Abstract
Background
The D antigen is the most immunogenic antigen in the Rh system. D variants must be considered if there is a significant discrepancy in the strength of reaction obtained with different anti-D reagents, a discrepancy between current and historical test results and if anti-D is detected in an individual serologically typed as RhD positive. A panel of monoclonal anti-D reagents can be used to identify partial D and weak D variants. The aim of this study was to develop a strategy for RhD typing in discrepant cases.
Materials and methods
Sixty RhD discrepant samples referred to our Institute for confirmation of RhD status were tested with a panel of 12 monoclonal anti-D reagents (ALBAclone advanced partial RhD typing kit) and Rh phenotype was determined using C, c, D, E, and e antisera.
Results
Ninety-three percent of the RhD discrepant cases were classified into weak and partial D using this kit. Among the D variants characterised, 37% belonged to DFR, 23% to DOL, 12% to weak D, and the remaining 21% to DAR, DV, DMH, DCS and DVI categories. Ninety-seven percent of the D variants were “C” antigen positive. Out of the panel of 12 monoclonal anti-D used, cell line LHM-70/45 gave negative reactions with all RhD discrepant cases and cell lines LHM-76/59, LHM-76/55 and ESD-1 gave positive reactions with all 60 RhD discrepant cases studied.
Discussion
The Advanced partial D kit was very useful in characterising and identifying D variants in the Indian population. A preliminary strategy for the detection and identification of D variants in discrepant cases could be to test for the presence of “C” antigen with anti-C, and for “D” antigen with anti-D of cell line LHM 70/45. A more comprehensive, but simple way to identify D variants in routine RhD typing is to use two anti-D reagents i.e LHM 70/45 and one out of LHM-76/59, LHM-76/55 and ESD-1. D variants can be further characterised by using the partial D typing kit and molecular genotyping in specialised laboratories.
Keywords: RhD typing, D variants, diagnostic strategy, Indian population
Introduction
Rh is one of the most important and clinically significant blood group system. D antigen is the most immunogenic and clinically important of this system because of the ability of anti-D to cause transfusion reactions and haemolytic disease of the foetus and newborn1. Rh blood group discrepancies may arise when an individual has a variant of D antigen. Partial D and weak D are the most commonly found D variants2. Partial D variants lack one or more epitopes of D antigen while weak D have all epitopes present but express a significantly reduced amount of D antigen per red blood cell and are usually identified by the indirect antiglobulin test (IAT)3,4. Both these variants may be mistyped as D negative by an immediate spin test. D antigen discrepancies need to be resolved in order that the correct D antigen status can be assigned and appropriate (D positive or D negative) blood products can be administered. RhD discrepancies can also create confusion over the use of Rh immunoglobulin prophylaxis in pregnant women with D variant5,6.
Earlier, when polyclonal anti-D reagents were in routine use, weak D (or Du as they were called in the past) were identified by performing sensitive tests such as the IAT. Blood banks have been encountering more cases of discrepancies in RhD grouping since they started using monoclonal anti-D reagents which are available as IgM, IgG and IgM+IgG7,8. Our experience has been similar as an increasing number of cases have been referred to our Institute for confirmation of RhD status in the past few years.
The incidences of D positive, D negative and D variants vary in different populations as documented in the literature9–11. The Indian population is considered ideal for genetic studies as it has enormous genetic, cultural and linguistic diversities12,13. Our earlier study, in which we tested 5,315 individuals with a panel of 30 epitope-specific monoclonal anti-D to determine the distribution of epitopes present in our population, showed a varied pattern of reactivity in different Indian castes and communities14. We have also tested 60 partial D variants confirmed by serology and molecular techniques with a panel of commercial anti-D reagents available on the Indian market and found that no single monoclonal anti-D reagent could identify all the D variants. A combination of two monoclonal anti-D reagents could identify only 66% of the D variants which were interpreted by their weaker reactions or discrepant results, thus showing the limitations of commercial anti-D reagents in identifying D variants in our population15.
Specific panels of monoclonal anti-D reagents have become popular for identifying partial D and some weak D variants and help laboratories (through identification and classification of these variants) to make informed decisions on D antigen status16,17. Molecular analysis of RHD gene has been used to resolve RhD discrepancies, but is not routinely used even in western countries. Our study on partial D variants using a panel of six monoclonal anti-D reagents (Diagnostics Scotland, Edinburgh, UK) showed that 37% of these could not be characterised18. When a panel of 12 monoclonal anti-D reagents with the potential to characterise more of the most common partial D and weak D variants19 became available, we started to use this kit to test all further samples referred to us with Rh discrepant results. This has helped us to select suitable anti-D reagents to identify most common D variants encountered in the Indian population by serological methods, which can be used in any Indian blood bank or laboratory.
Materials and methods
Two different Food and Drug Administration (FDA)-approved reagents from two different manufacturers or different batches from the same manufacturer are used to type D antigen in the majority of the blood banks and laboratories in India20. Most of the laboratories prefer using a blend of IgM+ IgG monoclonal anti-D reagents as this can be used for both immediate spin testing and IAT. A D variant is considered possible if there is: (i) a significant discrepancy in the strength of reactions obtained with different anti-D reagents, (ii) a weak agglutination reaction by IAT, (iii) a discrepancy between current and historical test results, and (iv) anti-D detected in an individual who is serologically RhD positive. Samples tested by two monoclonal anti-D reagents (IgM and IgM+IgG by the tube technique) were referred by various blood banks and laboratories to our Institute for confirmation of RhD status.
Sixty samples (from blood donors and antenatal women), referred to the Institute for investigation of discrepancies in RhD grouping were tested with the ALBAclone Advanced Partial RhD Typing Kit (Alba Bioscience Limited, Edinburgh, United Kingdom) by an IAT (tube technique) according to the manufacturer’s instructions. This kit contains a panel of 12 monoclonal anti-D reagents which can identify 15 partial D variants and weak D types 1 & 2 depending on the reactivity pattern (Table I). Rh phenotyping on all RhD discrepant samples was carried out using monoclonal anti-C, anti-c, anti-D, anti-E and anti-e antisera. The most probable Rh genotype was deduced from the Rh phenotype21.
Table I.
Reaction profile of the ALBAclone Advanced Partial D Typing kit.
| Kit ID. | Anti-D cell line | Weak D type 1 & 2 | DII & DNU | DIII | DIV | DV | DCS | DVI | DVII | DOL | DFR | DMH | DAR | DAR-E | DHK &DAU-4 | DBT | RoHar |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | LHM 76/58 | + | + | + | + | +/0 | + | 0 | + | + | + | + | + | 0 | 0 | 0 | (+)/0 |
| B | LHM 76/59 | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + | 0 | 0 |
| C | LHM 174/102 | (+)/0 | + | + | 0 | 0 | + | 0 | + | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 |
| D | LHM 50/28 | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | 0 |
| E | LHM 169/81 | + | + | + | 0 | 0 | + | 0 | + | + | + | + | 0 | 0 | 0 | 0 | 0 |
| F | ESD-1 | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + | 0 | 0 |
| G | LHM 76/55 | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + | 0 | 0 |
| H | LHM 77/64 | + | 0 | + | 0 | + | + | + | + | + | + | + | + | + | +/0 | 0 | 0 |
| I | LHM 70/45 | (+)/0 | + | + | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| J | LHM 59/19 | + | + | + | + | + | + | 0 | 0 | 0 | 0 | (+) | 0 | (+) | + | + | 0 |
| K | LHM 169/80 | + | + | + | + | + | + | 0 | + | + | + | + | + | + | 0 | 0 | 0 |
| L | LHM 57/17 | + | + | + | + | + | 0 | 0 | + | + | 0 | + | + | 0 | 0 | + | 0 |
Results
Sixty samples giving RhD discrepant results were further investigated for their characterisation into weak D and partial D. Ninety-three percent of these cases were classified into different D variants using the ALBAclone Advanced Partial RhD Typing kit, whereas four RhD discrepant cases (6.66%) could not be classified according to the reactivity pattern given in Table I. Sixty percent of D variants belonged to the DFR or DOL category, 12% to weak D type 1 or 2 and only 3% belonged to the DVI category (Figure 1). Ninety-seven percent of the D variants showed the presence of “C” antigen. In the present study using a kit with 12 monoclonal anti-D reagents (Table II), only 7% of the discrepant cases could not be characterised compared to 37% in our earlier study using a kit with six monoclonal anti-D reagents.
Figure 1.
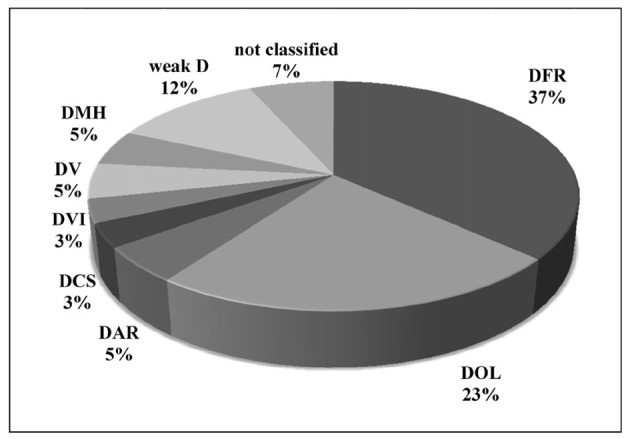
Classification of D variants in RhD discrepant cases studied.
Table II.
Characterisation of D variants with two partial D kits.
Further analysis of the reactivity pattern of the D variants against 12 monoclonal anti-D in the kit showed that the cell lines C (LHM 174/102), I (LHM 70/45) and J (LHM 59/19) gave negative reactions with the majority of the D variants. Monoclonal anti-D cell lines B (LHM 76/59, F (ESD-1) and G (LHM 76/55) showed positive reactions with all D variants identified in this study. Table III shows the percentage of RhD discrepant cases giving negative reactions with some monoclonal anti-D of the kit. All D variants gave negative reactions with cell line LHM 70/45 and cell lines LHM 174/102 and LHM 59/19 gave negative reactions with 87% and 83% of RhD discrepant cases studied, respectively. Monoclonal anti-D LHM 76/59, ESD-1 and LHM 76/55 gave positive reactions with all RhD discrepant cases.
Table III.
Number of D variants showing negative reactions with some monoclonal anti-D of the Advanced Partial D Typing kit.
| Monoclonal anti-D kit ID | B | F | G | I | C | J | Total n. of samples tested |
|---|---|---|---|---|---|---|---|
| Cell line | LHM 76/59 | ESD-1 | LHM 76/55 | LHM 70/45 | LHM 174/102 | LHM 59/19 | 60 |
| Number of D variants showing negative reaction | 0 | 0 | 0 | 60 | 52 | 50 | |
| % | 0 | 0 | 0 | 100 | 87 | 83 |
Discussion
The appropriate assignment of RhD antigen status is essential in order to administer Rh immunoglobulin injections to D negative women and to transfuse Rh negative blood to Rh negative recipients. However the serological distinction between D positive and D negative red blood cells is not always straightforward in the case of D variants5. Partial and weak D variant phenotypes give discrepant RhD group results when different commercially available monoclonal anti-D reagents are used in the laboratories15,22. Depending on the presence or absence of D epitopes on red cells, individuals with partial D may type as D positive or D negative with commercially available anti-D reagents23. Weak D variants may give strong, weak or negative reactions depending on the sensitivity of the anti-D reagents and techniques used and can, therefore, give discrepant results in different laboratories7,10.
Although it is difficult in blood banks to differentiate between partial D and weak D, it is important to identify a donor as having a D variant as the red cells of such a donor could elicit an immune response if transfused to a D negative recipient. However, a partial D and weak D recipient can be safely considered as Rh negative. Therefore, in many laboratories different anti-D reagents are used for Rh typing of red cells from donors and from recipients/patients. The intent of FDA-approved anti-D serological typing reagents for recipients/patients is to ensure that appropriate D antigen status is assigned so that most common partial D variants are non-reactive in an immediate spin test and reported as RhD negative. The reagent used for Rh typing of donors should be such that even very weak reactions are detected. Donor red cells showing weak reactions with anti-D or D variants should be considered as Rh positive and this blood must not be transfused to D negative recipients as it may produce anti-D24. Commercially available FDA-approved anti-D reagents can react differently with D variant antigens depending on the epitopes against which the antibody is produced and the epitopes present in partial D variants.
In the present study 60 samples, referred to us for confirmation of RhD grouping due to RhD discrepant results, were tested with the ALBAclone Advanced Partial D Typing kit to identify the D variants. We observed that this kit was very useful for the identification and characterisation of D variants. In our earlier study18, using a partial D kit containing a panel of six monoclonal anti-D, we identified 63% of D variants whereas in the present study 93% of D variants could be classified given the potential of the kit to identify weak D types 1 and 2 and 15 different partial D variants (Table II). The remaining 7% (four D variants) showed a new pattern of weak reactions not classified by the kit. A similar observation was reported by Pereira et al., who identified a pattern of results with an anti-D panel which did not correspond to any described before, suggesting the presence of a new D variant17.
The analysis of the reactivity pattern of the 60 RhD discrepant cases with the 12 monoclonal anti-D reagents of the kit showed that none of them reacted with the cell line LHM 70/45. This cell line can, therefore, be used for Rh typing of red cells from antenatal patients and blood transfusion recipients so that most of the D variants can be identified as D negative and that these recipients can be given D negative transfusions and antenatal women can be given Rh prophylaxis. On the other hand, the cell lines LHM 76/59, ESD-1 and LHM 76/55 showed positive reactions with all the D variants identified in this study. It can, therefore, be considered quite appropriate to use these cell lines for typing red cells from donors so that they are typed as Rh positive.
Different countries have their own policies for the selection of blood grouping reagents and have their own transfusion and diagnostic strategies10,24,25. Determination of RHD alleles carried by weak D/partial D individuals would be the best procedure for typing D variants and making the right decisions. However, only a few laboratories use molecular workup routinely when serology does not give the correct status, even in western countries26. Pirenne et al. observed that serological typing of D antigen can be safe if a strategy based on the frequency of D variants and clinical situation is implemented, as it is impossible to determine the cut-off for agglutination strength to decide D negative or D positive assignment for transfusion or pregnancy27. Polin et al. have developed a molecular RhD typing strategy for the Austrian population for samples showing weak reactions or discrepant results with different anti-D reagents26.
Based on our findings in this study, it seems appropriate to select anti-D reagents which will identify the majority of D variants in our population by simple serological techniques, which will be useful in blood banks for the determination of correct RhD status. Thus the shortcomings of our commercial anti-D reagents can be overcome and future implementation of molecular testing of Rh will reduce and may potentially eliminate alloimmunisation to Rh.
Testing for the presence of the “C” antigen with anti-C and for the D antigen with anti-D reagent using cell line LHM 70/45 could be the simple serological diagnostic strategy for the detection and identification of D variants in RhD discrepant cases. A more comprehensive and simple way to identify D variants in routine RhD typing would be to include two anti-D reagents i.e (i) LHM 70/45 and (ii) any one of the cell lines LHM 76/59, ESD-1 and LHM 76/55. As the use of this combination is expected to give discrepant results with the majority of the D variants in the Indian population, identification of D variants would become possible. Further characterisation of D variants can be performed using all the anti-D reagents of the Advanced Partial D Typing kit and molecular genotyping by specialised laboratories.
Acknowledgments
The Authors are very grateful to Janine Robb, Alba Bioscience Limited, Edinburgh, UK for providing the ALBAclone Advanced Partial RhD Typing kit and various blood banks and laboratories for sending RhD discrepant cases for confirmation of RhD status.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Bowman J. The management of hemolytic disease in the fetus and newborn. Sem Perinatology. 1997;21:39–44. doi: 10.1016/s0146-0005(97)80018-3. [DOI] [PubMed] [Google Scholar]
- 2.Flegel WA, Wagner F. Molecular biology of partial D and weak D: implications for blood bank practice. Clin Lab. 2002;48:53–9. [PubMed] [Google Scholar]
- 3.Flegel WA, Wagner FF. RHD epitope density profiles of RHD variant red cells analyzed by flow cytometry. Transfus Clin Biol. 1996;3:429–31. doi: 10.1016/s1246-7820(96)80058-9. [DOI] [PubMed] [Google Scholar]
- 4.Wagner FF, Frohmajer A, Ladewig B, et al. Weak D alleles express distinct phenotypes. Blood. 2000;95:2699–708. [PubMed] [Google Scholar]
- 5.Fung Kee Fung K, Eason E, Crane J, et al. Maternal Fetal Medicinal Committee, Genetics Committee Prevention of Rh alloimmunisation. J Obstet Gynaecol Can. 2003;25:765–73. doi: 10.1016/s1701-2163(16)31006-4. [DOI] [PubMed] [Google Scholar]
- 6.Denomme GA, Dake LR, Vilensky D, et al. Rh discrepancies caused by variable reactivity of partial and weak D types with different serologic techniques. Transfusion. 2008;48:473–8. doi: 10.1111/j.1537-2995.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams M. Monoclonal reagents for rhesus-D typing of Irish patients and donors: a review. Br J Biomed Science. 2000;57:142–9. [PubMed] [Google Scholar]
- 8.Denomme GA, Wagner FF, Fernande BJ, et al. Partial D, weak D types, and novel RHD alleles among 33,864 multiethnic patients: implications for anti-D alloimmunization and prevention. Transfusion. 2005;45:1554–60. doi: 10.1111/j.1537-2995.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Kim SY, Kim CA, et al. Molecular characterization of D-Korean persons: development of a diagnostic strategy. Transfusion. 2005;45:345–52. doi: 10.1111/j.1537-2995.2005.04311.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Lane C, Quillen K. Prevalence of RhD variants, confirmed by molecular genotyping, in a multiethnic prenatal population. Am J Clin Path. 2010;134:438–42. doi: 10.1309/AJCPSXN9HQ4DELJE. [DOI] [PubMed] [Google Scholar]
- 11.St-Louis M, Richard M, Cote M, et al. Weak D type 42 cases found in individuals of European descent. Immunohaematology. 2011;27:20–4. [PubMed] [Google Scholar]
- 12.Majumder PP. Ethnic populations of India as seen from an evolutionary perspective. J Biosci. 2001;26(4 Suppl):533–45. doi: 10.1007/BF02704750. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia HM, Rao VR. Genetic Atlas of Indian Tribes. Bombay: Institute of Immunohaematology (ICMR) publications; 1987. [Google Scholar]
- 14.Kulkarni SS, Gupte SC, Vasantha K, et al. Varied distribution of RhD epitopes in the Indian population. Natl Med J India. 2007;20:169–71. [PubMed] [Google Scholar]
- 15.Kulkarni SS, Vasantha K, Gupte SC, et al. Potential of commercial anti-D reagents in the identification of partial D variants in Indian population. Indian J Med Res. 2007;125:641–4. [PubMed] [Google Scholar]
- 16.Rupreht RR, Pretnar Hartman K, Galvani V, et al. Weak D and partial D in Slovenian population through serology and genotyping. Pflügers Arch. 2000;440(Suppl. 5):R195–6. [PubMed] [Google Scholar]
- 17.Pereira JC, Rodrigues MJ, Tilley L, et al. RhD variant caused by an in-frame triplet duplication in the RHD gene. Transfusion. 2011;51:570–3. doi: 10.1111/j.1537-2995.2010.02856.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni S, Colah R, Gorakshakar A, et al. Frequency of partial D in Western India. Transfus Med. 2008;18:91–6. doi: 10.1111/j.1365-3148.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 19.Wagner FF, Eicher NI, Jørgensen JR, et al. DNB: a partial D with anti-D frequent in Central Europe. Blood. 2002;100:2253–6. doi: 10.1182/blood-2002-03-0742. [DOI] [PubMed] [Google Scholar]
- 20.Standards for Blood Banks & Blood Transfusion Services National AIDS Control Organization . New Delhi: Ministry of Health and Family Welfare, Government of India; 2007. [Google Scholar]
- 21.Bhatia HM. Procedures in Blood Banking and Immunohaematology. Bombay: Blood Group Reference Centre (ICMR); 1977. [Google Scholar]
- 22.Jones J, Scott ML, Voak D. Monoclonal anti-D specificity and Rh D structure: criteria for selection of monoclonal anti-D reagents for routine typing of patients and donors. Transfus Med. 1995;5:171–84. doi: 10.1111/j.1365-3148.1995.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones JW, Voak D, Scott ML, Sonneborn HH. Policies for the selection of monoclonal RHD typing reagents. Biotest Bull. 1997;5:485–94. [Google Scholar]
- 24.Flegel WA, Denomme GA, Yazer MH. On the complexity of D antigen typing: a handy decision tree in the age of molecular blood group diagnostics. J Obstet and Gynaecol Can. 2007;29:746–52. doi: 10.1016/s1701-2163(16)32606-8. [DOI] [PubMed] [Google Scholar]
- 25.AABB . Standards for Blood Banks and Transfusion Services. 26th edition. Bethesda, MD: American Association of Blood Banks; 2009. [Google Scholar]
- 26.Polin H, Danzer M, Hofer K, et al. Effective molecular RHD typing strategy for blood donations. Transfusion. 2007;47:1350–5. doi: 10.1111/j.1537-2995.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- 27.Pirenne FN, Verdier M, Lejealle A, et al. Weak D phenotypes and transfusion safety: where do we stand in daily practice? Transfusion. 2007;47:1616–20. doi: 10.1111/j.1537-2995.2007.01332.x. [DOI] [PubMed] [Google Scholar]


