenuation values of the X-ray counts, micro-CT was calibrated during the first scan of each session using a phantom made of an epoxy-based resin that mimics hydroxyapatite and contains water and air inclusions. The phantom was placed in the field of view of the scanned specimen. Before volume reconstruction, the data were calibrated against the phantom using the reconstruction software. For micro-CT scans, the mice were anesthetized with isoflurane. Anesthesia was maintained by mask inhalation of isoflurane vaporized at concentrations of up to 3% in the induction phase and at 1.5% during acute imaging procedure. Imaging was performed with animals placed in the ventral position using a MicroCAT-II in vivo X-ray micro-CT scanner (Imtek, Inc.). The imaging protocol was 9 min long with real-time reconstruction, a voxel resolution of 80 μm with 719 views, and a relative mouse irradiation dose of 5 cGy (X-ray voltage: 80 kVp; anode current: 450 μA). Selection of the scan energy and voxel size was based on optimizing the requirements of scanning time and tissue detail, and to minimize exposure to radiation. Scout images were acquired to verify the correct positioning within the scanner. Mice were scanned at 3 days, and then 1, 2, 4, 6, and 8 weeks following injection. Four mice were also scanned at 12 weeks following injection. Data were reconstructed into 3D surfaces using the MicroCAT Reconstruction, Visualization, and Analysis Software (Imtek, Inc.). On CT scans, fat could be distinguished from skin and bone by its density or Hounsfield units. Ex vivo micro-CT imaging of fresh lipoaspirate specimens was used to determine the preliminary range of voxel values that define fat (−300 to +300 HU). The fresh lipoaspirate specimens only served to provide a broad estimate of the threshold. Subsequently, in the 2D coronal and sagittal slices, a region of interest was user-defined by setting an upper and a lower threshold value for pixel intensity such that all voxels within the threshold range would represent fat. To reduce potential bias, consistency in threshold definition was aided by each dataset being calibrated with a phantom. In addition, all analyses were performed by a single person (M.T.C.) to eliminate inter-user variability. Fat volume was rendered by reconstructing a 3D surface through cubic-spline interpolation (Fig. 2A).
FIG. 2.
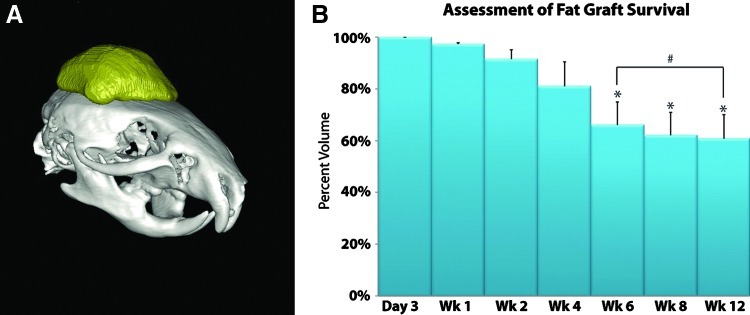
(A) Three-dimensional volume reconstruction of injected fat (in yellow). By setting a threshold for pixels such that all voxels within this range represent fat, the region of fat could be demarcated. Cubic-spline interpolation was then performed to render fat graft volume. (B) Micro-computed tomography (CT) volumetric analysis of the harvested fat demonstrated that there was a gradual reduction of fat graft volume, with 62.2% survival at 8 weeks and 60.9% at 12 weeks (*p<0.05; #p>0.05). Color images available online at www.liebertpub.com/tec
Histological analysis of adipose tissue
At 1-, 4-, 8-, and 12-week time points, one mouse was chosen to be euthanized for morphological and histological analysis of the lipoaspirate. The skull with adherent fat and overlying skin of the scalp was fixed immediately in 10% formalin for paraffin embedding. Ten-micron sections were cut and stained with hematoxylin and eosin (H&E) and the sections were analyzed.
For cannabinoid receptor 1 (CB1) staining, the same fat specimens harvested and sectioned above were deparaffinized and unmasked by the use of heat treatment in sodium citrate buffer (10 mM) at 95°C for 15 min. Sections were preincubated with serum-free protein block (Dako Corporation) and exposed to a 1:50 dilution of polyclonal anti-human CB1 antibody (Cayman Chemical Company). A no primary antibody control was also performed for comparison. Sections were then incubated with a 1:200 dilution of secondary antibody conjugated with DyLight 488 (Rockland Immunochemicals), and washed and mounted with Vectashield® mounting medium containing DAPI (Vector Laboratories). Bright-field and fluorescence images were obtained with a 40×objective at room temperature using a Zeiss Axioplan 2 immunofluorescence microscope (Carl Zeiss) equipped with a AxioCam HRc camera. The images were analyzed using Axiovision 4.2 software (Carl Zeiss).
At the 8- and 12-week time points (n=4 and n=2, respectively), fat was carefully dissected from the scalp and measured for weight and volume. The volume of each fat graft was calculated from the weight (in grams) and density of human fat (0.9 g/mL). The calculated volume of each excised fat graft was then compared with volumes obtained from the 3D surface reconstruction images. Finally, following volume assessment, one sample from the 8- and 12-week time points was fixed overnight in 4% paraformaldehyde, washed with phosphate buffered saline, and embedded in Optimal Cutting Temperature for cryosectioning. Sections were cut at 8 μm thickness and staining was performed with anti-CD68 antibody (AbD Serotec; 1:200 dilution) or IgG control. Counterstaining of nuclei was performed with a Hoechst stain (Invitrogen) and images were obtained by confocal microscopy. Sections of mouse spleen were similarly evaluated for macrophages as a positive control.
Statistical analysis
Data are presented as means±standard deviations. A one-way analysis of variance was used to look for significant differences in percentage of fat graft volume retention over time. Since the data were not normally distributed, we used a nonparametric Wilcoxon rank sum test to compare the actual fat graft volume with the radiographic volume obtained from 3D surface reconstruction of the fat graft. A value of *p<0.05 was considered significant. A Pearson correlation analysis was also performed in MATLAB (Mathworks) to compare initial baseline volumes with those at 8 and 12 weeks.
Results
Gross analysis
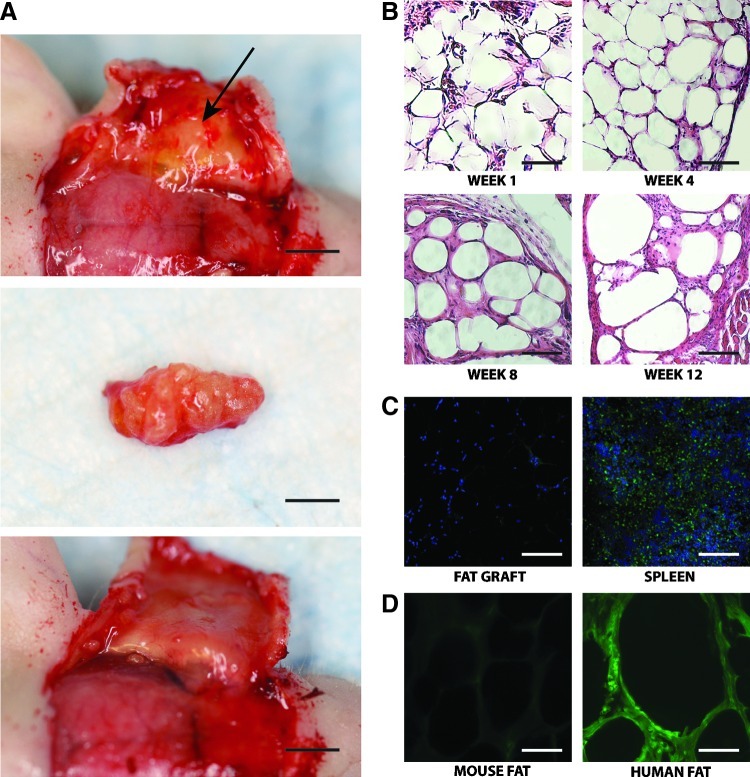
All 10 animals tolerated the procedure without complication or mortality and were healthy at the time of harvest. With a maximal total dose of 30 cGy delivered to any one animal, no signs of radiation damage to the scalp were observed, including redness, dryness, desquamation, or hair loss. Fat grafts were dissected out from the scalp in one piece (Fig. 3A). Successful take of the fat graft was grossly observed at the 8- and 12-week time points, as demonstrated by a well-circumscribed subcutaneous lump on the scalp. Visual analysis of the injected lipoaspirate demonstrated that the fat appeared viable and vascularized. No liponecrotic pseudocysts were observed on explantation.
FIG. 3.
(A) Photograph of fat explantation. Top photograph shows injected fat on undersurface of reflected skin (arrow) (scale bar represents 3.5 mm), middle photograph shows explanted fat (scale bar represents 2.0 mm), and bottom photograph shows undersurface of skin with fat removed (scale bar represents 3.5 mm). (B) Analysis of the grafted fat at 1-, 4-, 8-, and 12-week time points by hematoxylin and eosin staining. Large numbers of mature adipocytes with fibrous septa and minimal inflammatory reaction was observed (scale bars represent 50 μm). (C) Staining for macrophage marker CD68 in grafted fat (left) and control spleen (right). Counterstaining was performed with Hoechst (scale bars represent 100 μm). (D) Immunofluorescence staining at 12 weeks with anti-cannabinoid receptor 1 (CB1) confirmed human origin of the adipocytes (scale bars represent 30 μm). Color images available online at www.liebertpub.com/tec
Volume measurements
Many previous studies that have examined fat graft viability have demonstrated wide variability among volumes maintained over time. In this study, baseline volumes obtained 3 days following initial injection ranged from 28.0 μL to 216.9 μL, and were determined using micro-CT scans. Micro-CT volumetric analysis of the injected fat at 8- and 12-week time points showed an average of 62.2% and 60.9% survival, respectively (Fig. 2B). There was no significant difference in the amount of fat resorption or percentage of volume retention between these time points (p-value=0.946). Differences between the actual measured fat graft volume and the calculated radiographic volume obtained from 3D surface reconstruction of the fat graft were evaluated using the Wilcoxon rank sum test (Table 1). There was no significant difference between the actual volume and the radiographic volume (two-sided p-value=0.9362). Finally, to determine whether initial fat injection volumes correlated with measured retention rates at 8 or 12 weeks, a Pearson correlation analysis was performed. A correlation coefficient of 0.405 was noted between initial volumes and at 8 weeks with a p-value of 0.595. Comparing initial volumes and those measured at 12 weeks, the correlation coefficient was 0.441 with a p-value of 0.559.
Table 1.
Calculated Micro-CT Volume Versus Actual Measured Fat Volume
| Mouse | Micro-CT volume (μL) | Actual measured volume (μL) |
|---|---|---|
| 1 | 160.53 | 158.89 |
| 2 | 56.43 | 58.89 |
| 3 | 81.35 | 82.22 |
| 4 | 71.78 | 72.22 |
| 5 | 133.98 | 125.56 |
| 6 | 101.13 | 96.67 |
CT, computed tomography.
Histologic analysis
Analysis of the explanted grafted fat at 1-, 4-, 8-, and 12-week time points by H&E staining showed large numbers of mature adipocytes with fibrous septa and minimal inflammatory reaction (Fig. 3B). There was no substantial capsular reaction and minimal infiltration of macrophages, as shown by staining for the macrophage marker CD68 (Fig. 3C). Other markers of inflammatory response, such as presence of cysts, were also not noted. The adipocytes appeared healthy and viable. To confirm human origin of adipocytes, we performed immunofluorescence staining with anti-human anti-CB1 antibody (Fig. 3D). As expected, the region of injected fat stained heavily for anti-CB1 whereas mouse tissue did not. This same antibody has been used in other studies to identify human fat.20,21 These findings demonstrate that the injected fat engrafts remain viable in our mouse model.
Discussion
Autologous fat obtained by liposuction has many of the ideal properties desirable for repair of soft tissue defects: it is widely available, easily obtained, inexpensive, biocompatible, and can be harvested repeatedly. Nevertheless, the survival of autologous fat grafts is still a controversial subject. Autologous fat grafts often lose volume over time due to tissue resorption, most likely due to the lack of a well-vascularized nutrient bed into which they can engraft. Long-term survival rates of fat grafts have been reported to vary anywhere between 10% and 80% of the original volume. The lack of an objective measure in the literature regarding longevity, predictability, and survivability of autologous fat grafts may be the reason for this inconsistency.
To date, few studies have quantified the viability of fat grafts in vivo by imaging techniques. Horl et al. used magnetic resonance imaging (MRI) to quantitate the loss of volume in human fat grafts over time.15 Har-Shai et al. suggested the use of CT to quantify the volume of fat and employed this evaluation method in one of four cases in their study.22 Following the suggestion of Har-Shai et al., Fontdevila used CT scans to volumetrically measure fat graft viability in patients with HIV-associated facial lipodystrophy syndrome.14 Because computerized analysis of CT images is a standardized evaluation technique, it can provide an objective method for comparing the efficacy of various procedures used in autologous fat grafts. In light of this, Meier et al. attempted to quantitate the amount of autologous fat graft survival in the midface by using 3D imaging software that compared the volume difference between preoperative and postoperative images.16
Until now, most studies assessing the long-term viability of fat grafts in animals have focused on nonimaging modalities, including histological and biochemical analyses, which require euthanasia of the animals.23 Recently, Thanik et al. described injection of fat into the dorsum of immunocompromised mice and by serially removing specimens at 2, 4, 6, and 8 weeks; they noted 82% volume retention at their last time point.24 Using similar time points and similar sample sizes, in our present study we observed fat graft retention to be around 62% at 8 weeks. Some of this discrepancy may be attributed to donor-specific variability and slight differences in processing technique. The local environment of the injection site has also been shown to dramatically alter fat graft survival.25–28 And similar to Thanik et al. who reported stable survival at 8 weeks, we noted a nonsignificant change in fat volume between weeks 6 and 8. An additional measurement was made at 12 weeks (representing over 10% of the animal's typical life expectancy) to further confirm stability of our findings.18
Aside from the actual specific amount of fat graft survival observed, more importantly we demonstrated the validity of in vivo volume measurements by micro-CT in our mouse model. Karacaoglu et al. previously described the use of MRI to evaluate fat volume in animals; however, their studies were performed in larger New Zealand white rabbits.29 In addition, larger, unprocessed inguinal fat pads were implanted and no direct validation of measured volumes was provided in their report. In our present study, we compared the ability for micro-CT scanning with 3D reconstruction to accurately measure in vivo fat relative to more standard techniques involving explantation of samples. The volume of explanted fat was calculated from the commonly used density factor of 0.92 g/cm3 rather than the water displacement method.30–33 This is based on the finding by Fidanza et al. that the differences in fat density between individuals and between locations are trivial.30 Importantly, this differs from the water displacement method for measuring fat volume. Using a fat density approach, we observed no significant difference between the calculated radiographic volume and the actual measured volume of fat. The use of micro-CT for such an analysis may be limited; however, in that early fat necrosis cannot be easily distinguished from viable fat. Histological staining or MRI would provide a more definitive presentation of this. Nonetheless, at our late time points of 8 and 12 weeks, we observed healthy human adipocytes populating the fat graft with minimal inflammatory reaction and normal-appearing surrounding tissue.
In our study, we have thus demonstrated the ability to objectively measure in vivo fat graft volume retention in a murine model. Interestingly, analysis of explanted fat and histological studies confirmed that what we have measured by micro-CT correlated with actual injected human fat. To our knowledge, this is the first study to employ the application of micro-CT for 3D reconstruction and volumetric analysis of autologous fat grafts in an animal model. We believe that this modality will serve as a useful platform with which to study many of the persistent questions in autologous fat grafting. It is our hope that this approach may provide a sound base to subsequently study different perturbations of adipocytes (e.g., cytokines, growth factors, adipose-derived stromal cells, etc.) in an effort to maximize volume retention and allow for more predictable results following fat grafting for soft tissue defects.
Acknowledgments
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grants 1 R21 DE019274-01 and RC2 DE020771-01, the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine to M.T.L.
Authors' Contributions
M.T.C. and D.C.W. contributed to project design, data acquisition, drafted and revised the article, and approved the final version. M.T.C., J.S.H, D.D.L., D.T.M., and M.H. contributed to acquisition of data, revision of the article, and final approval. M.T.C. and B.L. contributed to analysis of data, revision of the article, and final approval. M.J. contributed to analysis of data. D.C.W. and M.T.L. contributed to project design and conception, interpretation of data, revision of the article, and approval of the final version.
Disclosure Statement
None of the authors have any competing financial interest to report.
References
- 1.Klein A.W. Elson M.L. The history of substances for soft tissue augmentation. Dermatol Surg. 2000;26:1096. [PubMed] [Google Scholar]
- 2.Atik B. Ozturk G. Erdogan E. Tan O. Comparison of techniques for long-term storage of fat grafts: an experimental study. Plast Reconstr Surg. 2006;118:1533. doi: 10.1097/01.prs.0000240806.19404.a8. [DOI] [PubMed] [Google Scholar]
- 3.Coleman S.R. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 4.Pereira L.H. Sterodimas A. Long-term fate of transplanted autologous fat in the face. J Plast Reconstr Aesthet Surg. 2010;63:e68. doi: 10.1016/j.bjps.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Khouri R.K. Eisenmann-Klein M. Cardoso E. Cooley B.C. Kacher D. Gombos E., et al. Brava(R) and autologous fat transfer is a safe and effective breast augmentation alternative: results of a six-year, eighty-one patients prospective multicenter study. Plast Reconstr Surg. 2012;129:1173. doi: 10.1097/PRS.0b013e31824a2db6. [DOI] [PubMed] [Google Scholar]
- 6.Kim H. Yang E.J. Bang S.I. Bilateral liponecrotic pseudocysts after breast augmentation by fat injection: a case report. Aesthetic Plast Surg. 2011;36:359. doi: 10.1007/s00266-011-9790-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang C.F. Zhou Z. Yan Y.J. Zhao D.M. Chen F. Qiao Q. Clinical analyses of clustered microcalcifications after autologous fat injection for breast augmentation. Plast Reconstr Surg. 2011;127:1669. doi: 10.1097/PRS.0b013e318208d1e4. [DOI] [PubMed] [Google Scholar]
- 8.Rubin J.P. Coon D. Zuley M. Toy J. Asano Y. Kurita M., et al. Mammographic changes after fat transfer to the breast compared with changes after breast reduction: a blinded study. Plast Reconstr Surg. 2012;129:1029. doi: 10.1097/PRS.0b013e31824a2a8e. [DOI] [PubMed] [Google Scholar]
- 9.Rieck B. Schlaak S. Measurement in vivo of the survival rate in autologous adipocyte transplantation. Plast Reconstr Surg. 2003;111:2315. doi: 10.1097/01.PRS.0000060797.59958.55. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez A.M. Lobocki C. Kelly C.P. Jackson I.T. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg. 2007;120:285. doi: 10.1097/01.prs.0000264401.19469.ad. [DOI] [PubMed] [Google Scholar]
- 11.Shoshani O. Shupak A. Ullmann Y. Ramon Y. Gilhar A. Kehat I., et al. The effect of hyperbaric oxygenation on the viability of human fat injected into nude mice. Plast Reconstr Surg. 2000;106:1390. doi: 10.1097/00006534-200011000-00028. discussion 7. [DOI] [PubMed] [Google Scholar]
- 12.Yuksel E. Weinfeld A.B. Cleek R. Wamsley S. Jensen J. Boutros S., et al. Increased free fat-graft survival with the long-term, local delivery of insulin, insulin-like growth factor-I, and basic fibroblast growth factor by PLGA/PEG microspheres. Plast Reconstr Surg. 2000;105:1712. doi: 10.1097/00006534-200004050-00017. [DOI] [PubMed] [Google Scholar]
- 13.Coleman S.R. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24:347. [PubMed] [Google Scholar]
- 14.Fontdevila J. Serra-Renom J.M. Raigosa M. Berenguer J. Guisantes E. Prades E., et al. Assessing the long-term viability of facial fat grafts: an objective measure using computed tomography. Aesthet Surg J. 2008;28:380. doi: 10.1016/j.asj.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Horl H.W. Feller A.M. Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg. 1991;26:248. doi: 10.1097/00000637-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Meier J.D. Glasgold R.A. Glasgold M.J. Autologous fat grafting: long-term evidence of its efficacy in midfacial rejuvenation. Arch Facial Plast Surg. 2009;11:24. doi: 10.1001/archfacial.2008.518. [DOI] [PubMed] [Google Scholar]
- 17.Rohrich R.J. Sorokin E.S. Brown S.A. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 2004;113:391. doi: 10.1097/01.PRS.0000097293.56504.00. discussion 6. [DOI] [PubMed] [Google Scholar]
- 18.Smith P. Adams W.P., Jr. Lipschitz A.H. Chau B. Sorokin E. Rohrich R.J., et al. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg. 2006;117:1836. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro G.A. De Francesco F. Tirino V. Cataldo C. Rossano F. Nicoletti G., et al. Effects of a new centrifugation method on adipose cell viability for autologous fat grafting. Aesthetic Plast Surg. 2011;35:341. doi: 10.1007/s00266-010-9613-8. [DOI] [PubMed] [Google Scholar]
- 20.Roche R. Hoareau L. Bes-Houtmann S. Gonthier M.P. Laborde C. Baron J.F., et al. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol. 2006;126:177. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- 21.Bennetzen M.F. Nielsen T.S. Paulsen S.K. Bendix J. Fisker S. Jessen N., et al. Reduced cannabinoid receptor 1 protein in subcutaneous adipose tissue of obese. Eur J Clin Invest. 2010;40:121. doi: 10.1111/j.1365-2362.2009.02231.x. [DOI] [PubMed] [Google Scholar]
- 22.Har-Shai Y. Lindenbaum E.S. Gamliel-Lazarovich A. Beach D. Hirshowitz B. An integrated approach for increasing the survival of autologous fat grafts in the treatment of contour defects. Plast Reconstr Surg. 1999;104:945. doi: 10.1097/00006534-199909040-00008. [DOI] [PubMed] [Google Scholar]
- 23.Brucker M. Sati S. Spangenberger A. Weinzweig J. Long-term fate of transplanted autologous fat in a novel rabbit facial model. Plast Reconstr Surg. 2008;122:749. doi: 10.1097/PRS.0b013e3181815a41. [DOI] [PubMed] [Google Scholar]
- 24.Thanik V.D. Chang C.C. Lerman O.Z. Allen R.J., Jr. Nguyen P.D. Saadeh P.B., et al. A murine model for studying diffusely injected human fat. Plast Reconstr Surg. 2009;124:74. doi: 10.1097/PRS.0b013e3181a80509. [DOI] [PubMed] [Google Scholar]
- 25.Guerrerosantos J. Gonzalez-Mendoza A. Masmela Y. Gonzalez M.A. Deos M. Diaz P. Long-term survival of free fat grafts in muscle: an experimental study in rats. Aesthetic Plast Surg. 1996;20:403. doi: 10.1007/BF02390315. [DOI] [PubMed] [Google Scholar]
- 26.Butterwick K.J. Enhancement of the results of neck liposuction with the FAMI technique. J Drugs Dermatol. 2003;2:487. [PubMed] [Google Scholar]
- 27.Eremia S. Newman N. Long-term follow-up after autologous fat grafting: analysis of results from 116 patients followed at least 12 months after receiving the last of a minimum of two treatments. Dermatol Surg. 2000;26:1150. [PubMed] [Google Scholar]
- 28.Fulton J.E., Jr. Silverton K. Resurfacing the acne-scarred face. Dermatol Surg. 1999;25:353. doi: 10.1046/j.1524-4725.1999.07229.x. [DOI] [PubMed] [Google Scholar]
- 29.Karacaoglu E. Kizilkaya E. Cermik H. Zienowicz R. The role of recipient sites in fat-graft survival: experimental study. Ann Plast Surg. 2005;55:63. doi: 10.1097/01.sap.0000168246.75891.62. discussion 8. [DOI] [PubMed] [Google Scholar]
- 30.Fidanza F. Keys A. Anderson J.T. Density of body fat in man and other mammals. J Appl Physiol. 1953;6:252. doi: 10.1152/jappl.1953.6.4.252. [DOI] [PubMed] [Google Scholar]
- 31.Hill A.M. LaForgia J. Coates A.M. Buckley J.D. Howe P.R. Estimating abdominal adipose tissue with DXA and anthropometry. Obesity (Silver Spring) 2007;15:504. doi: 10.1038/oby.2007.629. [DOI] [PubMed] [Google Scholar]
- 32.Hillebrand J.J. Langhans W. Geary N. Validation of computed tomographic estimates of intra-abdominal and subcutaneous adipose tissue in rats and mice. Obesity (Silver Spring) 2010;18:848. doi: 10.1038/oby.2009.341. [DOI] [PubMed] [Google Scholar]
- 33.Watts G.F. Chan D.C. Barrett P.H. Adipose tissue compartments and the kinetics of very-low-density lipoprotein apolipoprotein B-100 in non-obese men. Metabolism. 2002;51:1206. doi: 10.1053/meta.2002.34718. [DOI] [PubMed] [Google Scholar]