Abstract
Background
It is essential that the quality of platelet metabolism and function remains high during storage in order to ensure the clinical effectiveness of a platelet transfusion. New storage conditions and additives are constantly evaluated in order to achieve this. Using glucose as a substrate is controversial because of its potential connection with increased lactate production and decreased pH, both parameters triggering the platelet lesion during storage.
Materials and methods
In this study, we analysed the morphological status and metabolic profile of platelets stored for various periods in autologous plasma enriched with increasing glucose concentrations (13.75, 27.5 and 55 mM). After 0, 2, 4, 6 and 8 days, high energy phosphates (ATP, GTP, ADP, AMP), oxypurines (hypoxanthine, xanthine, uric acid), lactate, pH, mitochondrial function, cell lysis and morphology, were evaluated.
Results
The data showed a significant dose-dependent improvement of the different parameters in platelets stored with increasing glucose, compared to what detected in controls. Interestingly, this phenomenon was more marked at the highest level of glucose tested and in the period of time generally used for platelet transfusion (0–6 days).
Conclusion
These results indicate that the addition of glucose during platelet storage ameliorates, in a dose-dependent manner, the biochemical parameters related to energy metabolism and mitochondrial function. Since there was no correspondence between glucose addition, lactate increase and pH decrease in our experiments, it is conceivable that platelet derangement during storage is not directly caused by glucose through an increase of anaerobic glycolysis, but rather to a loss of mitochondrial functions caused by reduced substrate availability.
Keywords: platelet storage, glucose, energy metabolism, mitochondrial functioning, transfusion
Introduction
A prolongation of the storage time of platelet concentrates (PC) and/or an increase in the quality of stored PC would be of great advantage for both thrombocytopenic individuals and blood banks because of the continuous increase in the need for platelet transfusions1,2. The development of new generation bags which greatly improved gas exchange3, along with very recent technologies for bacterial detection and inactivation4,5 were fundamental for standardising platelet storage conditions aimed at minimising the so-called “platelet storage lesion”6. Although mechanisms triggering this phenomenon are still under discussion, it is known that the lesion is characterised by several biomolecular and morphological changes related to platelet metabolism7, activation and apoptosis8 profoundly compromising platelet functions and survival2. Furthermore, different platelet additive solutions (PAS) have been proposed to reduce plasma-related transfusion reactions, decrease platelet derangement and prolong the time of PC storage with minimal loss of platelet function9,10.
Two main strategies, with opposite conceptual approaches, have been adopted to obtain these results: (i) “minimise” platelet metabolism by reducing temperature and depriving the PAS of energy sources in order to drastically reduce platelet energy demand during storage11; (ii) to support platelet metabolism by supplementing PAS with energy sources to satisfy platelet energy demand during storage12.
The latter approach has been attempted with energy substrates alternative to glucose, such as fatty acids13, despite the fact that glucose is the basic component commonly used in blood storage14,15 and organ preservation for transplantation16,17. The current reason for avoiding the use of glucose is based on the belief that the active glycolytic pathway is responsible for the platelet storage lesion, via lactate production and the consequent decrease in pH18. It has, however, been reported that the use of glycolytic inhibitors did not cause an improvement in the quality of stored platelets19, therefore rendering doubtful even the contribution of pH decrease to the damage of platelet metabolism and functions during storage20. Furthermore, recent studies performed by storing platelets with glucose-enriched PAS formulations have encouraged the use of this fuel as an essential constituent of storage media21.
Using this approach, we showed in a preliminary study that the storage of PC in the presence of autologous plasma enriched with glucose had beneficial effects on platelet metabolic profile, as evaluated by measuring parameters representative of energy metabolism (ATP, ADP, AMP, GTP, etc.) in PC stored with and without 0.5% glucose (27.5 mM)22. The results of that study also showed the utility of the metabolic evaluation of platelets performed by simultaneous high performance liquid chromatography (HPLC) determination of a conspicuous number of compounds in deproteinised platelet extracts22.
Prompted by these results, in the present study we investigated the dose-response effects of the addition of different glucose concentrations (13.75, 27.5 and 55 mM) on a comprehensive metabolic profile of PC, determined at different times during the entire period of storage in autologous plasma. Analysis of high-energy phosphates (ATP, ADP, AMP, GTP), purine-containing compounds (adenosine, inosine, guanosine, hypoxanthine, xanthine, uric acid), lactate and pH was carried out in parallel to the determination of the mitochondrial functionality, studied by determining the mitochondrial inner membrane potential with two different fluorophores. These parameters were correlated to mean platelet volume (MPV), platelet count and lysis (release of lactate dehydrogenase [LDH] in the storage medium).
Materials and methods
Preparation, treatment and storage conditions of the platelet concentrates
Six healthy volunteers, who gave their agreement to this study, were recruited as blood donors. Six PC were obtained by isolating the buffy coat layer23 from single fresh whole blood units collected in Compoflex™ Quadruple bags (Fresenius Kabi Italia S.r.l., Isola della Scala, Verona, Italy) (450 mL anticoagulated with 63 mL CPD-A). After the first centrifugation (4,200 rpm for 10 minutes at 22 °C; Jouan KR 4–22, Conquer Scientific, San Diego, CA, United States of America), red blood cells, plasma and the buffy coat layer were collected into different supports, the last being shaken gently for 2 hours. The second soft centrifugation (1,000 rpm for 10 minutes at 22 °C) produced a leucoreduced PC from the buffy coat which was finally resuspended in 60 mL of autologous plasma. At this stage, glucose in the storage medium was approximately 29 mM.
The number of platelets and MPV were measured in each concentrate using a full blood haematology analyser (Coulter LH 750 Analyzer, Beckman Coulter, Cassina de’ Pecchi, Milan, Italy). The number of platelets ranged between 4.5 and 8.5×1010/60 mL, with leucocyte contamination less than 0.05×109. Each PC (n=6) was aseptically divided into four sterile aliquots of 15 mL each and stored into PVC-made, Compoflex™ Single Bags (Fresenius Kabi Italia S.r.L., Isola della Scala, Verona, Italy) with a bag volume of 100 mL. Three of these aliquots were supplemented with 13.75, 27.5 or 55 mM glucose (Bieffe Medital, Grosotto, SO, Italy). One aliquot did not receive any additional substrate supplementation and was used as a control. The four PC aliquots were kept under agitation at 22 °C for a total period of 8 days, and sampled during storage on days 0, 2, 4, 6, 8. On these samples, pH measurement by a pH meter (micropH 2001, Crison Strumenti S.p.A, Carpi, MO, Italy), platelet count and determination of MPV were performed immediately. Subsequently, the samples were processed for biochemical analyses, as described below. At the end of the storage period, each bag of PC was checked for sterility by plating an aliquot of the concentrate in Columbia CAN/Mac Conkey/MSA2/Sabouraud growth media (Microbiol s.n.c., Cagliari, Italy).
Sample processing for biochemical analyses
Aliquots of PC were centrifuged at 3,500 rpm for 7 minutes at 4 °C (ALC International S.r.L, Cologno Monzese, MI, Italy) to separate platelets from the suspending medium. One hundred microlitres of the supernatant were used to determine LDH activity in the medium. The enzyme activity was measured by spectrophotometrically (Beckman DU-620, Beckman Coulter, Cassina de’ Pecchi, Milan, Italy) following the time-depending disappearance of NADH at 340 nm in a mixture containing 2 mM pyruvate, 200 μM NADH, Tris-HCl buffer pH 7.4. The remaining suspending medium was processed for the HPLC analysis of metabolites released by platelet metabolism during storage, as described below.
The platelet pellet was first washed twice with large volumes of phosphate-buffered saline and then processed as reported elsewhere22: briefly, proteins were precipitated using a precipitating solution composed of CH3CN/10 mM KH2PO4 pH 7.40 (3:1; v:v), followed by centrifugation at 13,000 rpm for 10 minutes at 4 °C. Supernatants were washed three times with an abundant quantity of chloroform to remove the organic solvent. After each washing of the aqueous phase, centrifugation was performed again as described above. The HPLC analysis of adenine nucleotide derivatives and lactate was performed on 200 μL of the aqueous platelet extracts, using a Hypersil C-18, 250×4.6 mm, 5 μm particle size HPLC column, provided with its own guard column (ThermoFisher Scientific, Milan, Italy), according to an ion-pairing method previously published24,25. A SpectraSystem P4000 pump system and a highly-sensitive UV6000LP diode array detector (ThermoFisher Scientific, Milan, Italy), equipped with a 5-cm light path flow cell and set up between 200 and 300 nm, were used as our HPLC apparatus. Data acquisition and analysis were performed by a personal computer using the ChromQuest™ software package provided by the HPLC manufacturer. The concentration and purity of the peaks were determined at 206 nm (lactate) and 260 nm (adenine derivatives) by comparing areas, retention times and absorption spectra with those of the peaks of freshly prepared standard solutions (Sigma-Aldrich, St. Louis, MO, United States of America) with known concentrations.
Mitochondrial transmembrane potential (ΔΨm) measurements
Mitochondrial membrane potential (ΔΨm) changes in platelets were measured by using either the aggregate-forming lipophilic cationic fluorochrome 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolcarbocyanine iodide (JC1) or tetrametylrhodamine methyl ester (TMRM) (Molecular Probes, Eugene, OR, USA). The JC1 dye accumulates in healthy mitochondria showing a shift in fluorescence from green (monomeric form) to red (aggregate form). In the case of a decrease in the ΔΨm, the concentration of JC1 within the mitochondrial matrix is not sufficient to cause its aggregation and the fluorescence, therefore, remains green. The TMRM dye accumulates within healthy mitochondria because of its positive charge, being released from mitochondria in consequence of a decrease in the ΔΨm. For both probes 0.5 mM stock solutions in DMSO were prepared. In experiments using these dyes, 1 mL of PC from each bag was withdrawn at various time points and centrifuged for 10 minutes at 3,500 rpm. Subsequently, two aliquots of 500 μL, containing approximately 0.5×107 platelets each, were loaded with either 0.5 μM JC1or 50 nM TMRM. As a drug-resistant pump inhibitor, 20 μM verapamil (Sigma-Aldrich, St. Louis, MO, USA) was added in each case. Fluorophores were loaded at 37 °C for 25 minutes, after which samples were immediately placed on a glass cover slip mounted in a Focht Chamber System 2 (FCS2™, Bioptechs Inc., Butler, PA, USA), for live cell micro-observation. The temperature was maintained at 25 °C and observations were carried out under an Olympus Fluoview™ FV1000 confocal microscope (Olympus America Inc, Center Valley, PA, USA). Image analysis and fluorescence calculations were performed using Fluoview™ 1.7 software. To optimise the detection of JC1, we preliminarily performed a scan record of the dye emission spectrum over a wavelength interval ranging from 500 nm to 650 nm, with excitation at 488 nm. Emission maxima were detected at 520 nm (for the green JC1 monomer) and 590 nm (for the red aggregate form of JC1). These two emission wavelengths were, therefore, used in the experiments using this dye. The excitation and emission wavelengths for TMRM imaging were set at 550 and 590 nm, respectively. The fluorescence intensity of each cell was measured in a region of interest (ROI) delimitating a single cell, by calculating the mean fluorescence intensity (MFI) at the emission wavelength established. For each sample, 1,000 cells from different fields were scored. The ΔΨm of platelets was estimated by gating platelets with high red (TMRM), or high red and green values (JC1), both characteristic of polarised mitochondria. The gate value for polarised mitochondria was fixed by using the uncoupling agent carbonylcyanide-4-trifluoromethoxyphenylhydrazone (FCCP) as a negative control.
Statistical analysis
The effect of storage and of the different incubation media on the various parameters of platelet metabolism were compared by analysis of variance for repeated measures (ANOVA), with Bonferroni’s correction. A P value less than 0.05 was considered statistically significant.
Results
Platelet count and mean platelet volume
Changes in platelet count and MPV over the 8 days of storage are presented in Table I. Decreases in platelet count were recorded in the different types of bags during storage. However, PC receiving glucose supplementation had higher platelet counts than those of control PC at any time point (P <0.05). It is worth underlining that while the platelet count in the control PC was about 25% lower than the initial count on days 4 and 6, i.e. during the standard transfusional period, PC supplemented with increasing concentrations of glucose had platelet counts at the same time points close to 90% of their respective zero time count (P <0.001 in comparison to the values recorded in control PC). Change in platelet count were accompanied by a significant difference in the MPV. In fact, depending on the substrate dosage received, glucose-treated PC had significantly lower MPV values than those of control PC (P <0.01) (Table I).
Table I.
Effect on platelet count and mean platelet volume (MPV) of the addition of increasing concentrations of glucose to platelet concentrates (PC) during storage with autologous plasma under standard conditions (22 °C, constant shaking). Before the addition of the indicated amounts, the glucose in PC was 29 mM.
| Days of storage | Platelet count (%) | MPV (%) | |
|---|---|---|---|
| Control | 0 | 100.00±1.59 | 100.00±2.12 |
| 2 | 90.41a±2.89 | 100.37±1.77 | |
| 4 | 77.26a±3.09 | 107.41a±2.40 | |
| 6 | 74.05a±1.87 | 114.20a±1.56 | |
| 8 | 62.57a±3.79 | 118.95a±2.85 | |
|
| |||
| 13.75 mM glucose | 0 | 100.00±1.46 | 100.00±1.70 |
| 2 | 93.57a±2.48 | 97.67±2.64 | |
| 4 | 92.94a,b±3.16 | 103.75a,b±3.87 | |
| 6 | 85.34a,b±2.49 | 107.50a,b±1.88 | |
| 8 | 70.42a,b±2.22 | 110.67a,b±1.46 | |
|
| |||
| 27.5 mM glucose | 0 | 100.00±1.10 | 100.00±1.25 |
| 2 | 95.18a,b±2.06 | 93.62a,b±1.90 | |
| 4 | 93.87a,b±1.43 | 101.20b±2.16 | |
| 6 | 89.50a,b±1.27 | 103.38a,b±2.64 | |
| 8 | 76.72a,b±2.41 | 107.44a,b± 1.77 | |
|
| |||
| 55 mM glucose | 0 | 100.00±1.32 | 100.00±1.50 |
| 2 | 96.47b±1.88 | 93.17a,b±2.02 | |
| 4 | 95.86a,b±2.19 | 99.18b±1.64 | |
| 6 | 91.84a,b±1.77 | 101.34b±2.00 | |
| 8 | 84.63a,b±1.65 | 103.14a,b±1.71 | |
Legend
Values are the mean ± standard deviation of six experiments with different PC and are expressed as percent changes with respect to each corresponding zero time.
significantly different from zero time (P <0.05);
significantly different from corresponding time recorded in the control PC (P <0.05).
Effect of substrate addition on platelet metabolism and lysis
Figure 1 illustrates the percent changes of ATP (Panel A) and GTP (Panel B) concentrations during storage in the different types of bags. Control untreated PC maintained ATP and GTP at levels not different from the zero time level only for the first 2 days of storage. From day 2 to day 8, an exponential decay of these two high-energy phosphates occurred in control PC. The addition of increasing glucose concentrations to the PC modified the time-course alterations of energy metabolism during storage. In fact, during the first 2 days of incubation there were significant rises in both ATP and GTP concentrations, with respect to the zero time values, in all glucose-supplemented bags (P <0.01), with maximal ATP (+107.3±35.4%) and GTP (+61.8±11.9%) increases in platelets stored with 55 mM glucose. Four days after incubation, values of ATP and GTP in all glucose-supplemented PC were significantly higher than corresponding values recorded in controls (P <0.01) or at each respective zero time (P <0.01). Compared to day 0, the levels for ATP and GTP at storage day 6 ranged between 87±13% and 77±9% for PC to which 13.75 mM glucose was added and between 115±22% and 110±27% for PC to which 55 mM glucose was added. In consequence, the pattern of ATP and GTP decays as a function of time of storage was represented by polynomial equations in all glucose-treated PC, clearly indicating that the substrate supplementation profoundly affected the energy metabolism of platelet and, therefore, the rate of ATP and GTP depletion. However, on day 8 no differences could be observed in the platelet content of these phosphorylated compounds in control PC and any of the three glucose-supplemented PC.
Figure 1.
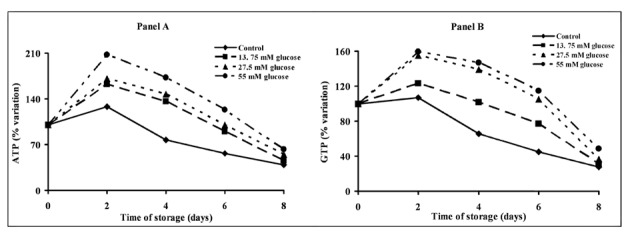
Alterations of energy metabolism of platelet concentrates stored for up to 8 days with autologous plasma only (control) and with autologous plasma supplemented with increasing concentrations of glucose. ATP (Panel A) and GTP (Panel B) were determined in deproteinised platelets at 0, 2, 4, 6 and 8 days of storage at 22 °C under constant shaking. Each point is the mean value for six platelet concentrates and is expressed as percent change with respect to the value determined at time 0, immediately before starting storage. Standard deviations have been omitted for the sake of clarity.
Figure 2 illustrates the changes in ADP, AMP and release in oxypurines (hypoxanthine+xanthine+uric acid) in the four types of PC during the 8 days of storage. Constant declines in ADP (Figure 2, Panel A) and AMP (Figure 2, Panel B) were observed in control PC over the whole period of incubation. Compared to the values in control PC, significantly higher values of both ADP (Figure 2, Panel A) and AMP (Figure 2, Panel B) were recorded in 27.5 and 55 mM glucose-supplemented PC at all time points (P <0.05). In consequence of a general improvement of energy metabolism, the release of oxypurines into the platelet suspension medium during storage of glucose-treated PC was significantly different from that recorded in control PC (Figure 2, Panel C). Although a steady increase in ATP catabolites was measured in all PC bags, the sum of oxypurines in the medium of control PC on day 8 was 5.7-fold higher than that recorded at zero time, while at the end of storage in PC stored with 13.75, 27.5 or 55 mM glucose it was 4.5-, 4.0- and 3.7-fold higher, respectively, than the value recorded at zero time (Figure 2, Panel C). In general, the addition of glucose significantly influenced the amount of ATP catabolites generated during storage in a dose-dependent manner (P <0.01).
Figure 2.
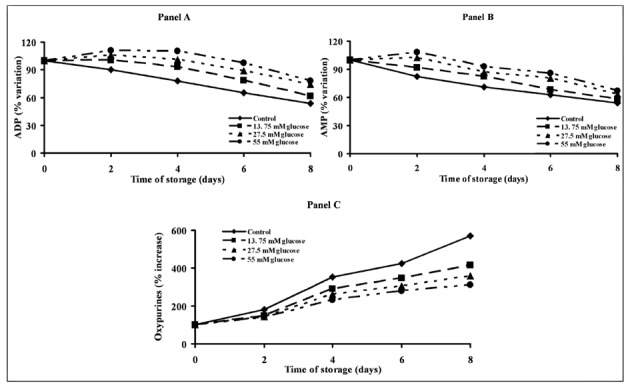
Alterations of energy metabolism of platelet concentrates stored for up to 8 days with autologous plasma only (control) and with autologous plasma supplemented with increasing concentrations of glucose. ADP (Panel A), AMP (Panel B) and oxypurines (hypoxanthine+xanthine+uric acid, Panel C) were determined in deproteinised platelets at 0, 2, 4, 6 and 8 days of storage at 22 °C under constant shaking. Each point is the mean value for six platelet concentrates and is expressed as percent change with respect to the value determined at time 0, immediately before starting storage. Standard deviations have been omitted for the sake of clarity.
Starting on day 4, the amount of lactate released by control PC (Figure 3, Panel A) was significantly higher than that released by PC stored with 27.5 or 55 mM glucose (P <0.05). Starting from day 4, this different increase in proton donors provoked a different decrease of pH which was more evident in control PC than in glucose-treated PC (Figure 3, Panel B).
Figure 3.
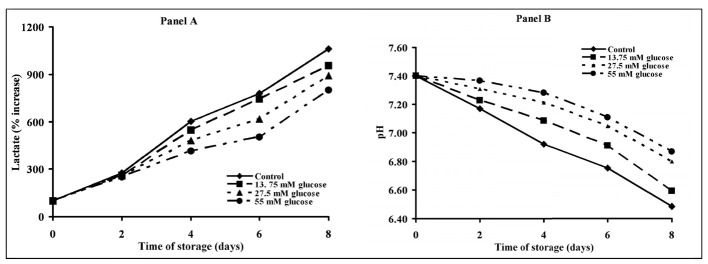
Alterations of metabolic parameters of platelet concentrates stored for up to 8 days with autologous plasma only (control) and with autologous plasma supplemented with increasing concentrations of glucose. Lactate (Panel A) and pH (Panel B) were determined in platelets at 0, 2, 4, 6 and 8 days of storage at 22 °C under constant shaking. Each point is the mean value for six platelet concentrates and is expressed as percent change with respect to the value determined at time 0, immediately before starting storage. Standard deviations have been omitted for the sake of clarity.
As a consequence of the improved energy metabolism of PC stored with increasing concentrations of glucose, there was a significant decrease in platelet lysis (evaluated by the release of LDH in the suspending medium) (Figure 4). Significant increase in LDH activity in the medium was observed starting from day 4 in both control and 13.75 mM glucose-supplemented PC (P <0.01 with respect to the corresponding zero time values), starting from day 6 in 27.5 mM glucose-supplemented PC (P <0.01 with respect to the zero time value) and only on day 8 in 55 mM glucose-supplemented PC (P <0.01 with respect to the zero time value). The LDH activity in the suspending medium of PC supplemented with 27.5 or 55 mM glucose was significantly lower than that measured in suspending medium of control PC at any time point during storage (P <0.01).
Figure 4.
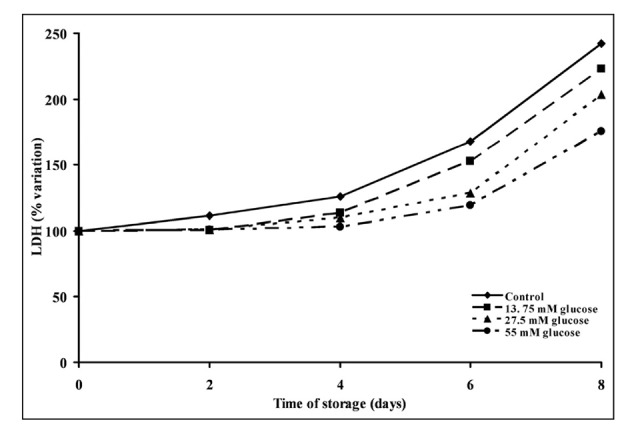
Change in platelet survival during storage of platelet concentrates for up to 8 days with autologous plasma only (control) and with autologous plasma supplemented with increasing concentrations of glucose. Lactate dehydrogenase (LDH) was determined in the platelet suspension medium at 0, 2, 4, 6 and 8 days of storage at 22 °C under constant shaking. Each point is the mean value for six platelet concentrates and is expressed as percent change with respect to the value determined at time 0, immediately before starting storage. Standard deviations have been omitted for the sake of clarity.
Platelet mitochondrial transmembrane potential (ΔΨm)
Figure 5 shows the kinetics of the uptake of the two different fluorochrome dyes JC1 (Panel A) and TMRM (Panel B) during the 8 days of storage. Changes in the mitochondrial transmembrane potential (ΔΨm), expressed as percent of cells with JC1 aggregates (Figure 5, Panel A) and percent of TMRM fluorescence emitting cells (Figure 5, Panel B) were dramatically influenced by time of storage in the absence of substrate addition. Control PC showed a steady decline in mitochondrial functions (already evident after 2 days of storage) with decreases in ΔΨm of 90±6% determined by JC1 and of 80±12% determined by TMRM, when measured after 8 days of storage. Consistently with the data referring to platelet metabolism, addition of glucose to the storage medium produced a dose-dependent protection of ΔΨm. In comparison with corresponding values measured in control PC, significantly higher values of ΔΨm were measured at all time points in PC stored with 13.75, 27.5 or 55 mM glucose. This last substrate concentration allowed platelets to maintain their ΔΨm almost unaltered during 6 days of storage, i.e. for the usual period of platelet storage before transfusion, with a 32±7% decrease measured by JC1 and a 14±5% decrease measured by TMRM, in line with data obtained on platelet metabolism and survival.
Figure 5.
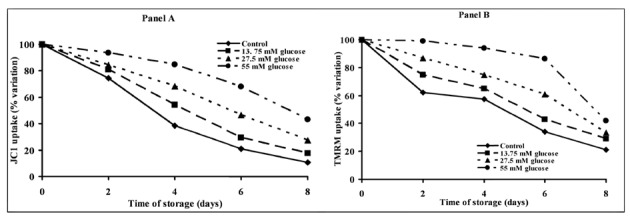
Alterations of mitochondrial functions of platelet concentrates stored for up to 8 days with autologous plasma only (control) and with autologous plasma supplemented with increasing concentrations of glucose. Mitochondrial membrane potential (ΔΨm) was determined in platelets using two different fluorophores (JC1, Panel A and TMRM, Panel B) at 0, 2, 4, 6 and 8 days of storage at 22 °C under constant shaking. Each point is the mean value for six platelet concentrates and is expressed as percent change with respect to the value determined at time 0, immediately before starting storage. Standard deviations have been omitted for the sake of clarity.
Discussion
The main goals of media used to suspend platelets during storage are either to prolong the time of platelet storage or to improve the quality of the PC. Contrasting approaches have been adopted in previous studies to ameliorate platelet storage: (i) the use of substrate-free PAS; (ii) the addition of different (multiple) substrates to the PAS13,21. To our knowledge, no data have been produced in which glucose was added to autologous plasma, derived from the preparation of PC, immediately before storage. The results of the present stud y indicate that the addition of glucose to autologous plasma significantly ameliorates, in a dose-dependent manner, metabolism, survival and morphology of stored platelets, even though it is ineffective in prolonging the time of platelet storage.
The metabolic evaluation of our PC during storage was carried out by measuring a broad spectrum of low molecular weight compounds representative of the cell energy state (high energy phosphates, oxypurines, lactate). Furthermore, to better evaluate mitochondrial functioning we also determined changes in ΔΨm as a function of both the time of storage and the substrate added to the platelets. The progressive rapid depletion of adenine and guanine nucleotides which we recorded in our control PC stored with autologous plasma only, in which a limited amount of glucose was present (about 29 mM at zero time), was accompanied by a steady increase in oxypurines (hypoxanthine+xanthine+uric acid). These results are in line with previously reported data26 showing comparable time-course metabolic depletion (adenine and guanine nucleotides) during platelet storage under similar experimental conditions. It is, therefore, evident that the amount of glucose initially present in the autologous plasma of the buffy coat-derived PC did not ensure the maintenance of an adequate energy supply during the whole period of platelet storage. This provoked an imbalance between ATP production and consumption during storage, leading to increased ATP catabolism (increased oxypurines in the medium). Glucose supplementation of PC produced a general improvement of platelet energy metabolism based on oxidative metabolism of glucose, as evidenced by higher values of ATP and GTP which were accompanied by a significant decrease in the release of oxypurines during storage.
The data from the present experiments clearly indicate the dose-dependent beneficial effects of glucose supplementation on metabolism and survival of platelets during storage; these effects were particularly evident when considering platelet energy metabolism and mitochondrial functions. As indicated by the values of the mitochondrial membrane potential, the increase in substrate availability allowed glucose-treated PC to maintain high values of their ΔΨm. In particular, while on day 6 of storage control PC showed a decrease in ΔΨm of 85% measured by JC1 and of 68% measured by TMRM, at the same time point the ΔΨm of PC supplemented with 55 mM glucose showed decrease of 32% measured by JC1 and of 13% measured by TMRM (values close to those of fresh resting platelets at zero time). This demonstrates that, for the whole duration of the standard period of storage (5–6 days), the mitochondrial function of 55 mM glucose-supplemented PC allowed a sufficient ATP supply through the oxidative phosphorylation coupled to the electron transfer chain. Results of the changes in high-energy phosphate content during storage (ATP, GTP, ADP, AMP) were consistent with the data recorded for the mitochondrial membrane potential and confirmed the dose-dependent effect of glucose in preserving the level of platelet energy stores.
A possible negative influence of glucose supplementation during platelet storage has certainly been attributed to the potential exacerbation of a pH decrease because of the possible acceleration in anaerobic lactate production26. In our experiments, the increase in glucose exogenously added to PC during storage was not accompanied by a parallel increase in lactate or decrease in pH (Figure 3). In fact, control PC, stored with autologous plasma only, showed the highest lactate concentration and lowest pH values at each time point. Even this finding might be related to the protection exerted by glucose on mitochondrial functions and energy metabolism, thereby ensuring glucose consumption based on aerobic glucose oxidation. This allowed maintenance of a high efficiency of the energy yield based on complete glucose oxidation, concomitantly reducing lactate production and decreasing pH with a consequent advantage for the general function of platelets. Hence, an alternative explanation for the increased lactate observed during platelet storage in various studies20,27 may be found in the failure of mitochondrial functions causing a reduction in oxygen consumption and decreased glucose oxidation in spite of sufficient oxygen availability.
Results reported elsewhere, indicating a loss of efficiency of the glucose transporter as one of the putative targets for platelet derangement28, support an additional explanation of our results. The presence of increasing glucose in the PC might have significantly counterbalanced the decrease in the efficiency of the glucose transporter occurring during platelet storage, thereby ensuring an adequate substrate supply for cellular energy needs. Such an effect can only be obtained by increasing the external concentration of glucose well above the concentration found in autologous plasma before platelet storage.
One of the main goals of the storage procedure of blood components, including PC, is to preserve the biological/biochemical characteristics of the different transfusion products in order to allow the maximal clinical efficacy7. In the case of platelets, the maximal time of storage before transfusion should not exceed 5 days29. During this period, the storage conditions applied also in our experiments, rapidly reduced the quality of the PC not only when considering biochemical parameters (decrease in high-energy phosphates, ΔΨm, pH; increase in oxypurines and lactate) but also when measuring morphological and vital parameters (see Table I and Figure 4). According to our results the limited substrate availability might represent the critical parameter that can be corrected to improve the overall quality of the PC, by adding a proper amount of glucose to autologous plasma. The general result is that of ensuring better quality PC during the standard transfusional period. It is, therefore, conceivable to postulate that this type of glucose-enriched PC might have greater clinical effectiveness than that of PC stored with autologous plasma only.
The main limitation of this study is the lack of evidence of a better therapeutic index of platelet transfusion of PC stored in autologous plasma and supplemented with a proper amount of glucose (13.5–55 mM). It is essential to have this clinical evidence before introducing the use of glucose for PC storage in transfusion practice. Studies are in progress to clarify these issues.
Acknowledgments
Angela M. Amorini, Stefano Gullotta, Michele Tuttobene and Flora M. Tomasello carried out the experiments taking care of preparation of the platelet concentrates and storage (Michele Tuttobene), preparation of samples and analysis of platelet metabolites by HPLC (Angela M. Amorini and Stefano Gullotta) and measurement of mitochondrial potential (Flora M. Tomasello). Filomena Biazzo and Vito De Pinto critically reviewed the manuscript. Barbara Tavazzi and Giuseppe Lazzarino supervised the experiments and wrote the manuscript.
This work was supported in part by research funds from Catania University (Giuseppe Lazzarino).
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Gulliksson H. Defining the optimal storage conditions for the long-term storage of platelets. Transfus Med Rev. 2003;17:209–15. doi: 10.1016/s0887-7963(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 2.Rinder HM, Smith BR. In vitro evaluation of stored platelets: is there hope for predicting posttransfusion platelet survival and function? Transfusion. 2003;43:2–6. doi: 10.1046/j.1537-2995.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 3.Ezuki S, Kanno T, Ohto H, et al. Survival and recovery of apheresis platelets stored in a polyolefin container with high oxygen permeability. Vox Sang. 2008;94:292–8. doi: 10.1111/j.1423-0410.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Dikeman R, Molini B, et al. Photochemical treatment of platelet concentrates with amotosalen and long-wavelength ultraviolet light inactivates a broad spectrum of pathogenic bacteria. Transfusion. 2004;44:1496–504. doi: 10.1111/j.1537-2995.2004.04125.x. [DOI] [PubMed] [Google Scholar]
- 5.Ortolano GA, Freundlich LF, Holme S, et al. Detection of bacteria in WBC-reduced PLT concentrates using percent oxygen as a marker for bacteria growth. Transfusion. 2003;43:1276–85. doi: 10.1046/j.1537-2995.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 6.Seghatchian J, Krailadsiri P. The platelet storage lesion. Transfus Med Rev. 1997;11:130–44. doi: 10.1053/tm.1997.0110130. [DOI] [PubMed] [Google Scholar]
- 7.Rinder HM, Snyder EL, Tracey JB. Reversibility of severe metabolic stress in stored platelets after in vitro plasma rescue or in vivo transfusion: restoration of secretory function and maintenance of platelet survival. Transfusion. 2003;43:1230–7. doi: 10.1046/j.1537-2995.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 8.Albanyan AM, Harrison P, Murphy MF. Markers of platelet activation and apoptosis during storage of apheresis- and buffy coat-derived platelet concentrates for 7 days. Transfusion. 2009;49:108–17. doi: 10.1111/j.1537-2995.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JG, Carter CJ, Culibrk B. Buffy-coat platelet variables and metabolism during storage in additive solutions or plasma. Transfusion. 2008;48:847–56. doi: 10.1111/j.1537-2995.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- 10.De Wildt-Eggen J, Gulliksson H. In vivo and in vitro comparison of platelets stored in either synthetic media or plasma. Vox Sang. 2003;84:256–64. doi: 10.1046/j.1423-0410.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 11.Badlou BA, van der Meer PF, Akkerman JW. Metabolic energy reduction by glucose deprivation and low gas exchange preserves platelet function after 48 h storage at 4 degrees C. Vox Sang. 2007;92:311–8. doi: 10.1111/j.1423-0410.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 12.Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 °C: development and current experience. Transfus Med Rev. 2006;20:158–64. doi: 10.1016/j.tmrv.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Guppy M, Whisson ME, Sabaratnam R. Alternative fuels for platelet storage: a metabolic study. Vox Sang. 1990;59:146–52. doi: 10.1111/j.1423-0410.1990.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 14.Hess JR, Rugg N, Knapp AD. The role of electrolytes and pH in RBC ASs. Transfusion. 2001;41:1045–51. doi: 10.1046/j.1537-2995.2001.41081045.x. [DOI] [PubMed] [Google Scholar]
- 15.Hess JR, Greenwalt TG. Storage of red blood cells: new approaches. Transfus Med Rev. 2002;16:283–95. doi: 10.1053/tmrv.2002.35212. [DOI] [PubMed] [Google Scholar]
- 16.Ostrowska A, Gu K, Bode DC, Van Buskirk RG. Hypothermic storage of isolated human hepatocytes: a comparison between University of Wisconsin solution and a hypothermosol platform. Arch Toxicol. 2009;83:493–502. doi: 10.1007/s00204-009-0419-x. [DOI] [PubMed] [Google Scholar]
- 17.Janssen H, Janssen PH, Broelsch CE. Celsior solution compared with University of Wisconsin solution (UW) and histidine-tryptophan-ketoglutarate solution (HTK) in the protection of human hepatocytes against ischemiareperfusion injury. Transpl Int. 2003;16:515–22. doi: 10.1007/s00147-003-0583-5. [DOI] [PubMed] [Google Scholar]
- 18.Van der Meer PF. Platelet additive solutions: a future perspective. Transfus Clin Biol. 2007;14:522–5. doi: 10.1016/j.tracli.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Goodrich L, Hansen E, et al. Platelet glycolytic flux increases stimulated by ultraviolet-induced stress is not the direct cause of platelet morphology and activation changes: possible implications for the role of glucose in platelet storage. Transfusion. 2005;45:1750–8. doi: 10.1111/j.1537-2995.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 20.Dekkers DW, De Cuyper IM, van der Meer PF, et al. Influence of pH on stored human platelets. Transfusion. 2007;47:1889–95. doi: 10.1111/j.1537-2995.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 21.Gyongyossy-Issa MI, Zhang JG, Culibrk B. Novel system for storage of buffy-coat derived platelet concentrates in a glucose-based platelet additive solution: parameters and metabolism during storage and comparison to plasma. Vox Sang. 2009;97:102–9. doi: 10.1111/j.1423-0410.2009.01196.x. [DOI] [PubMed] [Google Scholar]
- 22.Amorini AM, Tuttobene M, Lazzarino G. Evaluation of biochemical parameters in platelet concentrates stored in glucose solution. Blood Transfus. 2007;5:24–32. doi: 10.2450/2007.0019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietersz RNI, Reesink HW, Dekker WJA, Fijen FJ. Preparation of leukocyte-poor platelet concentrates from buffy coats. Vox Sang. 1987;53:203–7. doi: 10.1111/j.1423-0410.1987.tb05067.x. [DOI] [PubMed] [Google Scholar]
- 24.Tavazzi B, Amorini AM, Fazzina G, et al. Oxidative stress induces impairment of human erythrocyte energy metabolism through the oxygen radical-mediated direct activation of AMP-deaminase. J Biol Chem. 2001;276:48083–92. doi: 10.1074/jbc.M101715200. [DOI] [PubMed] [Google Scholar]
- 25.Tavazzi B, Lazzarino G, Leone P, et al. Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N-acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clin Biochem. 2005;38:997–1008. doi: 10.1016/j.clinbiochem.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Edenbrandt CM, Murphy S. Adenine and guanine nucleotide metabolism during platelet storage at 22 degrees C. Blood. 1990;76:1884–92. [PubMed] [Google Scholar]
- 27.Baker JM, Candy DJ, Hawker RJ. Influences of pH on human platelet metabolism. Platelets. 2001;12:333–42. doi: 10.1080/09537100120078412. [DOI] [PubMed] [Google Scholar]
- 28.Sorbara LR, Davies-Hill TM, Koehler-Stec EM. Thrombin-induced translocation of GLUT3 glucose transporters in human platelets. Biochem J. 1997;328:511–6. doi: 10.1042/bj3280511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow JF, Braine HG, Kickler TS. Septic reactions to platelet transfusions: A persistent problem. JAMA. 1991;266:555–8. [PubMed] [Google Scholar]


