Abstract
Neuropeptide Y (NPY) is a 36-amino-acid peptide that attenuates seizure activity following direct infusion or adeno-associated virus (AAV)-mediated expression in the central nervous system. However, NPY activates all NPY receptor subtypes, potentially causing unwanted side effects. NPY13-36 is a C-terminal peptide fragment of NPY that primarily activates the NPY Y2 receptor, thought to mediate the antiseizure activity. Therefore, we investigated if recombinant adeno-associated virus-mediated expression and constitutive secretion of NPY or NPY13-36 could alter limbic seizure sensitivity. Rats received bilateral piriform cortex infusions of AAV vectors that express and constitutively secrete full-length NPY (AAV-FIB-NPY) or NPY13-36 (AAV-FIB-NPY13-36). Control rats received no infusion, as we have previously shown that vectors expressing and secreting reporter genes like GFP (AAV-FIB-EGFP), as well as vectors expressing peptides that lack secretion sequences (AAV-GAL) have no effect on seizures. One week later, all animals received kainic acid (10 mg kg−1, intraperitoneally), and the latencies to wet dog shakes and limbic seizure behaviors were determined. Although both control and vector-treated rats developed wet dog shake behaviors with similar latencies, the latencies to class III and class IV limbic seizures were significantly prolonged in both NPY- and NPY13-36-treated groups. Thus, AAV-mediated expression and constitutive secretion of NPY and NPY13-36 is effective in attenuating limbic seizures, and provides a platform for delivering therapeutic peptide fragments with increased receptor selectivity.
Keywords: adeno-associated virus, seizures, kainic acid, neuropeptide Y, NPY13-36
Epilepsy is an attractive target for recombinant adeno-associated virus (rAAV) gene therapy, because the temporal lobe structures involved in seizure genesis and propagation have been shown to be permissive to AAV gene transfer.1–6 Recently, galanin and neuropeptide Y (NPY) have been delivered as transgenes in rAAV vectors, and are shown to be effective in several epilepsy paradigms.1,3,6,7 While previous approaches utilize pre-pro cDNA sequences, which rely on the cell to modulate release of the gene product, Haberman et al.3 were the first to use a novel secretion strategy whereby the secretion signal sequence from the constitutively secreted laminar protein fibronectin (FIB) is combined with the coding sequence for the active therapeutic peptide. Results from Haberman et al.3 and McCown1 show that expression and constitutive secretion of galanin is not only achieved, but also effective in attenuating focal and limbic seizure activity. In contrast, expression of galanin without the secretory signal or expression and secretion of the reporter gene, GFP had no effect on seizure sensitivity.1,3
In addition to galanin, rAAV delivery of NPY has also been shown to attenuate limbic seizures;6 however, there is still some question as to which of the NPY receptors (Y1–Y5) mediates the antiseizure activity. Several studies have suggested a critical role for the Y2 receptor in mediating antiepileptic actions.8–14 In addition, it has been demonstrated that in epileptic tissue from patients15 and rodents16,17 Y1 receptors are downregulated, whereas Y2 receptors are upregulated. Taken together, these results suggest that when designing antiepileptogenic therapeutics, it may be advantageous to deliver Y2-preferring agonists. In fact, acute intracerebral delivery of the Y2 receptor preferring agonist NPY13-36 reduces seizure susceptibility following systemic kainic acid administration.18,19 These data suggest NPY13-36 would be a good candidate to use in an rAAV vector to treat epilepsy. By using the FIB secretion strategy, we have the capability of expressing and constitutively secreting peptide fragments, which have demonstrated receptor selectivity. Thus, we evaluated the effects of AAV-mediated expression and constitutive secretion of NPY and the peptide fragment NPY13-36 on kainic acid-induced limbic seizures.
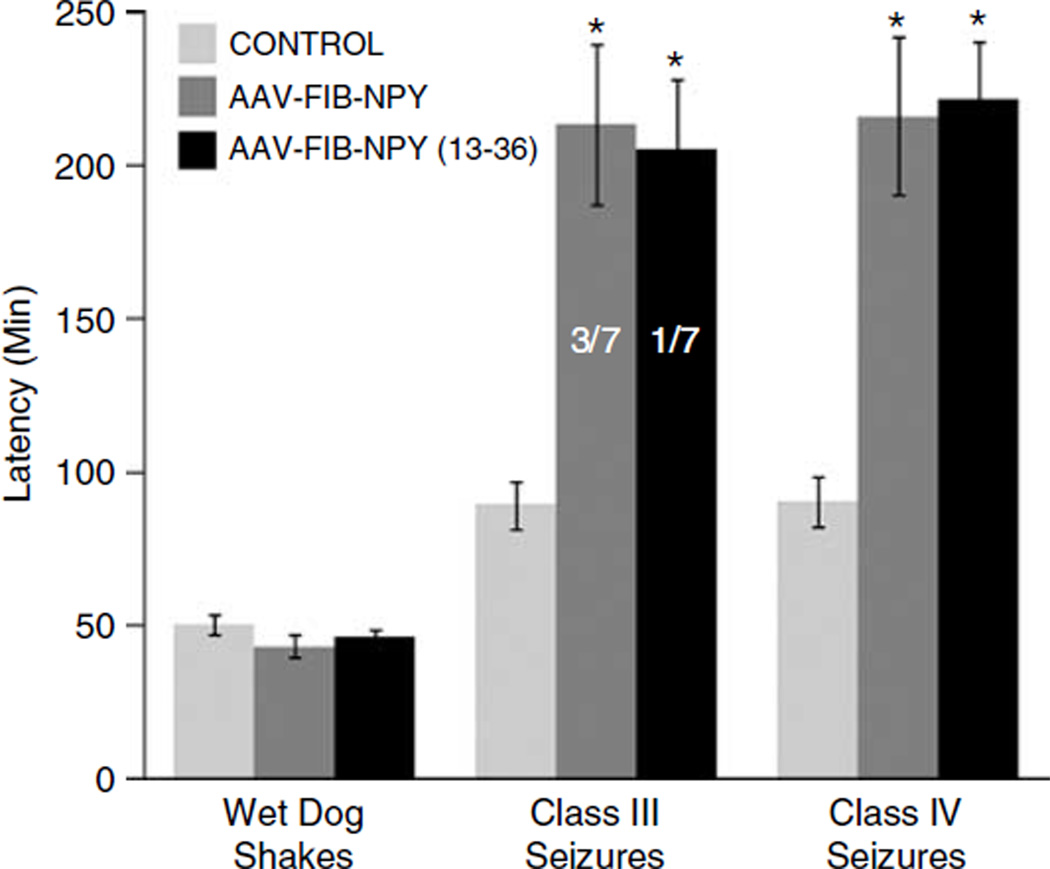
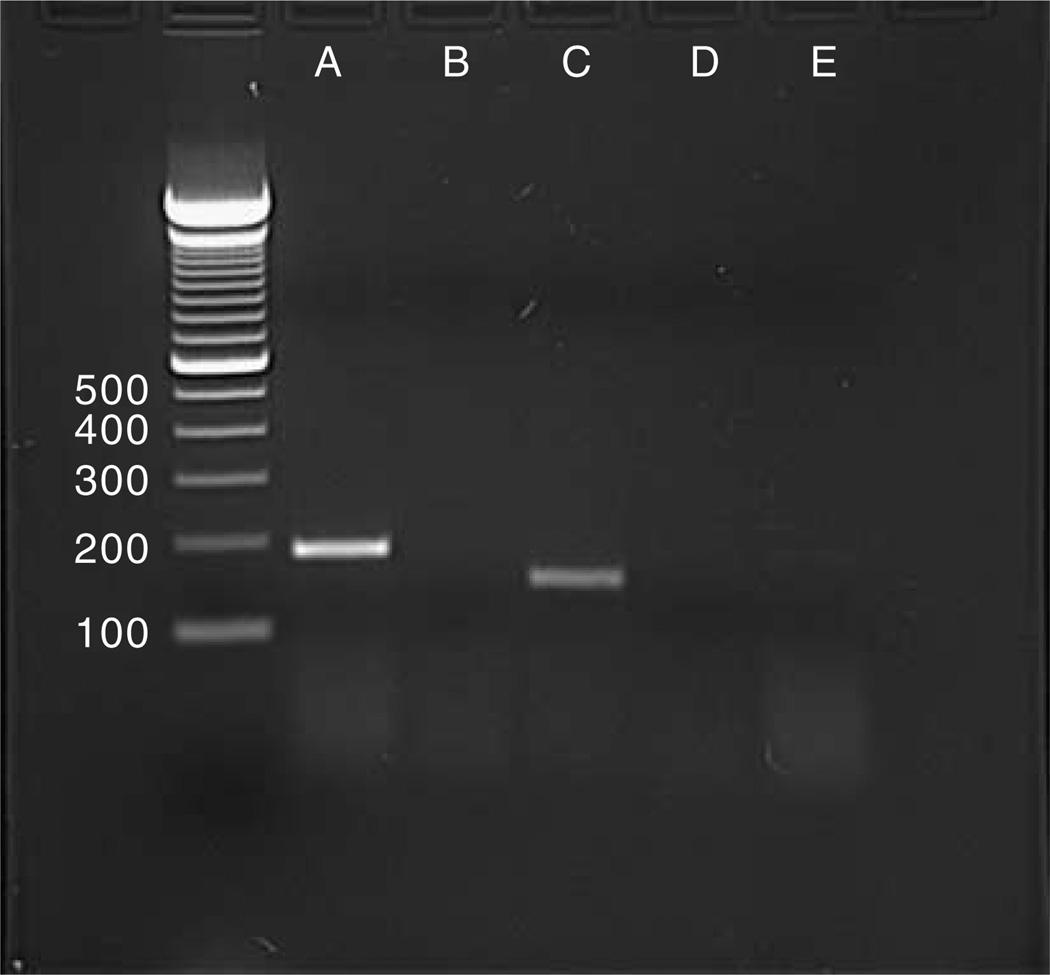
Although previous studies validated the effectiveness of gene expression and constitutive secretion using galanin, the same approach might not necessarily prove successful with NPY or the peptide fragment NPY13-36. Therefore, we used a similar approach with the following modifications: AAV 2 vectors were constructed where the hybrid chicken β-actin promoter drives expression of the FIB-NPY- or FIB-NPY13-36-coding sequences. Then, 2 µl of recombinant AAV-FIB-NPY (3.3 × 1012 viral particles per ml) or AAV-FIB-NPY13-36 (3.0 ×1012 viral particles per ml) was infused bilaterally into the piriform cortex of rats, as described previously.1 Control rats received no infusion, as we have previously shown that vectors expressing and secreting reporter genes like GFP (AAV-FIB-EGFP), as well as vectors expressing peptides that lack secretion sequences (AAV-GAL) have no effect on seizure sensitivity, seizure behaviors or seizure-induced cell death.1,3 One week later, the rats received a dose of 10 mg kg−1 intraperitoneal kainic acid, and subsequently the time to limbic seizure behaviors was recorded. As seen in Figure 1, both AAV treatment groups and the untreated control group developed wet dog shake behaviors with the same latency. Because the origin of wet dog shakes appears to be the hippocampus,20 a local action of the AAV-FIB-NPY or AAV-FIB-NPY13-36 in the piriform cortex would not be expected to influence these kainic acid-induced behaviors. However, the findings do validate the uniformity of kainic acid administration across the different groups. In marked contrast, the latency to class III or class IV limbic seizure activity was significantly increased in the AAV-FIB-NPY- or AAV-FIB-NPY13-36-treated groups. In fact, the majority of rats in both vector-treated groups did not exhibit any seizure behavior at all, whereas all rats in the untreated group develop class III and class IV seizures within 90 min (see Figure 1). Thus, like findings with expression and secretion of galanin, NPY actions in the piriform cortex significantly attenuated seizure activity induced by the peripheral administration of the chemical convulsant kainic acid. As previously discussed, the constitutive secretion of these peptides renders immunohistochemical identification untenable,3 but as previously shown for galanin,1,3 the appropriate vector-derived NPY or NPY13-36 mRNA was present in the area of AAV infusion (see Figure 2). Thus, not only does this gene therapy approach prove effective with NPY, but also it is now possible to express and secrete active fragments of peptides, allowing for more selective receptor targeting. In addition, the use of smaller peptide fragments in an AAV context may allow for combinations of therapeutic peptides to be expressed from a single vector cassette. It is likely that combination therapy will be the key to treating many complex neurological disorders.
Figure 1.
The effects of AAV-FIB-NPY and AAV-FIB-NPY13-36 vectors on the expression of limbic seizure behaviors. Rats received bilateral infusions of AAV-FIB-NPY (2 µl per 20 min; 3.3 × 1012 viral particles per ml; N = 7) or AAV-FIB-NPY13-36 (2 µl per 20 min; 3.0 × 1012 viral particles per ml; N = 7) into the piriform cortex as described previously.1 Seven days later both vector-treated groups and an untreated control group (N = 8) received an intraperitoneally injection of kainic acid (10 mg kg−1). The latencies to limbic seizure behaviors were determined for 240 min post-kainic acid. Seizures were scored according to the Racine Motor Seizure Grading Scale.21 Briefly, class I seizures were scored when rats exhibited facial twitches and chewing, class II when head nodding, class III when contralateral forelimb clonus was observed and class IV when bilateral forelimb clonus and rearing was observed. All groups developed wet dog shakes with the same latencies, indicating the uniformity of kainic acid administration across the different groups. In marked contrast, however, onset of class III and class IV seizures was significantly delayed or completely blocked in rats receiving either AAV-FIB-NPY or AAV-FIB-NPY13-36 compared to control (*t-test; P < 0.01). In fact, only 3/7 AAV-FIB-NPY-treated rats and 1/7 AAV-FIB-NPY13-36-treated rats developed any class III or class IV seizure behaviors over the entire 240 min observation period. All eight rats in the untreated control group developed class III and class IV seizures within 90 min. AAV, adeno-associated virus; FIB, fibronectin; NPY, neuropeptide Y.
Figure 2.
The in vivo presence of FIB-NPY and FIB-NPY13-36 mRNA 1 week after vector infusion into the piriform cortex. The appropriate 208 bp FIB-NPY (lane A) or 172 bp FIB-NPY13-36 (lane C) product is present in the injected piriform cortex, while no product was found in an area slightly distal to the piriform cortex (lane B). Omission of the reverse transcriptase step (lanes D and E) indicated the absence of contaminating viral DNA. The left outside lane contains a 100 bp DNA stepladder (Invitrogen, Carlsbad, CA, USA) with the size indicated on the left. Rats received bilateral infusions of AAV-FIB-NPY (2 µl per 20 min; 3.3 × 1012 viral particles per ml; N = 2) or AAV-FIB-NPY13-36 (2 µl per 20 min; 3.0 × 1012 viral particles per ml; N = 2) into the piriform cortex as described previously.1 Then, 1 week later, the animals received an overdose of pentobarbital (100 mg kg−1, intraperitoneally) and were subsequently decapitated. The brain was removed, and the piriform cortex was dissected out. The tissue was stored in RNAlater (Ambion, Austin, TX, USA) at −20°C. Subsequently, the RNA was extracted from the tissue (Promega SV-40 total RNA Isolation kit; Promega, Madison, WI, USA) and reverse transcribed using AMV reverse transcriptase and oligo (dT) primers. The subsequent PCR primers were designed to span the FIB-NPY (and thus the FIB-NPY13-36) sequence, which can only be derived from the rAAV vector (FIB, 5′-CTAGCAGTCCTGTGCCTG; NPY and NPY13-36, 3′-GCTCAATATCTCTGTCTGGTG). AAV, adeno-associated virus; FIB, fibronectin; NPY, neuropeptide Y; rAAV, recombinant adeno-associated virus.
Acknowledgements
We thank Julie Hamra for expert technical assistance. The study was supported by NIH grant NINDS NS 35633 to TJM.
References
- 1.McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. MOL Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Haberman RP, Samulski RJ, McCown TJ. Attenuation of seizures and neuronal death by adeno-associated virus vector galanin expression and secretion. Nat Med. 2003;9:1076–1080. doi: 10.1038/nm901. [DOI] [PubMed] [Google Scholar]
- 4.Freese A, Kaplitt MG, O'connor WM, Abbey M, Langer D, Leone P, et al. Direct gene transfer into human epileptogenic hippocampal tissue with an adeno-associated virus vector: implications for a gene therapy approach to epilepsy. Epilepsia. 1997;38:759–766. doi: 10.1111/j.1528-1157.1997.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 5.Vezzani A, Michalkiewicz M, Michalkiewicz T, Moneta D, Ravizza T, Richichi C, et al. Seizure susceptibility and epilepto-genesis are decreased in transgenic rats overexpressing neuro-peptide. Neuroscience. 2002;110:237–243. doi: 10.1016/s0306-4522(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 6.Richichi C, Lin EJ, Stefanin D, Colella D, Ravizza T, Grignaschi G, et al. Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin EJ, Richichi C, Young D, Baer K, Vezzani A, During MJ. Recombinant AAV-mediated expression of galanin in rat hippocampus suppresses seizure development. Eur J Neurosci. 2003;18:2087–2092. doi: 10.1046/j.1460-9568.2003.02926.x. [DOI] [PubMed] [Google Scholar]
- 8.Colmers WF, Klapstein GJ, Fournier A, St-Pierre S, Treherne KA. Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br J Pharmacol. 1991;102:41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont Y, Cadieux A, Doods H, Fournier A, Quirion R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIB03304 (Y1) and CGP71683A (Y5) Can J Physiol Pharmacol. 2000;78:116–125. [PubMed] [Google Scholar]
- 10.Dumont Y, Cadieux A, Doods H, Pheng LH, Abounader R, Hamel E, et al. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y(2) receptor antagonist. Br J Pharmacol. 2000;129:1075–1088. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greber S, Schwarzer C, Sperk G. Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br J Pharmacol. 1994;113:737–740. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin EJ, Young D, Baer K, Herzog H, During MJ. Differential actions of NPY on seizure modulation via Y1 and Y2 receptors: evidence from receptor knockout mice. Epilepsia. 2006;47:773–780. doi: 10.1111/j.1528-1167.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 13.El Bahh B, Balosso S, Hamilton T, Herzog H, Beck-Sickinger AG, Sperk G, et al. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y and not Y receptors. Eur J Neurosci. 2005;22:1417–1430. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- 14.El Bahh B, Cao JQ, Beck-Sickinger AG, Colmers WF. Blockade of neuropeptide Y(2) receptors and suppression of NPY’s anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br J Pharmacol. 2002;136:502–509. doi: 10.1038/sj.bjp.0704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kofler N, Kirchmair E, Schwarzer C, Sperk G. Altered expression of NPY-Y1 receptors in kainic acid induced epilepsy in rats. Neurosci Lett. 1997;230:129–132. doi: 10.1016/s0304-3940(97)00492-8. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzer C, Kofler N, Sperk G. Up-regulation of neuropeptide Y-Y2 receptors in an animal model of temporal lobe epilepsy. Mol Pharmacol. 1998;53:6–13. doi: 10.1124/mol.53.1.6. [DOI] [PubMed] [Google Scholar]
- 18.Vezzani A, Moneta D, Mule F, Ravizza T, Gobbi M, French-Mullen J, et al. Plastic changes in neuropeptide Y receptor subtypes in experimental models of limbic seizures. Epilepsia. 2000;41(Suppl 6):S115–S121. doi: 10.1111/j.1528-1157.2000.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 19.Vezzani A, Rizzi M, Conti M, Samanin R. Modulatory role of neuropeptides in seizures induced in rats by stimulation of glutamate receptors. J Nutr. 2000;130:1046S–1048S. doi: 10.1093/jn/130.4.1046S. [DOI] [PubMed] [Google Scholar]
- 20.Frush DP, McNamara JO. Evidence implicating dentate granule cells in wet dog shakes produced by kindling stimulations of entorhinal cortex. Exp Neurol. 1986;92:102–113. doi: 10.1016/0014-4886(86)90128-7. [DOI] [PubMed] [Google Scholar]
- 21.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]