Abstract
Neuroblastoma is a highly heterogeneous tumor accounting for 15 % of all pediatric cancer deaths. Clinical behavior ranges from the spontaneous regression of localized, asymptomatic tumors, as well as metastasized tumors in infants, to rapid progression and resistance to therapy. Genomic amplification of the MYCN oncogene has been used to predict outcome in neuroblastoma for over 30 years, however, recent methodological advances including miR-NA and mRNA profiling, comparative genomic hybridization (array-CGH), and whole-genome sequencing have enabled the detailed analysis of the neuroblastoma genome, leading to the identification of new prognostic markers and better patient stratification. In this review, we will describe the main genetic factors responsible for these diverse clinical phenotypes in neuroblastoma, the chronology of their discovery, and the impact on patient prognosis.
Keywords: Neuroblastoma, MYCN, MiRNA, DNA methylation, ALK, PTPRD
Introduction
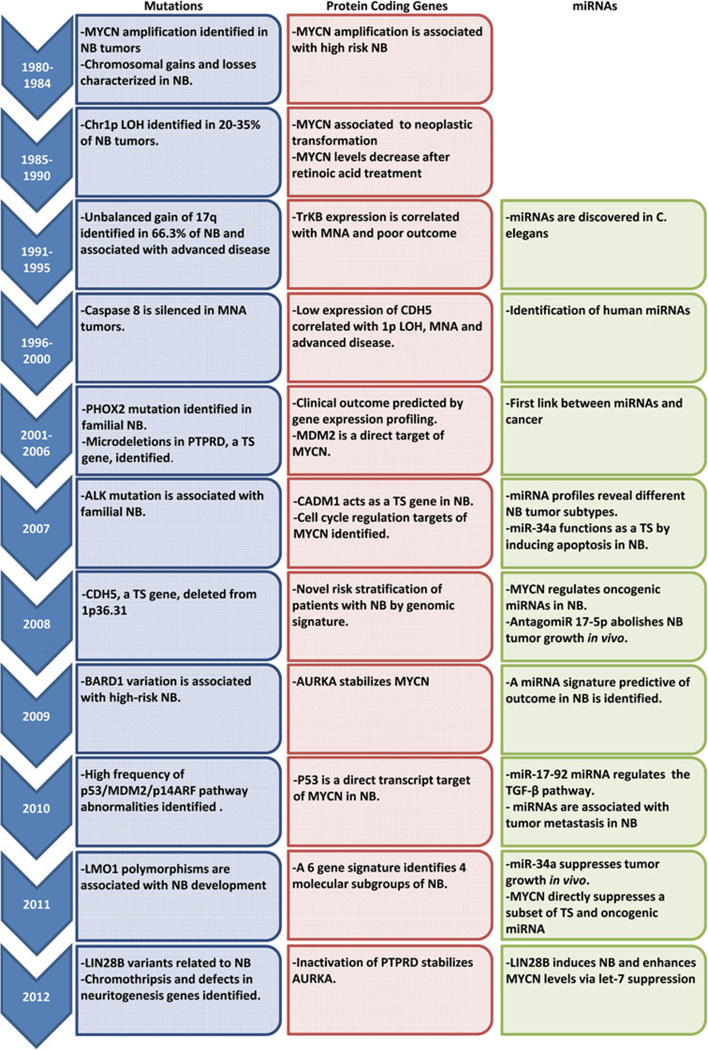
Neuroblastoma is a highly malignant pediatric cancer derived from precursor or immature cells of the sympathetic nervous system. Despite the relatively low incident level (6–10 children per million) [1, 2], approximately 15 % of all childhood cancer deaths can be attributed to the disease [3]. Long since recognized as a genetically complex form of cancer, neuroblastoma displays profound genetic heterogeneity (Fig. 1). As a result, strikingly different outcomes are observed across tumor subtypes. These range from spontaneous regression without therapy (developing into a benign ganglioneuroma) to rapid progression and death due to disease. It is clear therefore that in order to combat neuroblastoma, we must understand the genetics of the disease.
Fig. 1.
Chronology of neuroblastoma genetics
The significance of MYCN amplification (MNA) in neuroblastoma pathogenesis was first established in the early 1980’s with its association with high risk tumors and poor patient survival [4]. Since that time, multiple recurrent genetic alterations have been associated with neuroblastoma, including whole chromosome gains and a large number of large-scale chromosome imbalances, such as loss of heterozygosity at chromosome arms 1p, 3p, 14q and 11q, unbalanced gain of 1q, 11p and 17q and numerous mutations in key genes such as ALK, PHOX2B and PTPRD [5–9].
Numerous studies have now demonstrated that genomic and transcriptomic profiles can be predictive clinical disease course, so that a combination of mRNA, miRNA and arrayCGH are now being used to better define prognostic signatures and may provide insight into the molecular basis of clinical heterogeneity [10–16]. This progress is some-what reflected in the International Neuroblastoma Risk Group (INRG) staging system which takes into account both clinical characteristics and tumor biology to identify clinical risk groups with statistically different event-free survival rates [17]. Independently prognostic baseline characteristics included in this system are patient age, disease stage, histology, grade of differentiation, DNA index, MYCN amplification status, and the presence of copy number aberrations at chromosome arm 11q.
In this review, we will discuss the key genetic factors contributing to neuroblastoma as identified over the past 30 years, and the significance of such in relation to improved understanding of neuroblastoma predisposition in both familial and sporadic cases.
Chromosomal aberrations
The key to elucidating the means by which chromosomal aberrations reduce overall survival is to identify oncogenes and tumor suppressor genes located in the regions of alteration. Here, we take a look at some of the most frequent aberrations found in neuroblastoma tumors and the key protein-coding genes located at cancer-associated genomic regions (CAGRs) or in fragile sites.
DNA ploidy
Generally, tumors from patients with low stage disease are hyperdiploid or near-triploid and have few, if any, structural aberrations [18]. This genetic subtype of tumor is frequent in patients of less than 1 year of age, where tumors are localized and have good prognosis [19]. Although ploidy can be predictive of outcome in infants, the prognostic significance of ploidy is lost for patients older than 1–2 years [20], probably because chromosomal aberrations in diploid tumors have contributed to the deregulation of cancer-related pathways.
Many neuroblastoma tumors display DNA diploid status and bear partial gains, losses, amplifications or other structural chromosome aberrations. Recurrent structural chromosomal alterations commonly associated with advanced stage of disease and poor outcome in neuroblastoma include MYCN amplification, deletion of chromosome arms 1p, 3p, 4p and 11q, and gain of chromosome arm 17q (for review see [21]). In addition, a recent INRG report on non-MYCN amplified tumors determined that it is not single genetic markers, but the overall segmental genomic profile of tumors that adds information to patient prognosis [22].
Amplification of MYCN oncogene
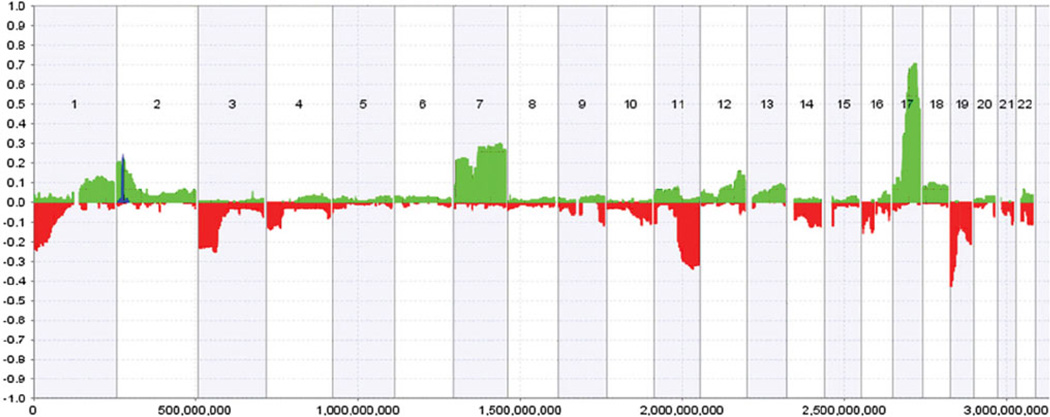
Amplification of the MYCN gene, mapping to 2p24.1 (Fig. 2), is present in approximately 20 % of neuroblastoma tumors and since its discovery in the early 1980’s remains one of the most important genetic abnormalities associated with advance stages of disease and a highly malignant phenotype [4, 23]. Numerous studies focusing on identifying the signalling pathways influenced by MYCN have established that high level enhances the expression of numerous genes involved in cell proliferation, and also represses expression of differentiation- and apoptosis-related genes either in a direct or indirect fashion [24–26]. Targets directly induced by MYCN include the high mobility group A (HMGA1) [27], the minichromosome maintenance complex component 7 (MCM7) [28], the Mdm2-p53 binding protein homolog (MDM2) [29], p53 [30], and the multidrug resistance-associated protein MRP1 [31].
Fig. 2.
DNA copy number alterations from 160 primary neuroblastoma tumors across the genome. Regions of hemizygous loss (red bar) and hemizygous gains (green bar) are plotted for each autosome with the observed frequency (%) of each aberration detected in the tumor series plotted on the Y axis. The frequency of MYCN and ALK amplifications is also plotted (blue). Figure taken from [78]
Perhaps most significantly, the overall impact of MYCN was revealed in an early study by Weiss et al. [32] confirming over-expression of MYCN alone was sufficient to initiate neuroblastoma formation in mice. Despite this, MYCN status cannot predict all cases of poor survival in neuroblastoma, and 80 % of neuroblastomas do not display MYCN amplification. Several studies have reported over-expression of MYCN in the absence of amplification [33, 34]. A recent study revealed a functional 157-gene signature in neuroblastoma consisting of relevant genes that are regulated by MYCN and predictive of outcome. Interestingly, a sub-group of the tumors displaying this signature and poor outcome did not have MYCN amplification or high MYCN mRNA levels, but high nuclear MYCN protein levels [35]. This suggests that the aggressive phenotype of MYCN might not only be associated with MYCN copy numbers, but with other signals that regulate MYCN expression, such as are RNA binding proteins (RBP) and microRNAs, which we will discuss in more detail later.
Chromosome 1p deletions
Loss of heterozygosity (LOH) of the short arm of chromosome 1p is found in 20–35 % of neuroblastoma tumors [36, 37]. This aberration is frequently associated with amplification of MYCN, is found approximately in 70 % of aggressive neuroblastomas [38], and it has been reported that 1p LOH is independently associated with poor outcome [39].
Pinpointing candidate tumor suppressor genes in the 1p LOH genetic subtype were aided by the identification of the shortest region of consistent heterozygous deletion (spanning 261 kb) at 1p36.3 [40–42]. One of the first genes identified was the chromodomain helicase DNA binding domain 5 (CHD5), mapping to 1p36.31 [43]. Very low levels of CDH5 were observed in 137 neuroblastoma primary tumors and cell lines, and that low expression of CHD5 was highly correlated with 1p LOH, MYCN amplification, advanced stage, and unfavorable histology. Consistently, Fujita et al. [44] reported that tumor growth was inhibited in mice over-expressing CHD5. In addition, their data strongly suggested that inactivation of the second allele of CHD5 in neuroblastoma occurs by means of epigenetic silencing. Based on the positive correlation between 1p LOH and MYCN amplification found in neuroblastoma tumors, the authors suggested that CHD5 promoter methylation could be a MYCN-mediated effect [45].
Recently, CAMTA1, a transcription factor mapping to 1p36, was also identified as a tumor suppressor gene in neuroblastoma. Multivariate survival analysis, based on CAMTA1 mRNA expression profiling data in a cohort of 251 neuroblastoma tumors, confirmed that low CAMTA1 was a predictor of poor clinical outcome independently of MYCN status, 1p LOH, and age of the patient at diagnosis [46]. In a follow-up study, transcriptome analysis using a CAMTA1-inducible cell model revealed that expression of CAMTA1 induces the transcription of genes involved in neuronal differentiation, and inhibits genes related to cell proliferation. In addition, subcutaneous inoculation of athymic nude mice with CAMTA1-inducible neuroblastoma cells resulted in a significant reduction of the tumors, demonstrating a role for CAMTA1 as a tumor suppressor in an in vivo model [47].
Zinc-finger transcription factor CASZ1, located on 1p36.22, has also been suggested to play a role in cell differentiation. A study by Liu et al. [48] reported that low expression of CASZ1 mRNA was found in 77 % of neuroblastomas of patients older than 18 months (n = 59), and significantly associated with decreased overall survival. Low CASZ1 mRNA levels, as measured by quantitative real-time PCR, were associated with neuroblastomas with a poor differentiated histopathology and significantly correlated with increased age (≥18 months), 1p LOH, MYCN amplification and advanced disease. Consistently, restoration of CASZ1 in neuroblastoma cell lines induced the cell differentiation, enhanced cell adhesion, and suppressed cell growth [48]. Subsequent studies demonstrated that silencing of the second allele of CASZ1 was mediated by the aberrant up-regulation of the polycomb protein histone methyltransferse EZH2, which regulates differentiation in many tissues [49].
Other strong candidate 1p tumor suppressor genes include the ubiquitination factor E4B (UBE4B) and apoptosis-inducing, TAF9-like domain 1 (APITD1). The expression of UBE4B, a gene implicated in the ubiquitin/proteasome pathway, is markedly decreased in high-stage/poor-prognosis tumors compared to low-stage/favorable-prognosis tumors [50]. In functional studies, APITD1, which is also lowly expressed in neuroblastoma, reduced the cell growth in the neuroblastoma cell lines SK-N-AS and SK-N-BE [51].
Loss of chromosome 11q
Another common structural chromosome aberration associated with aggressive clinical behavior is 11q LOH, occurring in approximately 40–45 % of cases [52]. Although inversely correlated with MYCN amplification [52–55], a small sub-set of tumors display both an 11q LOH and MYCN amplification. Numerous studies suggest that MYCN amplified and 11q LOH represent two distinct subtypes of neuroblastoma tumors, both of which can be associated with poor clinical outcome [52, 56, 57]. As a result, in 2009, aberrations of chromosome 11q were included in the international neuroblastoma risk group (INRG) classification system [17].
Therefore, identifying genes on the 11q chromosome that contributes to neuroblastoma aggressiveness is crucial to understand the pathways deregulated in these tumors. However, in spite of the intensive effort, only a few genes have been identified to date. A study by Caren et al. [58] reported that in tumors with 11q LOH, the frequency of segmental aberrations was significantly higher than in MNA tumors as determined using high-density SNP microarrays. This fact was explained in part by the loss of the H2AFX gene, located in the 11q23.3 deleted region. This gene has been shown to play a role in genomic stability modification, and enhanced susceptibility to cancer in mice [58, 59].
Other studies identified cell adhesion molecule 1 (CADM1), which transcribes a cellular adhesion protein involved in neural cell development, as a candidate tumor suppressor gene in the 11q23 deleted region. CADM1 was significantly down-regulated in tumors with 11q LOH relative to 11q diploid tumors and significantly associated with advance stage of disease and poor survival [60, 61]. Over-expression of CADM1 revealed a significant inhibition of cell proliferation and colony forming ability in a panel of four different cell lines, demonstrating that CADM1 expression attenuates the malignant phenotype in cultured cells. No evidence for inactivating mutations of CADM1, or hypermethylation of its promoter was found, suggesting that other mechanisms such as haplosufficiency or post-transcriptional regulators of gene expression may be involved in the modulation of CADM1 expression [60, 62].
Constitutional rearrangements of 11q have also been reported in patients with neuroblastoma [63, 64], indicating that aberrations in 11q genes may also be involved in development of neuroblastoma. Although 11q LOH remains strongly associated with poor outcome in neuroblastoma, more recent work now demonstrates that both miRNA and mRNA expression profiles are more powerful predictors of clinical outcome than 11q status alone [14, 65].
17q gains
The most common aberration found in neuroblastoma tumors is the unbalanced gain of 17q (segment 17q21-qter), occurring in ~70 % of tumors [66–68]. Frequently, this aberration is caused by unbalanced translocations of segment 17q21-qter and the distal part of chromosomes 1p or 11q [66, 69–74], though other chromosomes can also be involved in 17q gains [75]. Numerous studies have reported that 17q gain is significantly associated with advanced stage of disease, increased patient age, 1p LOH, 11q LOH, and MYCN amplification [68, 71, 75–77]. However, the independence of 17q gain as a prognostic factor is controversial. A study by Bown et al. [68] investigated the prognostic independence of 17q gain by analyzing the 17q status and clinical data for 313 tumors from six different European institutes. Multivariate analysis including patient age, tumor stage, MYCN status, and 1p LOH demonstrated that 17q gain was an independent prognostic factor. However, MYCN status and patient age were not predictive of survival in this model. Contrary to this, Buckley et al. [78] determined that 17q gain was not an independent predictor of poor survival, as did Spitz et al. [76] who reported that when 17q gain, 11q LOH, and MYCN status were included in a multivariate analysis, 17q gain was not a significant prognostic factor, while MYCN and 11q LOH were. Whether an independent prognostic factor, or merely a modifying factor, identifying the gene aberrations caused by 17q unbalance will be crucial to fully understand neuroblastoma progression.
Other imbalances
In addition to these major genetic aberrations found in neuroblastoma, there are other recurrent imbalances that could also be important, such as gain of chromosomes 1q, 2p, 7q, 9p, and 11p, or loss of 3p, 4p, 14q, 16p, and 19q [77]. However, the biological significance of these aberrations and the genes contributing to neuroblastoma pathogenesis remain elusive. Figure 2 displays a summary of the aberrations identified across the genome on 160 primary neuroblastoma tumors, as determined in a study by Buckley et al. [78].
Genetic mutations
Mutations are one of the several alterations at genome level that can provoke malignant transformation or tumor progression, and heavily contribute to neuroblastoma clinical heterogeneity. Table 1 summarizes published clinical studies focused on mutations identified in neuroblastoma.
Table 1.
Clinically relevant mutations in neuroblastoma
| Chr | Gene | Gene name | Status | Predisposition | Function | References |
|---|---|---|---|---|---|---|
| 1p36.31 | CHD5 | Chromodomain helicase DNA binding protein | Deletion | Sporadical | Chromatin remodelling and gene transcription | [44] |
| 1p36.3–p36.2 | DFF45 | DNA fragmentation factor | Mutation | Sporadical | Apoptosis | [186] |
| 1q23.3 | DUSP12 | Dual specificity phosphatase 12 | SNP | Sporadical | Negatively regulate members of the mitogen-activated protein (MAP) kinase superfamily (MAPK/ERK, SAPK/JNK, p38) | [187] |
| 1p36.3 | UBE4B | Ubiquitination factor E4B | Mutation | Sporadical | Ubiquitination | [50] |
| 2p23 | ALK | Anaplastic lymphoma receptor tyrosine kinase | Mutation, amplification | Hereditable | Genesis and differentiation of the nervous system | [6, 7, 9, 188–197] |
| 2q35 | BARD1 | BRCA1-assosiated RING domain | SNPs | Sporadical | Interaction with the N-terminal region of BRCA1 | [87] |
| 4p12 | PHOX2B | Paired-like homeobox 2B | Germline missense or frameshift | Hereditable, sporadical | Transcription factor | [5, 83, 198–200] |
| 5q11.2 | DDX4 | DEAD box polypeptide 4 isoform | SNP | Alteration of RNA secondary structure | [187] | |
| 5q11.2 | IL31RA | Interleukin 31 receptor A precursor | SNP | Signaling via activation of STAT-3 and STAT-5 | [187] | |
| 6p22 | FLJ44180 | Long intergenic non-protein coding RNA 340 | SNP | – | [89] | |
| 6p22 | FLJ22536 | Long intergenic non-protein coding RNA 340 | SNP | – | [89] | |
| 9p21 | CKDNA | Cyclin-dependent kinase inhibitor 2A | Mutation, deletion | Sporadical | Cell cycle G1 control | [201–204] |
| 9p23–p24.3 | PTPRD | Protein tyrosine phosphatase, receptor type, D | Microdeletion | Signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation | [8, 9] | |
| 11q13 | CCND1 | Cyclin D1 | Amplification, rearrangement | Proto-oncogene, control of cell cycle/cellular proliferation | [56, 205] | |
| 11p11.2 | HSD17B12 | Hydroxysteroid (17-beta) dehydrogenase 12 | SNP | Converts estrone into estradiol in ovarian tissue | [187] | |
| 6q21 | LIN28B | Lin-28 homolog B | SNP | RNA binding protein, negatively regulates let-7 processing | [93] | |
| 11p15.4 | LMO1 | LIM domain only 1 | SNP, amplification | Sporadical | Cysteine-rich transcriptional regulator | [88] |
| 12q24 | PTPN11 | Protein tyrosine phosphatase, non- receptor type 11 | Mutation | Sporadical | Regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic | [206, 207] |
| 17q11.2 | NF1 | Neurofibromin 1 | Deletion | Negative regulator of the ras signal transduction pathway | [208] | |
| 17q13.1 | P53 | Tumor protein p53, TP53 | Mutation | Sporadical | DNA binding protein | [146] |
| 18q21.3 | DCC | Netrin 1 receptor | Mutation, deletion | Sporadical | Member of the immunoglobulin superfamily of cell adhesion molecules | [209] |
| 20p11 | SLC24A3 | Solute carrier family 24 | SNP | Sporadical | Plasma membrane sodium/calcium exchangers | [89] |
Familial neuroblastoma
Familial neuroblastoma is a rare event, as it only accounts for 1–2 % of cases. Inheritance seems to follow an autosomal dominant pattern with incomplete penetrance [79]. As in sporadic neuroblastoma, the familial cases also display a significant clinical heterogeneity ranging from tumors that spontaneously regress to tumors that rapidly metastasize [80]. This suggests that the different outcomes could be linked to differences in additional somatically acquired mutations. Inherited mutations in the homeodomain transcription factor, paired-like homeobox 2B (PHOX2B) and the anaplastic lymphoma kinase (ALK), have been reported to predispose to familial neuroblastoma [5–7]. PHOX2B is a homeodomain-containing protein which plays an essential role during early development promoting neuron formation and differentiation [81]. Missense or frame-shift mutations in the homeodomain of PHOX2B were described in a rare subset of neuroblastomas with congenital central hypoventilation syndrome (CCHS). However, this mutation was reported to occur in only 6.4 % of familial neuroblastomas [82]. In addition, another study screening 237 sporadic neuroblastoma tumors revealed that mutations in PHOX2B were found only in ~2 % of the cases [83]. This suggests that although PHOX2B could give selective advantages to tumor cells, it is likely not sufficient to drive neuroblastoma pathogenesis.
Contrary to this, activating mutations of ALK are found in the majority of familial cases of neuroblastoma, as well as in 12.4 % of high risk sporadic neuroblastomas [6]. Although the role of ALK during normal development remains to be elucidated, it is clear that activating mutations in ALK promote oncogenesis in neuroblastoma and in other type of cancers [7, 84]. DNA amplification and protein over-expression, as well as activating point mutations of ALK, have been described in neuroblastomas [85]. A recent study demonstrated that ALK (F1174L), which is the most frequent and aggressive ALK mutation, was sufficient to promote neuroblastoma development in mice. In addition, when ALK (F1174L) and MYCN were co-expressed, a synergic effect was displayed in tumor development. Interestingly, these tumors had minimal chromosomal aberrations, suggesting that these two genes are sufficient to drive neuroblastoma formation [86].
Sporadic neuroblastoma
Almost 98 % of neuroblastoma cases represent sporadically arisen tumors. Sporadic neuroblastoma is driven by multiple, low frequency polymorphisms. Advances in genome sequencing technologies have allowed genome-wide association studies (GWAS) resulting in the identification of risk polymorphisms in a number of large, independent studies [87–89]. Maris et al. [89] genotyped genomic DNA from 1,032 NB patients and 2,043 control subjects. Association analyses of chromosome 6p22 SNPs were performed for 883 patients where complete clinical data were available. The authors identified three repeated common SNPs within two overlapping genes, FLJ22536 and FLJ44180, a non-coding RNA gene, at 6p22 showing genome-wide significance for association with sporadic neuroblastoma. Homozygosity for any of these risk alleles was significantly associated with high-risk features including metastatic disease, MYCN amplification, and lower patient survival. The same dataset also identified 6 SNPs at 2q35 located within introns 1, 3, and 4 of the BRCA1-associated RING domain-1 (BARD1) gene [87]. Apparently, BARD1 plays an important role in the tumor suppressor function of BRCA1, a hereditary breast and ovarian cancer susceptibility gene, and pathogenic BRCA1 mutations have been shown to impede with the heterodimerization of BRCA1 and BARD1 [90]. An expansion of the original GWAS cohort included 2,251 neuroblastoma patients and 6,097 controls [88], documented an additional predisposition locus at 11p15, within the LIM domain only 1 (LMO1) gene, a transcriptional regulator. Duplication of the LMO1 locus was reported to occur in 12 % of primary neuroblastoma tumors and was associated with more advanced disease as well as two SNPs. Supplementary in vitro studies demonstrated that siRNA knock down of LMO1 inhibits cell growth, and LMO1 over-expression enhances cell proliferation, supporting the role of LMO1 as an oncogene involved in the pathogenesis of neuroblastoma.
Another group used a whole-genome paired-end sequencing platform to identify mutated genes associated with sporadic neuroblastoma in 87 untreated primary neuroblastomas, with an additional validation by SNP arrays of 52 sequenced tumors [9]. The sequencing data identified very few recurrent somatic mutations. In contrast, novel genetic defects were identified in high-risk tumors. First, massive genomic rearrangements, known as chromothripsis [91], were observed in 18 % of high-, but not low-stage tumors. Chromothripsis was significantly associated with poor-survival, MYCN amplification, 1p LOH, and affected genes involved in neuroblastoma pathogenesis. In addition, structural aberrations in neuritogenesis genes were also found in high-stage tumors without MYCN amplification, which could explain the aggressive behavior of this subtype of tumors.
Very recently, over-expression of the RNA binding protein LIN28B was reported to be associated with the presence of a SNP within an intron of the LIN28B gene in neuroblastoma [92]. LIN28B mRNA expression was significantly higher in cell lines homozygous for the risk allele compared to heterozygous cell lines, and over-expression of LIN28B was significantly associated with poor survival. However, none of the 12 neuroblastoma cell lines tested was homozygous for the protective allele. In addition, cell lines homozygous for the SNP of LIN28B had higher levels of MYCN expression. In addition, Molenaar et al. [93] reported that high level amplifications of the 6q21 region, where LIN28B maps to, occur at low frequency in neuroblastoma tumors. Regardless of the mechanism leading to LIN28B over-expression, Molenaar et al. demonstrated for first time that LIN28B over-expression was predictive of survival independently of MYCN and ALK status, patient age or tumor stage. Most importantly, this study demonstrated that transgenic mice expressing Lin28b in the neural crest developed the tumors characterized for a histology and location similar to human neuroblastomas. LIN28B has therefore emerged as a new oncogene in neuroblastoma and a novel therapeutic target. The mechanism of action of LIN28B in neuroblastoma will be further discussed below.
mRNA signatures
In an effort to further improve the risk estimation of neuroblastoma patients, many groups have identified mRNA expression patterns that allow more detailed subset classification and prediction of outcome. By comparing gene expression patterns in Stage 4S versus Stage 4 tumors (MYCN non-amplified) using serial analysis of gene expression (SAGE), Fischer et al. [94] found a predominance of genes involved in neuronal differentiation in 4S tumors compared to Stage 4 tumors, despite the poorly differentiated histopathology observed in both subtypes. They validated the expression levels of 18 genes by qPCR and used the combined analysis of these transcripts to show that patients with a favorable gene signature displayed significantly better EFS than those with an unfavorable gene signature. Asgharzadeh et al. [10] also carried out a study on MYCN non-amplified tumors, and identified a 55 genes signature capable of stratifying clinically identical high-risk tumors into sub-groups with different outcomes.
In a retrospective SIOPEN/COG/GPOH study, Vermeulen et al. [11] identified a 59-gene expression signature which could independently distinguish between patients with respect to progression-free survival (PFS) and overall survival (OS) in the SIOPEN and GPOH cohort. The signature was also tested within each SIOPEN treatment protocol and found to accurately identify patients with risk of progression or relapse. A 236 tumor cohort from the Children’s Oncology Group (COG) was used as a validation set, and found that the signature was the most significant variable for prediction. Forty-two genes in the 59-gene signature were also found to be present in at least two out of four published NB expression studies profiling high risk and low risk sub-groups [95–98]. The authors then used these 42 genes to define a cross-platform signature that was validated on four independent sets [12]. An additional study found 3 genes from the 42-gene classifier also contributed to a 6-gene signature (MYCN, ALK, BIRC5, CCND1, NTRK1 and PHOX2B) that distinguishes between four molecular subgroups of neuroblastoma [99]. In addition to the delineation of the established sub-groups of Type 1 (low risk, high NTRK1), Type 2A (intermediate risk, 11q–) and Type 2B (high risk, MNA), the authors identified a novel fourth sub-group using this 6-gene signature, consisting of high-stage 11q– tumors displaying low MYCN and ALK expression. The identification of this fourth sub-group is in concordance with the findings of Fischer et al. and Buckley et al. [78, 100] that 11q– tumors can be subdivided into two groups with distinguishing gene and miRNA expression profiles, predictive of outcome.
Non-coding RNA
miRNAs
MicroRNAs (miRNAs) are evolutionarily conserved, endogenous, small non-coding RNA molecules, approximately 22 nucleotides in length. They function as post-transcriptional gene regulators through targeting regions of partial sequence complementarity mainly at the 3′UTR (untranslated region) of the target mRNA resulting in the degradation of the mRNA or inhibition of protein translation [101]. This partial complementarity allows miRNAs to regulate multiple mRNA sequences. At the same time, a single mRNA can be regulated by multiple different miRNAs, resulting in a sophisticated gene regulatory network. MiRNAs are known to regulate oncogenes, tumor suppressor genes, genes involved in cell cycle control, cell migration, apoptosis, and angiogenesis. Subsequently, altered miRNA profiles are found in several human diseases, as well as in many forms of cancer [102–105]. In fact, select miRNA signatures can classify multiple cancers more accurately than data from ~16,000 mRNAs [106].
In 2007, Chen and Stallings [15] determined that many miRNAs were differentially expressed in different genomic subtypes of neuroblastoma, and that those miRNA profiles were correlated with prognosis, differentiation, and apoptosis. A continuation of this work was published in 2009 when an extended tumor cohort was analyzed for expression of 450 miRNA loci [16]. This study highlighted that over-expression of the MYCN transcription factor as well as large-scale chromosomal imbalances had contributed to the widespread dysregulation of miRNA expression in neuroblastoma tumors. Importantly, a miRNA expression signature predictive of clinical outcome was identified, emphasizing the potential for miRNA-mediated diagnostics and therapeutics. Consistent with these results, Schulte et al. [107] reported that seven miRNAs (miR-92, miR-106a, miR-let7b, miR-17-5p, miR-93, miR-99 and miR-221) were up-regulated byMYCNin neuroblastoma, and demonstrated that miR-221 was directly induced by MYCN in vitro.
Emerging functional studies have established that miRNAs regulate important genes involved in neuroblastoma disease. For example, over-expression of the miR-17-5p-92 cluster promotes tumorigenesis by regulating the pro-apoptotic gene BIM, the cell cycle regulator p21, transcription factor TGF-β, and the tumor suppressor Dickkopf [108–110]. Other miRNAs can act as tumor suppressor miRNAs, such as let-7 and miR-101 which directly regulate MYCN expression [93, 111], or have an anti-tumorigenic effect, such as the pro-apoptotic miR-34a [112–114], the anti-invasive miR-335 [115], novel tumor suppressor miR-542-5p [116], and several differentiation-related microRNA (miR-125b, miR-10a and miR-10b [117–119]). Other miRNAs have been shown to be related to drug sensitivity in neuroblastoma, such as miR-204 [120]. The list of specific miRNAs validated as contributors to neuroblastoma pathogenesis (Table 2) is ever expanding, but, what are the mechanisms underlying their deregulation in neuroblastoma?
Table 2.
Neuroblastoma-related miRNAs
| miRNA | Chr | Status in NB cells | Biological effect in NB cells | Validated targets in NB Cells | References |
|---|---|---|---|---|---|
| miR-34a | 1p36.22 | Deleted in 1pLOH tumors | Ectopic over-expression is pro-apoptotic Inhibits tumor growth in mouse model | E2F3, MYCN, BCL2 | [112, 113, 123, 124] |
| miR-184 | 15q25.1 | Down-regulated in MNA tumors | Ectopic over-expression is pro-apoptotic | AKT2 | [210] |
| miR-125a, miR-125b | 19q13.41, 11q24.1, 21q21.1 | Up-regulated upon ATRA treatment | Ectopic over-expression is anti-proliferative and pro-differentiation | TRKC, AP1M1, STK11IP, PSMD8, ITCH, TBC1D1, TDG, MKNK2, DGAT1, GAB2, SGPL1, LIN28B, p53 | [93, 117, 118, 211] |
| miR-128 | 2q21.3, 3p22.3 | Up-regulated upon ATRA treatment | Ectopic over-expression decreases cell motility and invasiveness | RELN, DCX | [158, 212] |
| miR-149* | 2q37.3 | Unknown | Ectopic over-expression is pro-apoptotic | AKT1, E2F1 | [213] |
| miR-214, miR-7 | 1q24.3 | Up-regulated upon ATRA treatment | Ectopic over-expression promotes neurite out growth in vitro | Unknown | [214] |
| miR-184 | 15q25.1 | Down-regulated in MNA tumors | Act as a tumor suppressor in mouse model | AKT2 | [210] |
| miR-10a, miR-10b | 17q21.32, 2q31.1 | Up-regulated upon ATRA treatment | Induce neural cell differentiation | NCOR2, SFRS1 | [119, 215] |
| miR-101 | 1p31.3, 9p24.1 | Unknown | Ectopic over-expression is anti-proliferative | MYCN | [111] |
| Let-7a | 9q22.32, 11q24.1, 22q13.31 | Down-regulated in LIN28B over-expressing tumors | Ectopic over-expression is anti-proliferative | MYCN | [93, 111] |
| Let-7e | 19q13.41 | Down-regulated in LIN28B over-expressing tumors | Ectopic over-expression is anti-proliferative | MYCN, DKK3 | [111, 216] |
| miR-124 | 8p23.1, 8q12.3, 20q13.33 | Up-regulated upon ATRA treatment | Ectopic over-expression promotes differentiation and neurite outgrowth | PTBP1, AHR | [217, 218] |
| miR-27b | 9q22.32 | Unknown | Ectopic over-expression is anti-proliferative and inhibits tumor growth in mouse model | PPARγ | [219] |
| miR-29a | 7q32.3 | Down-regulated in MNA tumors | Under-expression in tumors facilitates immune escape | B7H3 | [16, 220] |
| miR-542-5p | Xq26.3 | Down-regulated in MNA tumors | Over-expression reduces cell invasiveness in vitro and restricts tumor growth in mouse model | Unknown | [14] |
| miR-335 | 7q32.2 | MYCN repressed | Suppresses cell invasiveness | ROCK1, MAPK1, LRG1 | [15] |
| miR-204 | 9q21.12 | Down-regulated in MNA tumors | Ectopic over-expression increases sensitivity to cisplastin | BCL2, NTRK2 | [16] |
| miR-9 | 1q22, 5q14.3, 15q26.1 | MYC/MYCN activated | Ectopic over-expression is anti-proliferative, induces differentiation and inhibits migration, invasion, and angiogenesis | TRKC, ID, (MMP)-14 | [117, 221, 222] |
| miR-103 | 5q34, 20p13 | Up-regulated upon ATRA treatment | Ectopic over-expression is anti-proliferative, induces differentiation and reduces cell migration | ID2, CDK5R1 | [117, 221, 223] |
| miR-107 | 10q23.31 | Unknown | Ectopic over-expression reduces cell migration | CDK5R1 | [223] |
| miR-885 | 3p25.3 | Down-regulated on 3pLOH tumors | Ectopic over-expression inhibits cell cycle progression and cell survival | CDK2, MCM5 | [224] |
| miR-17-92 cluster | 13q31.3 | MYCN-induced cluster | pSMAD2, DKK3 | [109] | |
| miR-17-5p | 13q31.3 | Knock-down is anti-proliferative and pro-apoptotic | P21, BIM | [108] | |
| miR-17-5p, miR-20a | 13q31.3 | Endogenous down-regulation in response to ATRA | BCL2, MEF2D, MAP3K12 | [225] | |
| miR-18a, miR-19a | 13q31.3 | Endogenous knock-down impedes cell proliferation and promotes differentiation | ESR1 | [226] | |
| miR-92 | 13q31.3 | Inhibits tumor suppressor in mouse model | Unknown | [227] | |
| miR-92a, miR-19b | 13q31.3 | Unknown | DKK3 | [110] | |
| miR-340 | 5q35.3 | Inactivated by DNA methylation | Ectopic over-expression is anti-proliferative and pro-apoptotic | SOX2 | [17] |
| miR-380-5p | 14q32 | Over-expressed in MNA tumors | Knock-down induces apoptosis Systematic delivery of anti-miR-380-5p decreases tumor growth in mouse model | p53 | [18] |
| miR-7 | 9q21.32 | Down-regulated upon ATRA treatment | Endogenous knock-down promotes neurite out growth in vitro | Unknown | [9] |
| miR-210 | 11p15.5 | Unknown | Ectopic over-expression is pro-apoptotic | BCL2 | [19] |
| mir-21 | 17q23.1 | Up-regulated in MNA tumors | Ectopic over-expression increases resistance to cisplastin and cell proliferation | Unknown | [20] |
| miR-152 | 17q21.32 | Up-regulated upon ATRA treatment | Ectopic over-expression reduced cell invasiveness and anchorage independent growth | DNMT1 | [158] |
Alteration of miRNA expression: mechanisms involved
Altered expression of miRNAs can be caused by multiple mechanisms, including aberrations in DNA copy number, altered transcriptional activators/repressors, aberrant DNA methylation, or defects of the proteins involved in the miRNA biogenesis machinery and in the post-transcriptional regulation of miRNA expression. The elucidation of the mechanisms involved in miRNA deregulation is required not only to better understand the role miRNAs play in the development of the disease but also may help us to identify new therapeutic targets.
Copy number gains and losses
As already mentioned, alterations in miRNA expression can be altered by DNA gains and losses. However, in addition to simple dosage effects, imbalance of miRNAs leads to altered expression of their target genes resulting in significant dysregulation throughout the genome. The finding that miRNAs are frequently located at fragile sites and genomic regions involved in cancer further implicates their involvement with malignant diseases [121].
Integrated analysis of miRNA expression profiling together with oligonucleotide array CGH revealed that many of the recurrent large-scale chromosomal imbalances in neuroblastoma tumors, including loss of 1p, 3p, 11q and 14q, along with gain of 1q and 17q, have a major impact upon miRNA expression [16]. This same study identified a 15-miRNA signature predictive of survival in neuroblastoma. In a subsequent study it was demonstrated that 11q LOH tumors could be split into distinct subtypes using a miRNA signature that differed significantly in clinical outcome and the overall frequency of large-scale genomic imbalances, with the poor survival group having more imbalances [78]. However, this study also found cases where miRNA expression was inversely related to the genomic imbalance, i.e., miRNAs were under-expressed in spite of mapping to a region of DNA copy number gain. This strongly suggests that alternative mechanisms can in some instances counteract the effects of DNA dosage.
In neuroblastoma, 1p LOH is frequently associated with MYCN amplification and poor outcome [38, 39]. One of the first tumor suppressor miRNAs identified as mapping to the shortest region of overlap was miR-34a, mapping to chromosome 1p36. Initial studies demonstrated that miR-34a could induce apoptosis in neuroblastoma cells [112], a function explained somewhat by the fact that miR-34a is directly activated by p53 [122] and soon after, MYCN was also identified as a direct target of miR-34a [123]. MiR-34a functions as a tumor suppressor miRNA by inducing apoptosis of neuroblastoma cells [112, 123, 124]. The multi-gene targeting nature of miR-34a is well documented, with target transcripts including MYCN, BCL2, SIRT1, NOTCH1, JAG1, CCND1, CDK6, and E2F3 [112, 114, 123, 125–128]. It is no surprise than that the potential of miR-34a to act as a novel therapeutic in neuroblastoma was further explored. Targeted delivery of a miR-34a encapsulated anti-GD(2)-nanoparticles was accomplished in a neuroblastoma mouse model and confirmed miR-34a as an effective inhibitor of neuroblastoma tumor growth in vivo [113, 129].
Activators and repressors of microRNA transcription
MYCN and C-MYC The MYCN transcription factor exerts regulatory control over the activation or repression of a large group of oncogenic and tumor suppressor miR-NAs in neuroblastoma [16, 130]. Activation or repression of miRNAs is thought to occur by direct binding of MYCN to the proximal region of miRNAs loci. Amplification of the MYCN oncogene was shown to contribute to the widespread miRNA deregulation in neuroblastoma tumors, with miRNA profiles correlating with clinical outcome [16, 130]. These two independent studies reported that in the majority of cases, miRNAs were being down-regulated in MNA tumors. This posed the questions: is MYCN directly regulating these miRNAs, or is it the result of a secondary event? and What role do these differentially expressed miRNAs play in neuroblastoma cells?
MYCN binding to a large number of promoters and CpG islands was identified in neuroblastoma cell lines [131]. Chip–chip analysis of MYCN binding sites in neuroblastoma cell lines expressing both high and low levels of MYCN protein was performed in order to characterize the binding behavior of MYCN in these states. The analysis revealed that MYCN preferentially binds to non-canonical E-box sequence of CATGTG and to additional motifs when it is over-expressed; indicating that MYCN binding becomes less specific when highly abundant. However, to date the promoter regions of few miRNAs have been identified [132], and validation of this mechanism will not be possible until the exact promoter region of each miRNA is determined. Recently, the repression of a panel of miRNAs by MYCN binding was confirmed by CHIP-sequencing analysis in neuroblastoma cell lines. Of these miRNAs, miR-591 had tumor suppressive effects in an orthotopic neuroblastoma xenograft, while miR-558 revealed an oncogenic phenotype. This study reveals that MYCN can regulate the expression of both oncogenic and tumor suppressor miRNAs [133].
As our knowledge builds, we have also become aware that miRNAs can play both pro-oncogenic and tumor suppressor functions depending on the environmental context. An interesting example is the miR-17-5p-93 polycistronic cluster. This cluster located in chromosome 13 (13q31.3 loci) encodes 7 mature miRNAs (miR-17-5p, miR-17-3p, miR-18a, miR-19a, miR-19b, miR-20a and miR-92) that play different roles in the cell. Of particular interest is miR-17-5p, which has been shown to have a pro-oncogenic function in several types of cancer [134–138], while also being reported to act as a tumor suppressor by targeting the oncogene E2F1 [139]. Fontana et al. [108] established that this cluster was highly expressed in neuroblastoma cells over-expressing MYCN or having MYCN amplification, and verified MYCN binds directly to the 5′ and 3′ of the 17-5p cluster, promoting expression of these miRNAs. MiR-17-5p was found to enhance tumorigenesis by binding to the 3′ UTR of p21 and BIM, resulting in increased proliferation and inhibited apoptosis, respectively. A study by Mestdagh et al. [109] in 2010 examined global protein response to up-regulation of miR-17-92 and found that the TGF-β pathway is also suppressed in neuroblastoma upon miR-17-92 activation. Impaired TGF-b signaling subsequently contributes to poor prognosis in neuroblastoma patients. More recently, the tumor suppressor Dickkopf-3 (DKK3) was also validated as a direct target of miR-92a and miR-19b, both members of the miR-17-92 cluster [110].
According to the results of Bray et al. [16] and Mestagh et al. [130], most of the differentially expressed miRNAs between MYCN amplified or MYCN single copy tumors were down-regulated. However, relatively few of the under-expressed miRNAs (n = 4) appear to be directly regulated by binding of MYCN based on unbiased ChIP sequencing studies [133], indicating that many of these miRNAs are indirectly repressed.
p53
p53 is a tumor suppressor protein that plays a major role in the protection of genomic stability and prevention of tumor development by directly activating several genes, including miRNAs, which promote DNA repair, cell cycle arrest, and apoptosis. Direct binding of p53 is responsible for the activation of the miR-34 family of miRNAs [140] which, as already explained, functions as a tumor suppressor miRNA in neuroblastoma. In addition to the miR-34 family, p53 also directly regulates the transcriptional expression of other miRNAs through direct binding to their promoter, such as miR-145, miR-107, miR-192, and miR-215 [141]. Although the role of these miRNAs in neuroblastoma remains to be elucidated, other studies suggest that these miRNAs act as tumor suppressors in other forms of cancer [142–144].
Inactivating mutations or deletions in the p53 gene are found in >50 % of adult human cancers [145]. However, in neuroblastoma, this gene is seldom mutated, occurring in <2 % of cases at diagnosis, and ~15 % at relapse [146]. Nevertheless, inactivation of p53 can occur by an alternative mechanism in neuroblastoma tumors, for example, MDM2 has been reported to act as a negative regulator of p53 expression [147]. Although MYCN can promote the expression of p53 [30], over-expression of MDM2, occurring in 29 % of neuroblastomas, can counteract its effects, resulting in p53 inactivation and the aberrant expression of the p53-regulated genes [146, 148]. Taken together, this evidence suggests that inactivation of p53 in neuroblastoma can cause the deregulation of both protein-coding genes and miRNAs, resulting in the deregulation neuroblastoma related-pathways.
DNA methylation
DNA methylation consist in the addition of a methyl group 5 of the cytosine within the dinucleotide CpG. Gene silencing can occur by aberrant hypermethylation of CpG islands, which are dense clusters of CPG dinucleotides often present in gene promoters. Neuroblastoma genome displays distinct patterns of DNA methylation which can be associated with different risk groups [149]. One of the first genes reported to be differentially methylated in neuroblastoma tumors was the tumor suppressor RASSF1A, located at 3p21.3 [150]. Inactivation of RASSF1A was observed to occur in 55 % of a cohort of 67 neuroblastomas, suggesting that silencing of this tumor suppressor gene could contribute to neuroblastoma disease [150]. Another example of a commonly methylated and inactivated gene in neuroblastoma is CASP8. The CASP8 gene plays an important role in the tumor necrosis factor-related apoptosis pathway [151]. An investigation of a cohort of 70 neuroblastoma tumor samples displayed 56 % hypermethylation which was correlated to poor outcome in neuroblastoma [152]. In another study, involving clustering of a limited number of hypermethylated genes, CASP8 was found to be methylated in 77 % of the neuroblastoma cell lines investigated, further supporting the importance of the methylation status of this gene in vitro [153]. To date, more than 75 genes have been described as methylated in neuroblastoma tumors (for review see [154]), and more importantly, the methylation status of several genes has been shown to be associated with patient survival or neuroblastoma risk factors, such as MYCN amplification, patient age, and tumor stage [149, 151, 152, 155–157].
In neuroblastoma, all-trans retinoic acid (ATRA) treatment induces some neuroblastoma cell lines to differentiate, leading to profound changes in mRNA and miRNA expression [119]. In a study by Das et al. [158], DNA methylation changes were compared in SKNBE ATRA-treated versus untreated cells using methylated DNA immunoprecipitation applied to microarrays. The authors identified a total of 402 gene promoters de-methylated following ATRA treatment, while only 88 genes became hypermethylated. The demethylation events were explained in part by the down-regulation of the methyl-transferases DNTMT1 and DNTMT3 along with the upregulation of endogenous miRNAs targeting them, such as miR-152 and miR-26a/b. The question is: are miRNAs epigenetically regulated?
Similar to protein coding genes, miRNAs are susceptible to epigenetic regulation. A recent study, compiling the methylation data available from several different neoplasms revealed that in comparison to protein coding genes, miRNAs displayed a higher magnitude of methylation, with about 11.6 % of all known miRNAs being methylated [159]. However, very few studies in this area have been reported in relation to neuroblastoma disease. Recently, Das et al. [160] attempted to explore DNA methylation as a possible mechanism for the dysregulation of miRNA expression in neuroblastoma. In depth analysis of DNA methylation patterns in conjunction with miRNA and mRNA expression profiles in neuroblastoma, clinical samples allowed to identify a large set of epigenetically regulated miRNAs with significantly enriched target sites in the 3′-UTRs of genes over-expressed in unfavorable tumor subtypes. Notably, a high proportion of both the methylated miRNAs (42 %) and their associated mRNA targets (56 % of the highly redundantly targeted mRNAs) was highly associated with poor clinical outcome when under and over-expressed in tumors, respectively. The potential epigenetically regulated miRNAs included well-characterized tumor suppressor miRNAs in neuroblastoma such as some of the let-7 miRNAs, miR-29c, miR-101, miR-335, and miR-184. Importantly, many of the genes targeted by this panel of miRNAs are known to play oncogenic roles in neuroblastoma, such as AKT2, LIN28B, and CDK6, suggesting that epigenetic silencing of miRNAs could contribute to the over-expression of oncogenes in neuroblastoma.
RNA binding proteins: LIN28B
A number of proteins that regulate miRNA processing have been described as key elements in defining the characteristic expression patterns of miRNAs in different cells or during disease. RNA binding proteins (RBP) can bind to primary or precursor miRNAs to regulate their expression. It has been determined that 14 % of all human pri-miRNAs have terminal loops that are conserved throughout evolution, which may act as docks for RBPs regulating miRNA biogenesis [161]. Here, we focus on the RBP LIN28B, which has recently demonstrated to induce neuroblastoma development, and might represent a promising therapeutic target [93].
The RNA binding protein Lin28 was initially discovered in Caenorhabditis elegans as an important regulator of developmental timing. The mammalian homologs Lin28 and Lin28B are highly expressed in embryonic cells, and are responsible for maintaining the undifferentiated state of the cells [162]. Lin28 is a target of the let-7 family of miRNAs [163, 164], and as the cells start to differentiate, the let-7 levels are increased to down-regulate Lin28 expression [165, 166].
Recently, it was discovered that Lin28 blocks let-7 miRNA maturation by blocking let-7 processing at the level of DROSHA or DICER during miRNA biogenesis [167, 168]. Binding of LIN28B to the terminal loop of pre-let-7 induces the uridylation of let-7 precursor by recruiting the terminal uridylyl transferase (TUTases) to the pre-let-7 through a tetranucleotide sequence motif (GGAG), resulting in the addition of an oligouridine tail to the pre-let-7 [169–171]. Oligouridylation results in DICER blockage and degradation of the pre-miRNA by an unidentified nuclease. Lin28 can also repress DICER processing of the pri-let-7. Similarly, Lin28 recognizes the terminal loop of the pri-let-7 which inhibits DICER cleavage in vitro [164]. In addition to their role as negative regulators of the let-7 members, they are pluripotent stem cell factors, which together with three additional factors (Oct4, SOX2 and Nanog) are sufficient to reprogram somatic fibroblasts to become pluripotent stem cells [172]. The importance of LIN28 and LIN28B as regulators of the let-7 members emerged with studies demonstrating the up-regulation of LIN28 and LIN28B in several forms of cancer, including hepatocarcinoma, ovarian cancer, Wilm’s tumor, and chronic myeloid leukemia [173]. Over-expression of LIN28 and LIN28B has also been associated with cellular transformation and enhanced metastasis [173–175].
Very recently, two independent studies related LIN28B over-expression to neuroblastoma pathogenesis [92, 93]. Diskin et al. reported that SNPs in the LIN28B gene had contributed to the over-expression of LIN28B in neuroblastoma tumors. The mechanism by which LIN28B exerts its oncogenic roles was explained in the study by Molenaar et al. Consistent with the role of LIN28B being a negative regulator of let-7 expression, silencing of LIN28B in neuroblastoma cells was shown to cause the up-regulation of the let-7 members. Over-expression of LIN28B led to increased MYCN protein levels, which was explained by the fact that the let-7a was a direct post-transcriptional regulator of MYCN expression. Most importantly, this study demonstrated that MYCN was a down-stream target of LIN28 and silencing of LIN28B resulted in decreased cell viability and an increase in markers of cell differentiation. Transgenic mice expressing Lin28b in the neural crest developed the tumors characterized for the low expression of let-7, high MYCN levels, and a histology and location similar to human neuroblastomas. With these studies, LIN28B has emerged as a new oncogene in neuroblastoma and a novel therapeutic target.
Long non-coding RNAs
Compared with the extensive information available regarding miRNA, there is very little known about the expression or role of long non-coding RNAs (lncRNAs) in NB. This emerging area of research concerns RNAs of length >200 nt, which lack protein-coding features such as open-reading frames, are not translated into proteins and exert their functional role as RNA transcripts.
One sub-group of lncRNAs whose expression has been investigated to some extent in neuroblastoma is the transcribed ultra-conserved region (T-UCR), transcripts from DNA segments that are at least 200 bp in length and 100 % conserved between human, rat and mouse genomes. Four hundred and eighty-one such genomic regions have been identified [176]. Calin et al. [177] carried out the first analysis of T-UCRs in cancer, demonstrating that approximately 9 % of the 962 possible T-UCRs (sense ? antisense) were aberrantly transcribed in either carcinomas or leukemias relative to normal tissue. The authors further demonstrated the oncogenic potential of T-UC.73A by siRNA-mediated down-regulation, resulting in significantly increased apoptosis in a colorectal cancer cell line. Two neuroblastoma studies have demonstrated that analysis of UCR expression signatures can be applied to the evaluation of neuroblastoma tumors [178, 179]. Differential UCR expression profiles are associated with outcome in short-term versus long-term survivors with high-risk, stage 4 neuroblastoma [179]. In addition, Mestdagh et al. [178] found an expression signature of up-regulated T-UCRs in MNA compared to non-MNA tumors.
Genetic aberrations such as chromosomal gains and losses can contribute to the over- or under-expression, respectively, of transcripts encoded in these regions. A predominant unfavorable prognostic factor in neuroblastoma is the gain of chromosome arm 17q [3, 68]. The 2.3 kb RNA ncRAN (non-coding RNA expressed in aggressive neuroblastoma) is one such transcript mapped to 17q, whose over-expression is present in neuroblastomas with partial gain of 17q, but interestingly not present in those with whole chromosome 17 gain [180]. The oncogenic potential of this transcript was shown by siRNA knockdown of ncRAN in SH-SY5Y neuroblastoma cells, and ectopic over-expression in NIH3T3 mouse fibroblast cells, resulting in significantly inhibited cell growth and an increase in anchorage-dependent cell growth, respectively [180].
Differentially expressed in neuroblastoma (DEIN) is a non-coding transcript (mapping to 4q33–34) whose expression shows no significant association with the common neuroblastoma aberrations of MYCN amplification, 1p/3p/11q deletion or 17q gain. However, it is highly expressed in stage 4S tumors compared with localized stage 1, 3, and 4 tumors and expression is significantly associated with event-free survival, although not an independent prognostic marker [181].
The lack of information regarding the identification of new lncRNAs and their putative functions is due in part to the fact that they do not exhibit the same level of conservation as protein-coding genes. Consequently, it is not possible to assign possible function through sequence similarity, as can be done with some protein-coding genes. In addition, determining that a transcript does not code for a protein is a complex process. Moreover, studies indicate that certain transcripts can function at both RNA and protein level [182, 183]. However, with the evidence amassed so far, and the identification of functional roles for certain lncRNAs in the regulation of processes such as DNA methylation or apoptosis [177, 184, 185], it is clear that these RNAs can play important roles in both normal and pathological physiology and represent a challenging new area of research in both normal development and disease.
Conclusions
Since the early 1980’s, we have progressed significantly in our ability to diagnose, stratify, and treat neuroblastoma patients. Risk classification continues to be optimized, and clearly future approaches will need to integrate profiling of mRNA and microRNA expression, epigenetic modifications, and whole genome copy number variations with the current INRG system. This will require advances in technology which will allow us to screen patients in a time-effective, cost efficient manner. In parallel, novel therapeutics are being developed to target key regulators of the neuroblastoma genome and more refined treatment regimens are being designed based on our increasing knowledge of the pathogenesis of the disease. This progress is due greatly to our increased understanding of the fundamental genetic alterations associated with tumor behavior and patient outcomes.
Contributor Information
Raquel Domingo-Fernandez, Department of Cancer Genetics, Royal College of Surgeons in Ireland, Dublin, Ireland; Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland.
Karen Watters, Department of Cancer Genetics, Royal College of Surgeons in Ireland, Dublin, Ireland; Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland.
Olga Piskareva, Department of Cancer Genetics, Royal College of Surgeons in Ireland, Dublin, Ireland; Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland.
Raymond L. Stallings, Email: rstallings@rcsi.ie, Department of Cancer Genetics, Royal College of Surgeons in Ireland, Dublin, Ireland; Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland.
Isabella Bray, Department of Cancer Genetics, Royal College of Surgeons in Ireland, Dublin, Ireland; Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland.
References
- 1.Spix C, et al. Neuroblastoma incidence and survival in European children (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2081–2091. doi: 10.1016/j.ejca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Gurney JG, et al. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75(8):2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 5.Mosse YP, et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet. 2004;75(4):727–730. doi: 10.1086/424530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosse YP, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455(7215):930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455(7215):971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 8.Stallings RL, et al. High-resolution analysis of chromosomal breakpoints and genomic instability identifies PTPRD as a candidate tumor suppressor gene in neuroblastoma. Cancer Res. 2006;66(7):3673–3680. doi: 10.1158/0008-5472.CAN-05-4154. [DOI] [PubMed] [Google Scholar]
- 9.Molenaar JJ, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483(7391):589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 10.Asgharzadeh S, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98(17):1193–1203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen J, et al. Predicting outcomes for children with neuroblastoma using a multigene-expression signature: a retrospective SIOPEN/COG/GPOH study. Lancet Oncol. 2009;10(7):663–671. doi: 10.1016/S1470-2045(09)70154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Preter K, et al. Accurate outcome prediction in neuroblastoma across independent data sets using a multigene signature. Clin Cancer Res. 2010;16(5):1532–1541. doi: 10.1158/1078-0432.CCR-09-2607. [DOI] [PubMed] [Google Scholar]
- 13.Oberthuer A, et al. Subclassification and individual survival time prediction from gene expression data of neuroblastoma patients by using CASPAR. Clin Cancer Res. 2008;14(20):6590–6601. doi: 10.1158/1078-0432.CCR-07-4377. [DOI] [PubMed] [Google Scholar]
- 14.Ohira M, Nakagawara A. Global genomic and RNA profiles for novel risk stratification of neuroblastoma. Cancer Sci. 2010;101(11):2295–2301. doi: 10.1111/j.1349-7006.2010.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 2007;67(3):976–983. doi: 10.1158/0008-5472.CAN-06-3667. [DOI] [PubMed] [Google Scholar]
- 16.Bray I, et al. Widespread dysregulation of MiRNAs by MYCN amplification and chromosomal imbalances in neuro-blastoma: association of miRNA expression with survival. PLoS One. 2009;4(11):e7850. doi: 10.1371/journal.pone.0007850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn SL, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko Y, et al. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res. 1987;47(1):311–318. [PubMed] [Google Scholar]
- 19.Brodeur GM, Nakagawara A. Molecular basis of clinical heterogeneity in neuroblastoma. Am J Pediatr Hematol Oncol. 1992;14(2):111–116. doi: 10.1097/00043426-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko Y, Knudson AG. Mechanism and relevance of ploidy in neuroblastoma. Genes Chromosomes Cancer. 2000;29(2):89–95. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1021>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Brodeur GM, et al. Biology and genetics of human neuroblastomas. J Pediatr Hematol Oncol. 1997;19(2):93–101. doi: 10.1097/00043426-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Schleiermacher G, et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012;107(8):1418–1422. doi: 10.1038/bjc.2012.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeger RC, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 24.Lutz W, et al. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13(4):803–812. [PubMed] [Google Scholar]
- 25.Schweigerer L, et al. Augmented MYCN expression advances the malignant phenotype of human neuroblastoma cells: evidence for induction of autocrine growth factor activity. Cancer Res. 1990;50(14):4411–4416. [PubMed] [Google Scholar]
- 26.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16(20):2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannini G, et al. High mobility group A1 is a molecular target for MYCN in human neuroblastoma. Cancer Res. 2005;65(18):8308–8316. doi: 10.1158/0008-5472.CAN-05-0607. [DOI] [PubMed] [Google Scholar]
- 28.Shohet JM, et al. Minichromosome maintenance protein MCM7 is a direct target of the MYCN transcription factor in neuroblastoma. Cancer Res. 2002;62(4):1123–1128. [PubMed] [Google Scholar]
- 29.Slack A, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci USA. 2005;102(3):731–736. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, et al. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer Res. 2010;70(4):1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manohar CF, et al. MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma. Oncogene. 2004;23(3):753–762. doi: 10.1038/sj.onc.1207151. [DOI] [PubMed] [Google Scholar]
- 32.Weiss WA, et al. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16(11):2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edsjo A, et al. Neuroblastoma cells with overexpressed MYCN retain their capacity to undergo neuronal differentiation. Lab Invest. 2004;84(4):406–417. doi: 10.1038/labinvest.3700061. [DOI] [PubMed] [Google Scholar]
- 34.Chan HS, et al. MYCN protein expression as a predictor of neuroblastoma prognosis. Clin Cancer Res. 1997;3(10):1699–1706. [PubMed] [Google Scholar]
- 35.Valentijn LJ, et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci USA. 2012;109(47):19190–19195. doi: 10.1073/pnas.1208215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodeur GM, Fong CT. Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet. 1989;41(2):153–174. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- 37.Brodeur GM, et al. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- 38.Fong CT, et al. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci USA. 1989;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attiyeh EF, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353(21):2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 40.Martinsson T, et al. Delimitation of a critical tumour suppressor region at distal 1p in neuroblastoma tumours. Eur J Cancer. 1997;33(12):1997–2001. doi: 10.1016/s0959-8049(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 41.Bauer A, et al. Smallest region of overlapping deletion in 1p36 in human neuroblastoma: a 1 Mbp cosmid and PAC contig. Genes Chromosomes Cancer. 2001;31(3):228–239. doi: 10.1002/gcc.1139. [DOI] [PubMed] [Google Scholar]
- 42.Ohira M, et al. Identification and characterization of a 500-kb homozygously deleted region at 1p36.2-p36.3 in a neuroblastoma cell line. Oncogene. 2000;19(37):4302–4307. doi: 10.1038/sj.onc.1203786. [DOI] [PubMed] [Google Scholar]
- 43.Thompson PM, et al. CHD5, a new member of the chromodomain gene family, is preferentially expressed in the nervous system. Oncogene. 2003;22(7):1002–1011. doi: 10.1038/sj.onc.1206211. [DOI] [PubMed] [Google Scholar]
- 44.Fujita T, et al. CHD5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J Natl Cancer Inst. 2008;100(13):940–949. doi: 10.1093/jnci/djn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyama H, et al. Mechanisms of CHD5 inactivation in neuroblastomas. Clin Cancer Res. 2012;18(6):1588–1597. doi: 10.1158/1078-0432.CCR-11-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henrich KO, et al. Reduced expression of CAMTA1 correlates with adverse outcome in neuroblastoma patients. Clin Cancer Res. 2006;12(1):131–138. doi: 10.1158/1078-0432.CCR-05-1431. [DOI] [PubMed] [Google Scholar]
- 47.Henrich KO, et al. CAMTA1, a 1p36 tumor suppressor candidate, inhibits growth and activates differentiation programs in neuroblastoma cells. Cancer Res. 2011;71(8):3142–3151. doi: 10.1158/0008-5472.CAN-10-3014. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, et al. CASZ1, a candidate tumor-suppressor gene, suppresses neuroblastoma tumor growth through reprogramming gene expression. Cell Death Differ. 2011;18(7):1174–1183. doi: 10.1038/cdd.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C, et al. EZH2 mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012;72(1):315–324. doi: 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krona C, et al. Screening for gene mutations in a 500 kb neuroblastoma tumor suppressor candidate region in chromosome 1p; mutation and stage-specific expression in UBE4B/ UFD2. Oncogene. 2003;22(15):2343–2351. doi: 10.1038/sj.onc.1206324. [DOI] [PubMed] [Google Scholar]
- 51.Krona C, et al. A novel 1p36.2 located gene, APITD1, with tumour-suppressive properties and a putative p53-binding domain, shows low expression in neuroblastoma tumours. Br J Cancer. 2004;91(6):1119–1130. doi: 10.1038/sj.bjc.6602083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo C, et al. Allelic deletion at 11q23 is common in MYCN single copy neuroblastomas. Oncogene. 1999;18(35):4948–4957. doi: 10.1038/sj.onc.1202887. [DOI] [PubMed] [Google Scholar]
- 53.Plantaz D, et al. Comparative genomic hybridization (CGH) analysis of stage 4 neuroblastoma reveals high frequency of 11q deletion in tumors lacking MYCN amplification. Int J Cancer. 2001;91(5):680–686. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1114>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 54.Maris JM, et al. Allelic deletion at chromosome bands 11q14-23 is common in neuroblastoma. Med Pediatr Oncol. 2001;36(1):24–27. doi: 10.1002/1096-911X(20010101)36:1<24::AID-MPO1007>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Spitz R, et al. Loss in chromosome 11q identifies tumors with increased risk for metastatic relapses in localized and 4S neuroblastoma. Clin Cancer Res. 2006;12(11 Pt 1):3368–3373. doi: 10.1158/1078-0432.CCR-05-2495. [DOI] [PubMed] [Google Scholar]
- 56.Michels E, et al. ArrayCGH-based classification of neuroblastoma into genomic subgroups. Genes Chromosomes Cancer. 2007;46(12):1098–1108. doi: 10.1002/gcc.20496. [DOI] [PubMed] [Google Scholar]
- 57.Luttikhuis ME, et al. Neuroblastomas with chromosome 11q loss and single copy MYCN comprise a biologically distinct group of tumours with adverse prognosis. Br J Cancer. 2001;85(4):531–537. doi: 10.1054/bjoc.2001.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caren H, et al. High-risk neuroblastoma tumors with 11q–deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc Natl Acad Sci USA. 2010;107(9):4323–4328. doi: 10.1073/pnas.0910684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Celeste A, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114(3):371–383. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nowacki S, et al. Expression of the tumour suppressor gene CADM1 is associated with favourable outcome and inhibits cell survival in neuroblastoma. Oncogene. 2008;27(23):3329–3338. doi: 10.1038/sj.onc.1210996. [DOI] [PubMed] [Google Scholar]
- 61.Michels E, et al. CADM1 is a strong neuroblastoma candidate gene that maps within a 3.72 Mb critical region of loss on 11q23. BMC Cancer. 2008;8:173. doi: 10.1186/1471-2407-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ando K, et al. Expression of TSLC1, a candidate tumor suppressor gene mapped to chromosome 11q23, is downregulated in unfavorable neuroblastoma without promoter hypermethylation. Int J Cancer. 2008;123(9):2087–2094. doi: 10.1002/ijc.23776. [DOI] [PubMed] [Google Scholar]
- 63.Bown NP, Pearson AD, Reid MM. High incidence of constitutional balanced translocations in neuroblastoma. Cancer Genet Cytogenet. 1993;69(2):166–167. doi: 10.1016/0165-4608(93)90100-z. [DOI] [PubMed] [Google Scholar]
- 64.Koiffmann CP, et al. Neuroblastoma in a boy with MCA/ MR syndrome, deletion 11q, and duplication 12q. Am J Med Genet. 1995;58(1):46–49. doi: 10.1002/ajmg.1320580110. [DOI] [PubMed] [Google Scholar]
- 65.Schulte JH, et al. Accurate prediction of neuroblastoma outcome based on miRNA expression profiles. Int J Cancer. 2010;127(10):2374–2385. doi: 10.1002/ijc.25436. [DOI] [PubMed] [Google Scholar]
- 66.Meddeb M, et al. Additional copies of a 25 Mb chromosomal region originating from 17q23.1-17qter are present in 90 % of high-grade neuroblastomas. Genes Chromosomes Cancer. 1996;17(3):156–165. doi: 10.1002/(SICI)1098-2264(199611)17:3<156::AID-GCC3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 67.Caron H. Allelic loss of chromosome 1 and additional chromosome 17 material are both unfavourable prognostic markers in neuroblastoma. Med Pediatr Oncol. 1995;24(4):215–221. doi: 10.1002/mpo.2950240402. [DOI] [PubMed] [Google Scholar]
- 68.Bown N, et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med. 1999;340(25):1954–1961. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- 69.Van Roy N, et al. Molecular cytogenetic analysis of 1;17 translocations in neuroblastoma. Eur J Cancer. 1995;31A(4):530–535. doi: 10.1016/0959-8049(95)00004-3. [DOI] [PubMed] [Google Scholar]
- 70.Lastowska M, et al. Promiscuous translocations of chromosome arm 17q in human neuroblastomas. Genes Chromosomes Cancer. 1997;19(3):143–149. [PubMed] [Google Scholar]
- 71.Savelyeva L, Corvi R, Schwab M. Translocation involving 1p and 17q is a recurrent genetic alteration of human neuroblastoma cells. Am J Hum Genet. 1994;55(2):334–340. [PMC free article] [PubMed] [Google Scholar]
- 72.McConville CM, et al. Molecular cytogenetic characterization of two non-MYCN amplified neuroblastoma cell lines with complex t(11;17) Cancer Genet Cytogenet. 2001;130(2):133–140. doi: 10.1016/s0165-4608(01)00480-0. [DOI] [PubMed] [Google Scholar]
- 73.Stark B, et al. der(11)t(11;17): a distinct cytogenetic pathway of advanced stage neuroblastoma (NBL)—detected by spectral karyotyping (SKY) Cancer Lett. 2003;197(1–2):75–79. doi: 10.1016/s0304-3835(03)00083-1. [DOI] [PubMed] [Google Scholar]
- 74.Stallings RL, et al. Molecular cytogenetic analysis of recurrent unbalanced t(11;17) in neuroblastoma. Cancer Genet Cytogenet. 2004;154(1):44–51. doi: 10.1016/j.cancergencyto.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Schleiermacher G, et al. Variety and complexity of chromosome 17 translocations in neuroblastoma. Genes Chromosomes Cancer. 2004;39(2):143–150. doi: 10.1002/gcc.10313. [DOI] [PubMed] [Google Scholar]
- 76.Spitz R, et al. Gain of distal chromosome arm 17q is not associated with poor prognosis in neuroblastoma. Clin Cancer Res. 2003;9(13):4835–4840. [PubMed] [Google Scholar]
- 77.Brinkschmidt C, et al. Comparative genomic hybridization (CGH) analysis of neuroblastomas—an important methodological approach in paediatric tumour pathology. J Pathol. 1997;181(4):394–400. doi: 10.1002/(SICI)1096-9896(199704)181:4<394::AID-PATH800>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 78.Buckley PG, et al. Chromosomal and microRNA expression patterns reveal biologically distinct subgroups of 11qneuroblastoma. Clin Cancer Res. 2010;16(11):2971–2978. doi: 10.1158/1078-0432.CCR-09-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Claviez A, et al. Low occurrence of familial neuroblastomas and ganglioneuromas in five consecutive GPOH neuroblastoma treatment studies. Eur J Cancer. 2004;40(18):2760–2765. doi: 10.1016/j.ejca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Maris JM, et al. Molecular genetic analysis of familial neuroblastoma. Eur J Cancer. 1997;33(12):1923–1928. doi: 10.1016/s0959-8049(97)00265-7. [DOI] [PubMed] [Google Scholar]
- 81.Pattyn A, et al. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000;127(7):1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- 82.Raabe EH, et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene. 2008;27(4):469–476. doi: 10.1038/sj.onc.1210659. [DOI] [PubMed] [Google Scholar]
- 83.van Limpt V, et al. The Phox2B homeobox gene is mutated in sporadic neuroblastomas. Oncogene. 2004;23(57):9280–9288. doi: 10.1038/sj.onc.1208157. [DOI] [PubMed] [Google Scholar]
- 84.Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2(6):495–502. doi: 10.1158/2159-8290.CD-12-0009. [DOI] [PubMed] [Google Scholar]
- 85.Azarova AM, Gautam G, George RE. Emerging importance of ALK in neuroblastoma. Semin Cancer Biol. 2011;21(4):267–275. doi: 10.1016/j.semcancer.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heukamp LC, et al. Targeted Expression of Mutated ALK Induces Neuroblastoma in Transgenic Mice. Sci Transl Med. 2012;4(141) doi: 10.1126/scitranslmed.3003967. 141ra91. [DOI] [PubMed] [Google Scholar]
- 87.Capasso M, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41(6):718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang K, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469(7329):216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maris JM, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358(24):2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu LC, et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14(4):430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 91.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144(1):27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diskin SJ, et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet. 2012;44(10):1126–1130. doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molenaar JJ, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44(11):1199–1206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 94.Fischer M, et al. Differential expression of neuronal genes defines subtypes of disseminated neuroblastoma with favorable and unfavorable outcome. Clin Cancer Res. 2006;12(17):5118–5128. doi: 10.1158/1078-0432.CCR-06-0985. [DOI] [PubMed] [Google Scholar]
- 95.Berwanger B, et al. Loss of a FYN-regulated differentiation and growth arrest pathway in advanced stage neuroblastoma. Cancer Cell. 2002;2(5):377–386. doi: 10.1016/s1535-6108(02)00179-4. [DOI] [PubMed] [Google Scholar]
- 96.Oberthuer A, et al. Customized oligonucleotide micro-array gene expression-based classification of neuroblastoma patients outperforms current clinical risk stratification. J Clin Oncol. 2006;24(31):5070–5078. doi: 10.1200/JCO.2006.06.1879. [DOI] [PubMed] [Google Scholar]
- 97.Ohira M, et al. Expression profiling using a tumor-specific cDNA microarray predicts the prognosis of intermediate risk neuroblastomas. Cancer Cell. 2005;7(4):337–350. doi: 10.1016/j.ccr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Wang Q, et al. Integrative genomics identifies distinct molecular classes of neuroblastoma and shows that multiple genes are targeted by regional alterations in DNA copy number. Cancer Res. 2006;66(12):6050–6062. doi: 10.1158/0008-5472.CAN-05-4618. [DOI] [PubMed] [Google Scholar]
- 99.Abel F, et al. A 6-gene signature identifies four molecular subgroups of neuroblastoma. Cancer Cell Int. 2011;11:9. doi: 10.1186/1475-2867-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fischer M, et al. Integrated genomic profiling identifies two distinct molecular subtypes with divergent outcome in neuroblastoma with loss of chromosome 11q. Oncogene. 2009;29:865–875. doi: 10.1038/onc.2009.390. [DOI] [PubMed] [Google Scholar]
- 101.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 102.Hebert SS, De Strooper B. Molecular biology. miRNAs in neurodegeneration. Science. 2007;317(5842):1179–1180. doi: 10.1126/science.1148530. [DOI] [PubMed] [Google Scholar]
- 103.Iorio MV, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 104.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15(5):563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Kapsimali M, et al. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8(8):R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 107.Schulte JH, et al. MYCN regulates oncogenic microRNAs in neuroblastoma. Int J Cancer. 2008;122(3):699–704. doi: 10.1002/ijc.23153. [DOI] [PubMed] [Google Scholar]
- 108.Fontana L, et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3(5):e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mestdagh P, et al. The miR-17-92 microRNA cluster regulates multiple components of the TGF-beta pathway in neuroblastoma. Mol Cell. 2010;40(5):762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Brouwer S, et al. Dickkopf-3 is regulated by the MYCN-induced miR-17-92 cluster in neuroblastoma. Int J Cancer. 2012;130(11):2591–2598. doi: 10.1002/ijc.26295. [DOI] [PubMed] [Google Scholar]
- 111.Buechner J, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105(2):296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26(34):5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 113.Tivnan A, et al. MicroRNA-34a is a potent tumor suppressor molecule in vivo in neuroblastoma. BMC Cancer. 2011;11:33. doi: 10.1186/1471-2407-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cole KA, et al. A functional screen identifies miR-34a as a candidate neuroblastoma tumor suppressor gene. Mol Cancer Res. 2008;6(5):735–742. doi: 10.1158/1541-7786.MCR-07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch J, et al. MiRNA-335 suppresses neuroblastoma cell invasiveness by direct targeting of multiple genes from the noncanonical TGF-beta signalling pathway. Carcinogenesis. 2012;33(5):976–985. doi: 10.1093/carcin/bgs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bray I, et al. MicroRNA-542-5p as a novel tumor suppressor in neuroblastoma. Cancer Lett. 2011;303(1):56–64. doi: 10.1016/j.canlet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laneve P, et al. The interplay between microRNAs and the neurotrophin receptor tropomyosin-related kinase C controls proliferation of human neuroblastoma cells. Proc Natl Acad Sci USA. 2007;104(19):7957–7962. doi: 10.1073/pnas.0700071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Le MT, et al. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol. 2009;29(19):5290–5305. doi: 10.1128/MCB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Foley NH, et al. MicroRNAs 10a and 10b are potent inducers of neuroblastoma cell differentiation through targeting of nuclear receptor corepressor 2. Cell Death Differ. 2011;18(7):1089–1098. doi: 10.1038/cdd.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ryan J, et al. MicroRNA-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favourable clinical outcome. Br J Cancer. 2012;107(6):967–976. doi: 10.1038/bjc.2012.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calin GA, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang TC, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei JS, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27(39):5204–5213. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tivnan A, et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialogan-glioside GD2 coated nanoparticles. PLoS One. 2012;7(5):e38129. doi: 10.1371/journal.pone.0038129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bommer GT, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17(15):1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 126.Sun F, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582(10):1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 127.Atroshchenko ES, et al. Effect of xanthinol niacinate on the autoimmunity and capillary permeability in patients with stable stenocardia. Kardiologiia. 1991;31(3):18–21. [PubMed] [Google Scholar]
- 128.Pang RT, et al. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis. 2010;31(6):1037–1044. doi: 10.1093/carcin/bgq066. [DOI] [PubMed] [Google Scholar]
- 129.Brodeur GM, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6(12):1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 130.Mestdagh P, et al. MYCN/c-MYC-induced microRNAs repress coding gene networks associated with poor outcome in MYCN/c-MYC-activated tumors. Oncogene. 2010;29(9):1394–1404. doi: 10.1038/onc.2009.429. [DOI] [PubMed] [Google Scholar]
- 131.Murphy DM, et al. Global MYCN transcription factor binding analysis in neuroblastoma reveals association with distinct E-box motifs and regions of DNA hypermethylation. PLoS One. 2009;4(12):e8154. doi: 10.1371/journal.pone.0008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Corcoran DL, et al. Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS One. 2009;4(4):e5279. doi: 10.1371/journal.pone.0005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shohet JM, et al. A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma. Cancer Res. 2011;71(11):3841–3851. doi: 10.1158/0008-5472.CAN-10-4391. [DOI] [PubMed] [Google Scholar]
- 134.Hayashita Y, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65(21):9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 135.Busacca S, et al. MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol. 2009;42:312–319. doi: 10.1165/rcmb.2009-0060OC. [DOI] [PubMed] [Google Scholar]
- 136.Yu Z, et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182(3):509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mraz M, et al. MicroRNAs in chronic lymphocytic leukemia pathogenesis and disease subtypes. Leuk Lymphoma. 2009;50(3):506–509. doi: 10.1080/10428190902763517. [DOI] [PubMed] [Google Scholar]
- 138.Takakura S, et al. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008;99(6):1147–1154. doi: 10.1111/j.1349-7006.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Donnell KA, et al. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 140.He L, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Braun CJ, et al. p53-Responsive microRNAs 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68(24):10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sachdeva M, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106(9):3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Georges SA, et al. Coordinated regulation of cell cycle transcripts by p53-inducible microRNAs, miR-192 and miR-215. Cancer Res. 2008;68(24):10105–10112. doi: 10.1158/0008-5472.CAN-08-1846. [DOI] [PubMed] [Google Scholar]
- 144.Feng Z, et al. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3(1):44–50. doi: 10.1093/jmcb/mjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 146.Carr-Wilkinson J, et al. High frequency of p53/MDM2/ p14ARF pathway abnormalities in relapsed neuroblastoma. Clin Cancer Res. 2010;16(4):1108–1118. doi: 10.1158/1078-0432.CCR-09-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Momand J, et al. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 148.Slack A, Shohet JM. MDM2 as a critical effector of the MYCN oncogene in tumorigenesis. Cell Cycle. 2005;4(7):857–860. doi: 10.4161/cc.4.7.1790. [DOI] [PubMed] [Google Scholar]
- 149.Alaminos M, et al. Clustering of gene hypermethylation associated with clinical risk groups in neuroblastoma. J Natl Cancer Inst. 2004;96(16):1208–1219. doi: 10.1093/jnci/djh224. [DOI] [PubMed] [Google Scholar]
- 150.Astuti D, et al. RASSF1A promoter region CpG island hypermethylation in phaeochromocytomas and neuroblastoma tumours. Oncogene. 2001;20(51):7573–7577. doi: 10.1038/sj.onc.1204968. [DOI] [PubMed] [Google Scholar]
- 151.Banelli B, et al. Expression and methylation of CASP8 in neuroblastoma: identification of a promoter region. Nat Med. 2002;8(12):1333–1335. doi: 10.1038/nm1202-1333. (author reply 1335) [DOI] [PubMed] [Google Scholar]
- 152.Yang Q, et al. Methylation of CASP8, DCR2, and HIN-1 in neuroblastoma is associated with poor outcome. Clin Cancer Res. 2007;13(11):3191–3197. doi: 10.1158/1078-0432.CCR-06-2846. [DOI] [PubMed] [Google Scholar]
- 153.van Noesel MM. Clustering of hypermethylated genes in neuroblastoma. Genes Chromosomes Cancer. 2003;38(3):226–233. doi: 10.1002/gcc.10278. [DOI] [PubMed] [Google Scholar]
- 154.Decock A, et al. Neuroblastoma epigenetics: from candidate gene approaches to genome-wide screenings. Epigenetics. 2011;6(8):962–970. doi: 10.4161/epi.6.8.16516. [DOI] [PubMed] [Google Scholar]
- 155.Grau E, et al. Hypermethylation of apoptotic genes as independent prognostic factor in neuroblastoma disease. Mol Carcinog. 2011;50(3):153–162. doi: 10.1002/mc.20700. [DOI] [PubMed] [Google Scholar]
- 156.Michalowski MB, et al. Methylation of tumor-suppressor genes in neuroblastoma: the RASSF1A gene is almost always methylated in primary tumors. Pediatr Blood Cancer. 2008;50(1):29–32. doi: 10.1002/pbc.21279. [DOI] [PubMed] [Google Scholar]
- 157.Yagyu S, et al. Circulating methylated-DCR2 gene in serum as an indicator of prognosis and therapeutic efficacy in patients with MYCN nonamplified neuroblastoma. Clin Cancer Res. 2008;14(21):7011–7019. doi: 10.1158/1078-0432.CCR-08-1249. [DOI] [PubMed] [Google Scholar]
- 158.Das S, et al. MicroRNA mediates DNA demethylation events triggered by retinoic acid during neuroblastoma cell differentiation. Cancer Res. 2010;70(20):7874–7881. doi: 10.1158/0008-5472.CAN-10-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kunej T, et al. Epigenetic regulation ofmicroRNAs in cancer: an integrated review of literature. Mutat Res. 2011;717(1–2):77–84. doi: 10.1016/j.mrfmmm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 160.Das S, et al. Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene. 2012 doi: 10.1038/onc.2012.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Michlewski G, et al. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell. 2008;32(3):383–393. doi: 10.1016/j.molcel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in, C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88(5):637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 163.Boyerinas B, et al. Identification of let-7-regulated onco-fetal genes. Cancer Res. 2008;68(8):2587–2591. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 164.Rybak A, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10(8):987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 165.Richards M, et al. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22(1):51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 166.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320(5872):97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14(8):1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Piskounova E, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147(5):1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heo I, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138(4):696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 170.Lehrbach NJ, et al. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol. 2009;16(10):1016–1020. doi: 10.1038/nsmb.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol. 2009;16(10):1021–1025. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 173.Viswanathan SR, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41(7):843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.King CE, et al. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71(12):4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bejerano G, et al. Ultraconserved elements in the human genome. Science. 2004;304(5675):1321–1325. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 177.Calin GA, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12(3):215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 178.Mestdagh P, et al. An integrative genomics screen uncovers ncRNA T-UCR functions in neuroblastoma tumours. Oncogene. 2010;29(24):3583–3592. doi: 10.1038/onc.2010.106. [DOI] [PubMed] [Google Scholar]
- 179.Scaruffi P, et al. Transcribed-ultra conserved region expression is associated with outcome in high-risk neuroblastoma. BMC Cancer. 2009;9:441. doi: 10.1186/1471-2407-9-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu M, et al. High expression of ncRAN, a novel non-coding RNA mapped to chromosome 17q25.1, is associated with poor prognosis in neuroblastoma. Int J Oncol. 2009;34(4):931–938. doi: 10.3892/ijo_00000219. [DOI] [PubMed] [Google Scholar]
- 181.Voth H, et al. Identification of DEIN, a novel gene with high expression levels in stage IVS neuroblastoma. Mol Cancer Res. 2007;5(12):1276–1284. doi: 10.1158/1541-7786.MCR-06-0258. [DOI] [PubMed] [Google Scholar]
- 182.Chooniedass-Kothari S, et al. The steroid receptor RNA activator is the first functional RNA encoding a protein. FEBS Lett. 2004;566(1–3):43–47. doi: 10.1016/j.febslet.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 183.Candeias MM, et al. P53 mRNA controls p53 activity by managing Mdm2 functions. Nat Cell Biol. 2008;10(9):1098–1105. doi: 10.1038/ncb1770. [DOI] [PubMed] [Google Scholar]
- 184.Gupta RA, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rinn JL, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abel F, et al. Mutations in the N-terminal domain of DFF45 in a primary germ cell tumor and in neuroblastoma tumors. Int J Oncol. 2004;25(5):1297–1302. [PubMed] [Google Scholar]
- 187.le Nguyen B, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility loci. PLoS Genet. 2011;7(3):e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Duijkers FA, et al. High anaplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglio-neuroblastoma is an independent predictor of poor outcome. Am J Pathol. 2012;180(3):1223–1231. doi: 10.1016/j.ajpath.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 189.Bourdeaut F, et al. ALK germline mutations in patients with neuroblastoma: a rare and weakly penetrant syndrome. Eur J Hum Genet. 2012;20(3):291–297. doi: 10.1038/ejhg.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.de Pontual L, et al. Germline gain-of-function mutations of ALK disrupt central nervous system development. Hum Mutat. 2011;32(3):272–276. doi: 10.1002/humu.21442. [DOI] [PubMed] [Google Scholar]
- 191.Devoto M, et al. Genome-wide linkage analysis to identify genetic modifiers of ALK mutation penetrance in familial neuroblastoma. Hum Hered. 2011;71(2):135–139. doi: 10.1159/000324843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Passoni L, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69(18):7338–7346. doi: 10.1158/0008-5472.CAN-08-4419. [DOI] [PubMed] [Google Scholar]
- 193.Caren H, et al. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem J. 2008;416(2):153–159. doi: 10.1042/bj20081834. [DOI] [PubMed] [Google Scholar]
- 194.Janoueix-Lerosey I, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455(7215):967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 195.George RE, et al. Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS One. 2007;2(2):e255. doi: 10.1371/journal.pone.0000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schulte JH, et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin Cancer Res. 2011;17(15):5082–5092. doi: 10.1158/1078-0432.CCR-10-2809. [DOI] [PubMed] [Google Scholar]
- 197.Shukla N, et al. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin Cancer Res. 2012;18(3):748–757. doi: 10.1158/1078-0432.CCR-11-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Perri P, et al. PHOX2B mutations and genetic predisposition to neuroblastoma. Oncogene. 2005;24(18):3050–3053. doi: 10.1038/sj.onc.1208532. [DOI] [PubMed] [Google Scholar]
- 199.Bourdeaut F, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Cancer Lett. 2005;228(1–2):51–58. doi: 10.1016/j.canlet.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 200.McConville C, et al. PHOX2B analysis in non-syndromic neuroblastoma cases shows novel mutations and genotype-phenotype associations. Am J Med Genet A. 2006;140(12):1297–1301. doi: 10.1002/ajmg.a.31278. [DOI] [PubMed] [Google Scholar]
- 201.Ghiorzo P, et al. Impact of E27X, a novel CDKN2A germ line mutation, on p16 and p14ARF expression in Italian melanoma families displaying pancreatic cancer and neuroblastoma. Hum Mol Genet. 2006;15(18):2682–2689. doi: 10.1093/hmg/ddl199. [DOI] [PubMed] [Google Scholar]
- 202.Obana K, et al. Aberrations of p16INK4A, p14ARF and p15INK4B genes in pediatric solid tumors. Int J Oncol. 2003;23(4):1151–1157. [PubMed] [Google Scholar]
- 203.Caren H, et al. High-resolution array copy number analyses for detection of deletion, gain, amplification and copyneutral LOH in primary neuroblastoma tumors: four cases of homozygous deletions of the CDKN2A gene. BMC Genomics. 2008;9:353. doi: 10.1186/1471-2164-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Omura-Minamisawa M, et al. p16/p14(ARF) cell cycle regulatory pathways in primary neuroblastoma: p16 expression is associated with advanced stage disease. Clin Cancer Res. 2001;7(11):3481–3490. [PubMed] [Google Scholar]
- 205.Molenaar JJ, et al. Rearrangements and increased expression of cyclin D1 (CCND1) in neuroblastoma. Genes Chromosomes Cancer. 2003;36(3):242–249. doi: 10.1002/gcc.10166. [DOI] [PubMed] [Google Scholar]
- 206.Martinelli S, et al. Activating PTPN11 mutations play a minor role in pediatric and adult solid tumors. Cancer Genet Cytogenet. 2006;166(2):124–129. doi: 10.1016/j.cancergencyto.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 207.Mutesa L, et al. Germline PTPN11 missense mutation in a case of Noonan syndrome associated with mediastinal and retroperitoneal neuroblastic tumors. Cancer Genet Cytogenet. 2008;182(1):40–42. doi: 10.1016/j.cancergencyto.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 208.Origone P, et al. Homozygous inactivation of NF1 gene in a patient with familial NF1 and disseminated neuroblastoma. Am J Med Genet A. 2003;118A(4):309–313. doi: 10.1002/ajmg.a.10167. [DOI] [PubMed] [Google Scholar]
- 209.Kong XT, et al. Expression and mutational analysis of the DCC, DPC4, and MADR2/JV18-1 genes in neuroblastoma. Cancer Res. 1997;57(17):3772–3778. [PubMed] [Google Scholar]
- 210.Foley NH, et al. MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2. Mol Cancer. 2010;9:83. doi: 10.1186/1476-4598-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Le MT, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23(7):862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Evangelisti C, et al. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J. 2009;23(12):4276–4287. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- 213.Lin RJ, Lin YC, Yu AL. miR-149* induces apoptosis by inhibiting Akt1 and E2F1 in human cancer cells. Mol Carcinog. 2010;49(8):719–727. doi: 10.1002/mc.20647. [DOI] [PubMed] [Google Scholar]
- 214.Chen H, et al. miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development and embryonic stem cells differentiation, and control neurite outgrowth in vitro. Biochem Biophys Res Commun. 2010;394(4):921–927. doi: 10.1016/j.bbrc.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 215.Meseguer S, et al. MicroRNAs-10a and-10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ ASF) J Biol Chem. 2011;286(6):4150–4164. doi: 10.1074/jbc.M110.167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Haug BH, et al. MYCN-regulated miRNA-92 inhibits secretion of the tumor suppressor DICKKOPF-3 (DKK3) in neuroblastoma. Carcinogenesis. 2011;32(7):1005–1012. doi: 10.1093/carcin/bgr073. [DOI] [PubMed] [Google Scholar]
- 217.Huang TC, et al. Silencing of miR-124 induces neuroblastoma SK-N-SH cell differentiation, cell cycle arrest and apoptosis through promoting AHR. FEBS Lett. 2011;585(22):3582–3586. doi: 10.1016/j.febslet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 218.Makeyev EV, et al. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27(3):435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee JJ, et al. MiR-27b targets PPARgamma to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012;31(33):3818–3825. doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xu H, et al. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Annibali D, et al. A new module in neural differentiation control: two microRNAs upregulated by retinoic acid, miR-9 and-103, target the differentiation inhibitor ID2. PLoS One. 2012;7(7):e40269. doi: 10.1371/journal.pone.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang H, et al. MicroRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Mol Cancer Ther. 2012;11(7):1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 223.Moncini S, et al. The role of miR-103 and miR-107 in regulation of CDK5R1 expression and in cellular migration. PLoS ONE. 2011;6(5):e20038. doi: 10.1371/journal.pone.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Afanasyeva EA, et al. MicroRNA miR-885-5p targets CDK2 and MCM5, activates p53 and inhibits proliferation and survival. Cell Death Differ. 2011;18(6):974–984. doi: 10.1038/cdd.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beveridge NJ, et al. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cell Signal. 2009;21(12):1837–1845. doi: 10.1016/j.cellsig.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 226.Loven J, et al. MYCN-regulated microRNAs repress estrogen receptor-alpha (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc Natl Acad Sci USA. 2010;107(4):1553–1558. doi: 10.1073/pnas.0913517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chayka O, et al. Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas. J Natl Cancer Inst. 2009;101(9):663–677. doi: 10.1093/jnci/djp063. [DOI] [PMC free article] [PubMed] [Google Scholar]