Summary
Purpose
S-trans,trans-Farnesylthiosalicylic Acid (FTS, salirasib) inhibits Ras-dependent cell growth by dislodging all isoforms of Ras, including mutant Ras, from the plasma membrane. This study evaluated the activity, safety, and toxicity of salirasib in preclinical models and patients with metastatic pancreatic adenocarcinoma (PDA).
Patients and methods
In the preclinical study, salirasib was tested, alone and in combination with gemcitabine, in patient derived xenografts (PDX) of PDA. In the clinical study, treatment-naïve patients with advanced, metastatic PDA were treated with a standard dose schedule of gemcitabine and salirasib 200–800 mg orally (PO) twice daily (bid) for 21 days every 28 days. Tissue from preclinical models and patients’ biopsies were collected pre-treatment and on Cycle (C) 1, Day (D) 9 to characterize the effect of gemcitabine and salirasib on activated Ras protein levels. Plasma samples for pharmacokinetics were collected for salirasib administered alone and in combination.
Results
Salirasib inhibited the growth of 2/14 PDX models of PDA and modulated Ras signaling in these tumors. Nineteen patients were enrolled. No DLTs occurred. Common adverse events included hematologic and gastrointestinal toxicities and fatigue. The median overall survival was 6.2 months and the 1 year survival 37 %. In 2 patients in whom paired tissue biopsies were available, Ras and KRas protein levels were decreased on C1D9. Salirasib exposure was not altered by gemcitabine and did not correlate with PD outcomes.
Conclusion
The combination of gemcitabine and salirasib appears well-tolerated, with no alteration of salirasib exposure, and exerted clinical and PD activity in PDA.
Keywords: Salirasib, Gemcitabine, Pancreatic cancer, Phase I, RAS
Introduction
Chemotherapy treatment options for patients with metastatic pancreatic cancer (PDA) remain limited [1–4].
Ras mutations are seen in approximately one-third of human cancers, with the highest incidence of mutations seen in PDA [5]. Thus, the RAS signaling pathway stands as a strategic target for this disease [6]. Previous attempts to directly block RAS activity by inhibition of farnesyltransferase have been ineffective, as multiple escape pathways exist that allow for alternative prenylation of Ras protein [7, 8]. S-trans,trans-farnesylthiosalicylic acid (FTS, salirasib) inhibits Ras-dependent cell growth by dislodging all of the isoforms of RAS from the plasma membrane [9]. The in vitro activity of salirasib has been demonstrated in pancreatic cell lines and xenograft models [9, 10]. In the Panc-1 cell line, salirasib decreased the amount of RAS in a dose-dependent manner, with a maximum decrease in Ras of approximately 50 % seen at concentrations of 25 to 50 µM. Furthermore, in mouse xenograft models, salirasib inhibited Panc-1 tumor growth and was shown to be synergistic with gemcitabine, both inhibiting tumor growth and prolonging survival [9–11]. Salirasib was tested in a phase I study in patients with solid tumors twice daily for 21 days every 4 weeks. Doses were escalated from 100 to 200, 400, 600, and 800 mg. Dose-limiting toxicity was not reached, but all three patients treated with 800 mg experienced Grade 1–2 diarrhea, preventing further dose escalation. The recommended dose for phase II studies was 600 mg bid [12].
To further investigate the role of salirasib in PDA, we conducted this preclinical and clinical study. The objectives of the preclinical study were to determine the activity and signaling effects of salirasib alone and combined with gemcitabine in a set of patient derived xenografts (PDX) from the PancXenoBank collection [13]. The clinical study aimed to a) determine the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of salirasib in combination with gemcitabine in patients with advanced PDA; b) characterize the safety profile of the combination; c) explore the pharmacokinetic (PK) behavior and pharmacodynamic (PD) effects of the agents’ effects; and d) seek preliminary evidence of antitumor effects.
Patients and methods
Preclinical studies
In vivo tumor therapy studies
A set of 14 PDX from the PancXenoBank collection at Johns Hopkins were used for these studies. Mice (6-week-old male athymic nude mice, Harlan) were housed and maintained in accordance with the Institutional Animal Care and Use Committee and guidelines of the American Association of Laboratory Animal Care. Fresh pancreatic tumor specimens were implanted subcutaneously into the flanks of mice as reported [14]. Tumors were allowed to grow on both flanks of mouse from the same patient xenografts until the tumor reached a volume of ~200 mm3. Mice were randomized (5 mice with bilateral flank tumors; 10 evaluable tumors/group) and treated with vehicle (control) or salirasib 100 mg/Kg p.o., once daily for 4 weeks. In order to investigate whether salirasib could potentiate gemcitabine sensitivity, we treated A6L and Panc265 xenografts with gemcitabine 100 mg/Kg i.p., twice weekly for 4 weeks or gemcitabine in combination with salirasib. Tumor size was evaluated twice per week by caliper measurements and tumor volume was calculated using the following formula: tumor volume=[length × width2]/2. Tumor growth index (TGI) was calculated using the formula: (mean tumor volume of drug-treated group/mean tumor volume of control group) × 100. Xenografts with a TGI <50 % were considered sensitive, TGI >50 % were considered resistant to Salirasib. 50 % cut-off value was used as a criterion for sensitivity in prior published studies using PDA xenografts [15].
Protein extraction and western blotting
In order to investigate the PD effects of salirasib, we conducted immunoblotting using post treatment tumor samples. Protein extracts were prepared from tumors according to previously published method [16]. Primary antibodies for K-RAS (Proteintech Group Inc, 1:1500 dilution), total RAS, Akt, p-Akt Ser473, MAPK, p-MAPK Thr202/Tyr204, MEK, p-MEK Ser221, NF-κB, XIAP and c-PARP, (Cell Signaling Technology) were diluted at 1:1,000 in TBS containing 5 % protease-free bovine serum albumin (Sigma-Aldrich) and the membranes were incubated with primary antibodies overnight at 4°C with rocking. After washing three times with TBS, the membranes were incubated for 2 h at room temperature with ECL anti-rabbit IgG horseradish peroxidase-conjugated antibody (GE Healthcare, UK) at a final dilution of 1:2,000 in TBS containing 0.01 % Tween 20 and 5 % non fat dry milk. After washing three times with TBS, bound antibodies were detected by enhanced chemiluminescence (GE Healthcare, UK). β actin was used as a loading control.
Clinical study
Study design
This was a single-institution phase I study in patients with metastatic PDA conducted at the Johns Hopkins Hospital (Baltimore, Maryland). Eligible patients were treated with gemcitabine 1,000 mg/m2 administered intravenously (IV) over 30 min on days 1, 8 and 15 plus salirasib administered orally (PO) at doses ranging from 200 to 800 mg bid for 21 days of a 28-day cycle. The dose of salirasib was escalated using the continuous reassessment method (CRM) [17]. Patients were treated until disease progression (PD) or intolerable toxicity. A cohort of six patients was treated at the MTD to asses PD effects in tumor biopsies. The MTD of salirasib in combination with gemcitabine was defined as the highest dose level evaluated that achieved a DLT rate <33 %. DLT was defined as: 1) ANC<500/mm3 for >5 days or associated with fever (temperature, ≥38°C) or with infection; 2) platelet count <50,000/mm3 accompanied by clinically significant bleeding or any platelet count <25,000/mm3; 3) any drug-related grade 3 or grade 4 non-hematological toxicity, except diarrhea or nausea and vomiting in the absence of optimal medical management; and 4) treatment delay for >14 days due to study drug-related toxicities. Grade 4 vomiting or diarrhea that persisted despite maximal prophylaxis and treatment with anti-emetic or anti-diarrheal therapy, respectively, was considered dose-limiting. Intra-patient dose escalation was permitted.
Eligibility
Treatment-naïve patients with histologic confirmed advanced PDA; an Eastern Cooperative Oncology Group (ECOG) score of 0–2; liver function tests (LFTs)≤3×the upper limit of normal (ULN) or, if liver involvement, ≤5×ULN; absolute neutrophil count (ANC)≥1,500/mm3; platelet count ≥100,000/mm3; and hemoglobin ≥10 g/dL were eligible. Measureable disease was not required. Patients with central nervous system (CNS) metastases or a baseline QT/QTc interval ≥470 msec were excluded. Prior adjuvant therapy was allowed if >3 weeks since the last treatment. No prior treatment with gemcitabine was allowed.
Procedures and assessment
After obtaining informed consent, baseline studies were performed for tumor assessment (computed tomography [CT] scan and serum CA 19–9 levels), organ function (complete chemistry profile and complete blood counts [CBC] with differential and platelet count) and toxicity. Patients were monitored weekly during study participation including clinical and toxicity assessment, complete chemistry profile and CBC with differential. Safety was assessed by the incidence of treatment-related adverse events (AEs), as reported per the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, and incidence of dose modifications, dose interruptions, and/or premature discontinuation of study drug. Response, in patients with measureable disease, was assessed by CT scans at baseline and pretreatment every other cycle using the Response Evaluation Criteria in Solid Tumors (RECIST) at baseline and at 4 and 8 weeks based using the European Organization for Research and Treatment of Cancer (EORTC) criteria. Changes in CA 19–9 levels were also examined.
Pharmacokinetic and pharmacodynamic assessments
Salirasib pharmacokinetics were assessed at steady-state when administered alone (C1D7 or C1D22) and in combination (C1D8 or C1D15) with gemcitabine. Salirasib concentrations were determined in plasma samples by validated high pressure liquid chromatography with mass spectrometry detection (LC/MS/MS), with a lower limit of quantitation of 1 ng/ml [18]. Individual salirasib plasma concentration–time data were analyzed by noncompartmental methods using WinNonlin version 5.3 (Pharsight, Inc.) [19]. Tissue biopsies were collected pretreatment and on C1D9 to characterize the effect of treatment on RAS levels using a western blot assay as described above.
Statistical considerations
Toxicity and response, as determined using RECIST, was to be summarized using descriptive statistics. Overall survival was defined as the time from first study drug dose to patient death (any cause). Progression free survival (PFS) was defined as the time from first study drug dose to the start of disease progression or patient death (any cause), whichever occurred first. If the patient did not have PD or was still alive, then PFS was to be censored at the last date of contact with the patient. Time to progression (TTP) was defined as the time from first study drug dose to the start of PD or patient death due to PD. If the reason for a patient’s death was other than PD, then the TTP was to be censored at the date of death. PFS, OS, and TTP were analyzed using Kaplan-Meier analysis methods.
Pharmacokinetic parameters were summarized by descriptive statistics. Differences between pharmacokinetic parameters during sampling periods were compared by a Wilcoxon matched-pairs signed-rank test. Pearson’s correlation coefficient or Mann–Whitney U-tests were used to assess correlations between exposure (Cmax or AUC) and exploratory PD end points. These tests were performed using JMP Statistical Discovery software (version 7.0.1; SAS Institute, Cary, NC, USA). The a priori level of significance was p>0.05.
Results
Preclinical studies
Tumor growth inhibition in PDX models
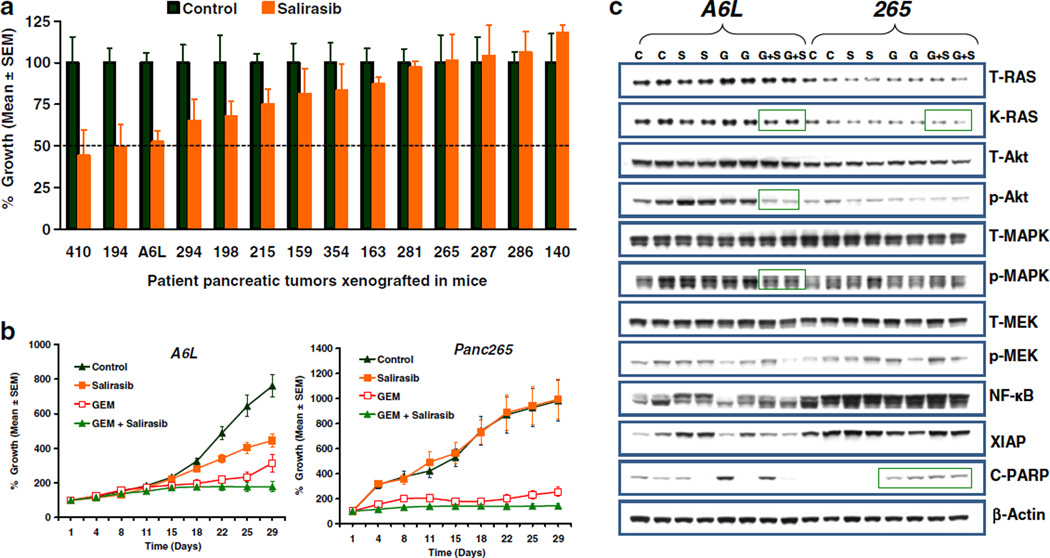
To investigate whether salirasib monotherapy has preclinical clinical activity in PDA, we treated 14 individual patient-derived PDA. As shown in Fig. 1a, salirasib monotherapy produce a wide range of tumor growth inhibition among xenografts with >50 % inhibition in tumor growth of 2/14 xenografts (410 and 194). We next determined if the combination of salirasib and gemcitabine resulted in heightened activity. As shown in Fig. 1b, the combined treatment improved the activity of gemcitabine supporting the further clinical development of the agent in combination.
Fig. 1.
Efficacy and pharmacodynamic effect of salirasib on PDA PDX. PDX from PDA patients were implanted in athymic mice. Animals with established tumors were treated with the agents as mentioned in the materials and method section. a Anti-tumor effect of salirasib on the tumor growth of fourteen xenografts. Error bars represent standard error of mean (SEM); N=10 tumors per group (5 mice with bilateral flank tumors). Dotted line represents 50 % TGI. Salirasib treatment could reduce tumor volume by 50 % in 410 and 194 xenograft compared to vehicle treated animals. b Growth curves of A6L and 265 xenografts treated with vehicle, salirasib, GEM or combination of GEM and Salirasib. c Immunoblots showing that a combination of GEM and Salirasib treatment inhibits the expression of K-Ras, p-Akt, p-MEK (blots in side the green rectangle). C-PARP expression was up-regulated in the tumors of GEM or combination of GEM and salirasib treatment as compared to vehicle or salirasib treated tumors. Two separate tumors from the vehicle, salirasib, GEM and GEM plus salirasib treatment were homogenized. Lyates were resolved in SDS-PAGE and probed with specific antibodies against indicated proteins. β-actin was used as a loading control
Pharmacodynamic effects of treatment
To investigate the effect of salirasib on KRAS pathway proteins, we conducted western blot analysis on whole tumor homogenates. Results obtained in the western blot analysis indicate that the combination of salirasib and gemcitabine decreased the levels of KRAS, p-Akt and p-MAPK compared to control and either single agents alone (Fig. 1c). Decreased levels of KRAS, p-Akt and p-MAPK in combination therapy treated tumors were obvious in A6L but not in 265 xenograft. In addition, the combination of gemcitabine and salirasib induced apoptosis as shown by elevated cleaved-PARP expression in 265 xenograft (Fig. 1c).
Clinical results
Patient characteristics
A total of 19 patients, whose pertinent clinical characteristics are listed in Table 1 were enrolled. The majority of the population was male (68 %) and the median age was 61 years (range 40 to 80 years). All 19 patients had stage 4 pancreatic cancer with metastases to the liver in 90 %, to the abdomen in 37 %, to the lung in 16 %, and to other sites in 16 %. No patient had received prior chemotherapy for advanced PDA.
Table 1.
Demographic and baseline disease characteristics
| Number of patients | 19 |
| Median age (range) | 64 (40–80) |
| Gender (M/F) | 13/6 |
| ECOG | |
| 0 | 15 |
| 1 | 3 |
| 2 | 1 |
| Metastases site | |
| Liver | 17 |
| Abdomen | 7 |
| Lung | 3 |
| Other | 3 |
| Prior treatment | |
| Surgery | 6 |
| Chemotherapy | 1 |
| Radiation therapy | 1 |
Dose and treatment administration
Three, 4, 3 and 9 patients received 200, 400, 600 and 800 mg or salirasib in combination with gemcitabine respectively. The mean number of cycles in which patients were treated was 4, with the majority (11 patients; 58 %) treated in at least 4 cycles. Two patients received >10 cycles; 1 of these 2 patients continued treatment for 19 cycles before study drug was discontinued due to pleural effusion and pulmonary embolus and the other continued treatment for 24 cycles without disease progression. Eight (42 %) patients received 1 or 2 cycles only. Ultimately, patients were discontinued from the study because of disease progression/worsening clinical status (58 %), unacceptable toxicity (26 %), or administrative reasons (patient or investigator decision, termination of the study by the sponsor, 16 %).
Dose-Limiting toxicity and safety assessments
No events meeting the DLT definition were observed; therefore, an MTD was not reached. Overall, as demonstrated in Table 2, the most common toxicities were hematologic, including anemia (68 %), neutropenia (58 %), leukopenia (53 %), and thrombocytopenia and lymphopenia (47 % each), as well as diarrhea (58 %) and abdominal pain and fatigue (42 % each). The most common grade 3 or 4 toxicity events were hematological and occurred in 47 % (neutropenia), and 11 % (thrombocytopenia) of patients respectively. Other grade 3 or 4 events reported in 2 or more patients included pulmonary embolism (16 %) and abdominal pain, diarrhea, fatigue, and increased aspartate transaminase (AST) and alkaline phosphatase (ALK) (11 % each). No clear dose or salirasib exposure (Cmax and AUC) relationship was apparent with regard to hematologic toxicity. However, diarrhea occurred at a higher incidence in the 2 highest salirasib dose cohorts (600 and 800 mg) compared to the lower dose cohorts (200 and 400 mg) but did not correlate to salirasib exposure. Nausea and vomiting (overall incidence 32 % and 26 %, respectively) also occurred at a higher incidence in the highest salirasib dose cohort (800 mg) compared to the lower dose cohorts (200, 400, or 600 mg) but did not correlate to salirasib exposure. There were no deaths or serious adverse events considered to be salirasib-related. No patterns of change in clinical laboratory tests or clinically meaningful changes in electrocardiogram findings or vital sign measurements were seen.
Table 2.
Summary of clinical adverse events
| Adverse event/ Intensity |
Cohort 1 200 mg bid N = 3 n (%) |
Cohort 2 400 mg bid N = 4 n (%) |
Cohort 3 600 mg bid N = 3 n (%) |
Cohort 4 800 mg bid N = 9 n (%) |
All N = 19 n (%) |
|---|---|---|---|---|---|
| Anemia | |||||
| All grades | 3 (100.0) | 2 (50.0) | 3 (100.0) | 5 (55.6) | 13 (68.4) |
| Grade 3–4 | 0 (0) | 0 (0) | 0 (0) | 3 (33.3) | 3 (15.8) |
| Neutropenia | |||||
| All grades | 3 (100.0) | 3 (75.0) | 2 (66.7) | 3 (33.3) | 11 (57.9) |
| Grade 3–4 | 3 (100.0) | 3 (75.0) | 2 (66.7) | 1 (11.1) | 9 (47.4) |
| Thrombocytopenia | |||||
| All grades | 2 (66.7) | 2 (50.0) | 2 (66.7) | 3 (33.0) | 9 (47.4) |
| Grade 3–4 | 0 (0) | 1 (25.0) | 0 (0) | 1 (11.1) | 2 (10.5) |
| Diarrhea | |||||
| All grades | 0 (0) | 2 (50.0) | 3 (100.0) | 6 (66.7) | 11 (57.9) |
| Grade 3–4 | 0 (0) | 0 (0) | 0 (0) | 2 (22.2) | 2 (10.5) |
| Abdominal pain | |||||
| All grades | 0 (0) | 2 (50.0) | 1 (33.3) | 5 (55.6) | 8 (42.1) |
| Grade 3–4 | 0 (0) | 1 (25.0) | 0 (0) | 1 (11.1) | 2 (10.5) |
| Nausea | |||||
| All grades | 0 (0) | 1 (25.0) | 0 (0) | 5 (55.6) | 6 (31.6) |
| Grade 3–4 | 0 (0) | 1 (25.0) | 0 (0) | 0 (0) | 1 (5.3) |
| Vomiting | |||||
| All grades | 0 (0) | 1 (25.0) | 0 (0) | 4 (44.4) | 5 (26.3) |
| Grade 3–4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | |||||
| All grades | 0 (0) | 2 (50.0) | 1 (33.3) | 5 (55.6) | 8 (42.1) |
Pharmacokinetic and pharmacodynamic endpoints
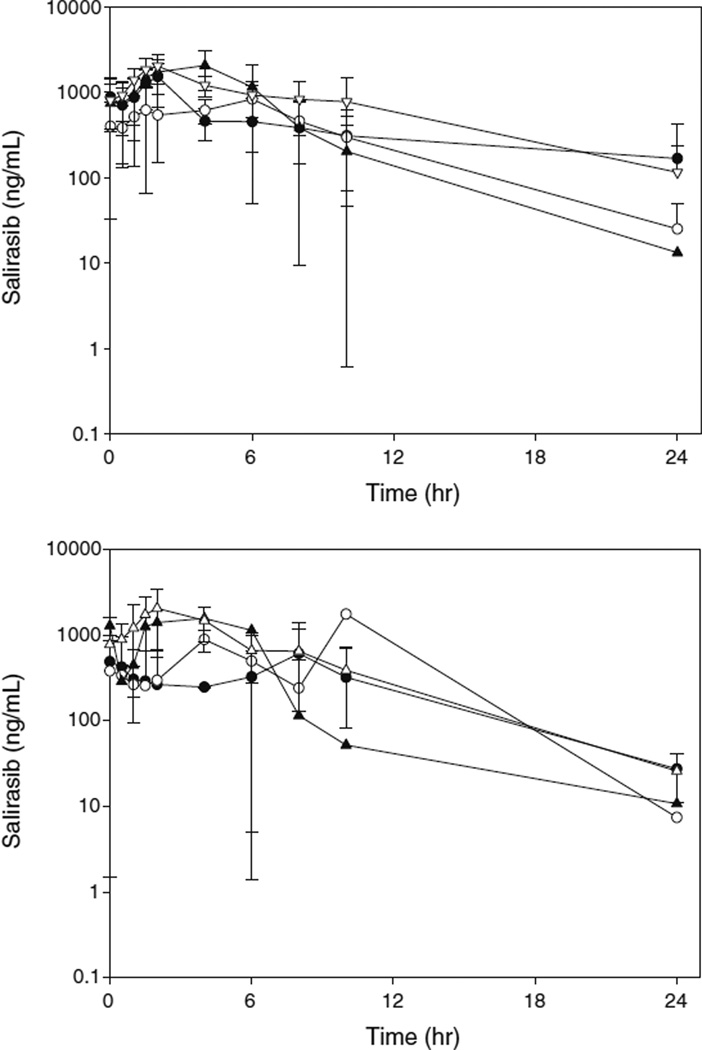
The PK profile of salirasib after oral administration was characterized by slow absorption and a rapid elimination phase (Fig. 2). Gemcitabine did not alter salirasib exposure (Cmax or AUCτ), which was similar when salirasib was administered alone (Day 7 or 22) or in combination with gemcitabine (Day 8 or 15) (p>0.05; matched pairs analysis). The T1/2 (mean±standard deviation [SD]) was 5.71±4.31 h when administered alone and 3.73±1.42 h when administered in combination with gemcitabine (p=0.44; matched pairs analysis). Thus, salirasib appears to be well tolerated, with no PK interaction.
Fig. 2.
Average salirasib concentration-time profile. Average plasma concentration-time for all patients after salirasib was administered alone (day 7 or 22; A) or in combination with gemcitabine (day 15 or 8; B). The solid circle (Black Circle), open circle (○), solid triangle (Black Down-Pointing Triangle), and open triangle (White Triangle) represent 200 mg, 400 mg, 600 mg, and 800 mg, respectively. The error bars depict the standard deviation. Concentrations that were BLQ are represented as 0.5 ng/mL (i.e., 1/2 LOQ). Concentrations that were not trough samples were not utilized to calculate the average concentration for that time point
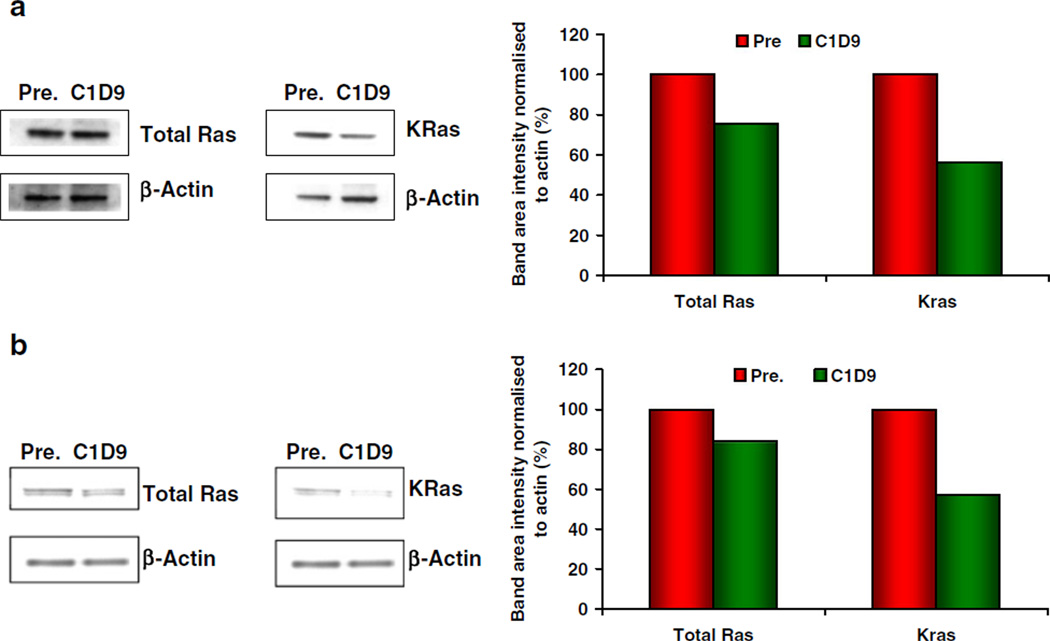
Biopsies of liver metastases were obtained pretreatment and on C1D9 from three patients in the 800 mg cohort. Total RAS in these 3 patients was lowered by up to 33 % after 9 days of salirasib treatment. KRAS, as determined in 2 patients, was lowered by up to 44 % after 9 days; representative changes in total RAS and KRAS in these 2 patients (Patients 019 and 023) are shown in Fig. 3.
Fig. 3.
Pharmacodynamic effects in paired tumor biopsies. a Data shown represent 24.75 % inhibition in total Ras and 43.65 % inhibition in KRas in Patient 019, a 67-year-old white male with metastases to the liver. b Data shown represent 16.09 % inhibition in total Ras and 42.81 % inhibition in KRas in Patient 023, a 55-year-old white female with metastases to the liver
Efficacy
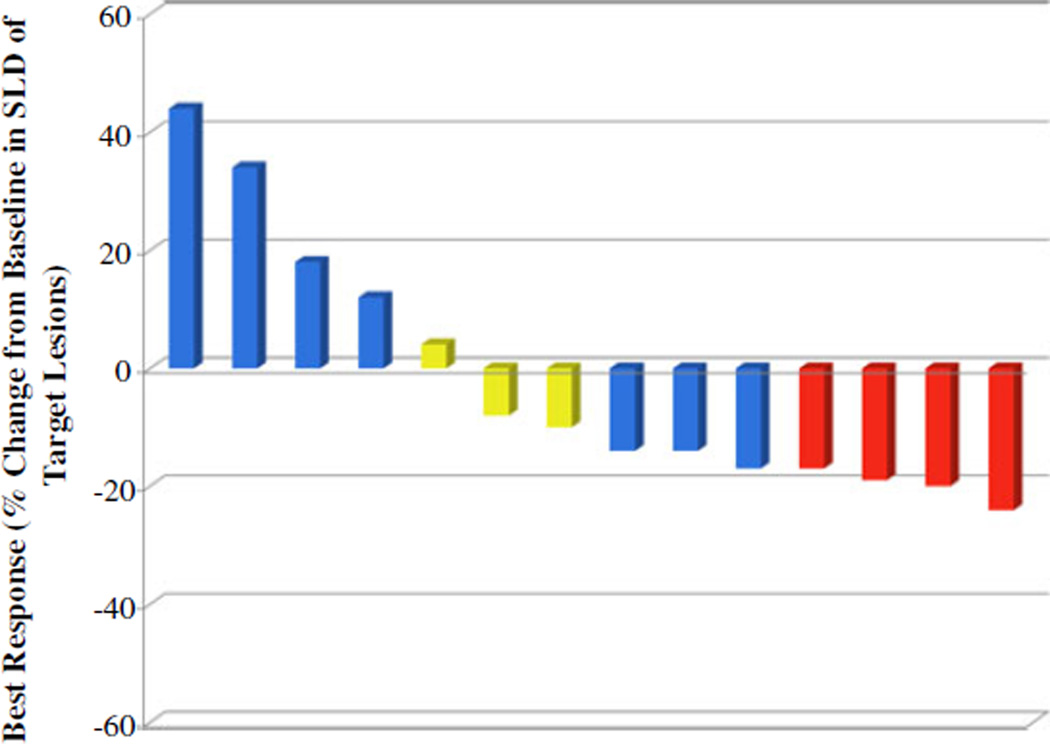
No objective responses were observed but 9 patients had reduction in tumor size (Fig. 4). The overall survival was 6.2 months (95 % confidence interval [CI]: 5.07, 18.42) and the median PFS was 3.9 months (95 % CI: 2.76, 5.99). The 1-year survival rate was 37 %. When the 600 mg cohort plus the 400 mg cohort were considered, the median OS was notably longer than that in the overall study population, at 20.2 months (95 % CI: 6.18, 24.11), and the 1-year survival rate was 71 % (5 of 7 patients). Two patients, with ECOG 0 survived beyond 2 years. The first patient is a 46-year-old white male who continued treatment salirasib+gemcitabine for 24 cycles over 1.8 years, and remains alive on alternate chemotherapy >1 year after his last salirasib dose. The second patient is a, a 62-year-old white female who received 4 cycles of treatment died of PDA 731 days (2.0 years) after starting treatment. There was no correlation between salirasib exposure and clinical outcomes (p>0.05).
Fig. 4.
Waterfall plot of tumor measurement. Data are shown for patients with both baseline and post-baseline target lesion and CA19-9 measurements. Best response=maximum decrease from baseline in the sum of the longest diameters (SLD) of target lesions; if a decrease from baseline was not seen, the smallest increase from baseline in the SLD of target lesions is presented. [≥25 % increase from baseline in CA19-9 (blue); 25 % change from baseline in CA19-9 (yellow); and ≥25 % decrease from baseline in CA19-9 (red)]
Discussion
The ultimate goal of this work was to gain data to support the further development of the agent in this disease. The results showed that salirasib, in combination with gemcitabine, inhibits the growth of PDX of PDA and modulates downstream Ras signaling. In the clinical study, the combination of salirasib with gemcitabine was well tolerated with neither DLT nor PK interaction observed in the dose-range tested. However, based on the overall rate of toxicities, a dose of 600 mg bid appears well tolerated and has been selected for further studies. The PD data, albeit limited, supports the mechanism of action of agent in patient tumors.
Activating mutations in the KRas oncogene constitute one of the main genetic alterations in PDA [5]. Indeed, targeting RAS has been actively sought in the development of drugs in PDA. Initial studies focused on agents that blocked Ras farnesylation and, therefore, anchorage to the plasma membrane. However, these agents failed to inhibit PDA growth likely because Ras could be activated by alternative prenylation reactions [20]. Salirasib works by a different mechanism of action dislodging all RAS isoforms from the plasma membrane. The agent exerted antitumor and mechanistic effects in PDA cell lines and was selected for clinical development [9–11].
More extensive preclinical testing may help to better identify potential active agents and to characterize mechanism of action and select biomarker of drug action. With this goal our group developed the PancXenoBank, which is a collection of well characterize PDX [12]. This model has been extensively used to screen for new drugs in PDA with recent data showing that positive results in the model predict for subsequent clinical efficacy [21]. The integrate approach presented here with an initial preclinical study followed, if activity warrants, by a focus clinical trial provides an strategy for a more rational drug development plan in PDA [22].
Salirasib was well tolerated and did not interact pharmacokinetically with gemcitabine. Though an MTD was not defined, a dose of 600 mg b.i.d is well tolerated and has been selected for further studies. Paired tumor biopsies showed target modulation. Unfortunately this endpoint was only assessable in two subjects. As shown in the data, the degree of KRas down regulation ranged was approximately 40 %. It is not possible to determine the biological significance of this observation with current data. This topic should be the focus of subsequent studies. In this study, the combination resulted in a 6.2 months median survival and a 37 % one-year survival. The corresponding parameters for single agent gemcitabine in the recent randomized clinical trials are 5–6 and 20–25 %. While comparisons across studies is difficult, the 1 year survival data is promising and supports continuing the development of the agent in a phase II study.
In summary, these data show that salirasib in combination with gemcitabine demonstrated antitumor activity and biomarker modulation in preclinical models of PDA. This combination was well tolerated in patients with advanced PDA neither serious toxicity nor pharmacokinetic interaction. The treatment down regulated Ras protein expression and resulted in meaningful treatment efficacy. A dose of 600 mg po bid is recommended for further studies of this combination. Based on these results, a randomized phase II study of gemcitabine plus salirasib versus gemcitabine alone is been considered.
Acknowledgments
Supported by: Concordia Pharmaceuticals, Inc. This research was supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 RR025005). The project described was supported in part by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: Disclaimers: Funding for the study described in this manuscript was provided by Concordia Pharmaceuticals. Concordia Pharmaceuticals is a client of ClinOps, LLC, and Averion International Corp. At the time of the study, Dr. Rudek was a paid consultant to Averion International.
The terms of this arrangement were being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Author’s Disclosures of Potential Conflicts of Interest Although all authors completed the disclosure declaration, the following author (s) indicated a financial or other interest that is relevant to the subject matter under consideration in this manuscript. Those relationships marked with a “C” were compensated.
Employment or Leadership position: None
Consultant or Advisory Role: Rudek (C)
Stock Ownership: None
Research Funding: Laheru, Rudek
Expert Testimony: None
Other remuneration: None
Contributor Information
Daniel Laheru, Email: laherda@jhmi.edu, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
Preeti Shah, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
N. V. Rajeshkumar, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA
Florencia McAllister, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
Gretchen Taylor, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
Howard Goldsweig, Biovex, Woburn, MA, USA.
Dung T. Le, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA
Ross Donehower, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
Sheila Linden, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
Ming Zhao, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA.
Michelle A. Rudek, Department of Medical Oncology, Skip Viragh Center for Pancreatic Cancer Research and Patient Care, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting- Blaustein Cancer Research Building, Room 4M09, 1650 Orleans Street, Baltimore, MD 21231, USA
References
- 1.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra C, Schuhmacher AJ, Canamero M, Schuhmacher AJ, Hernández-Porras I, Cañamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 8.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 9.Weisz B, Giehl K, Gana-Weisz M, Ben-Baruch G, Marciano D, Gierschik P, et al. A new functional Ras antagonist inhibits human pancreatic tumor growth in nude mice. Oncogene. 1999;18:2579–2588. doi: 10.1038/sj.onc.1202602. [DOI] [PubMed] [Google Scholar]
- 10.Haklai R, Elad-Sfadia G, Egozi Y, Kloog Y. Orally administered FTS (salirasib) inhibits human pancreatic tumor growth in nude mice. Cancer Chemother Pharmacol. 2008;61:89–96. doi: 10.1007/s00280-007-0451-6. [DOI] [PubMed] [Google Scholar]
- 11.Gana-Weisz M, Halaschek-Wiener J, Jansen B, Elad G, Haklai R, Kloog Y. The Ras inhibitor S-trans, trans-farnesylthiosalicylic acid chemosensitizes human tumor cells without causing resistance. Clin Cancer Res. 2002;8:555–565. [PubMed] [Google Scholar]
- 12.Tsimberidou A, Rudek MA, Hong D, Ng CS, Blair J, Goldsweih H, et al. Phase I first-i-human clinical study of S-trans, transfarnesylthiosalicylic acid (salirasib) in patients with solid tumors. Cancer Chemother Pharmacol. 2010;65(2):235–241. doi: 10.1007/s00280-009-1027-4. [DOI] [PubMed] [Google Scholar]
- 13.Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 14.Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–2806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15:4138–4146. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- 16.Rajeshkumar NV, Rasheed ZA, Garcia-Garcia E, López-Ríos F, Fujiwara K, Matsui WH, et al. A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther. 2010;9:2582–2592. doi: 10.1158/1535-7163.MCT-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimeno A, Rudek MA, Kulesza P, Ma WW, Wheelhouse J, Howard A, et al. Pharmacodynamic-guided modified continuous reassessment method-based, dose-finding study of rapamycin in adult patients with solid tumors. J Clin Oncol. 2008;26:4172–4179. doi: 10.1200/JCO.2008.16.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, He P, Xu L, Hidalgo M, Laheru D, Rudek MA. Determination of salirasib (S-trans, trans-farnesylthiosalicylic acid) in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;869:142–145. doi: 10.1016/j.jchromb.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibaldi M, Perrier D. Noncompartmental analysis based on statistical moment theory. In: Gibaldi M, Perrier D, editors. Pharmacokinetics. New York: Marcel Dekker; 1982. pp. 409–417. [Google Scholar]
- 20.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Hoff DD, Ramanathan RK, Borad M, Laheru D, Smith LS, Wood TE, et al. Gemcitabine plus nab-Paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011 Oct 3; doi: 10.1200/JCO.2011.36.5742. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrido-Laguna I, Tan AC, Uson M, Angenendt M, Ma WW, Villaroel MC, et al. Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br J Cancer. 2010;103:649–655. doi: 10.1038/sj.bjc.6605819. [DOI] [PMC free article] [PubMed] [Google Scholar]