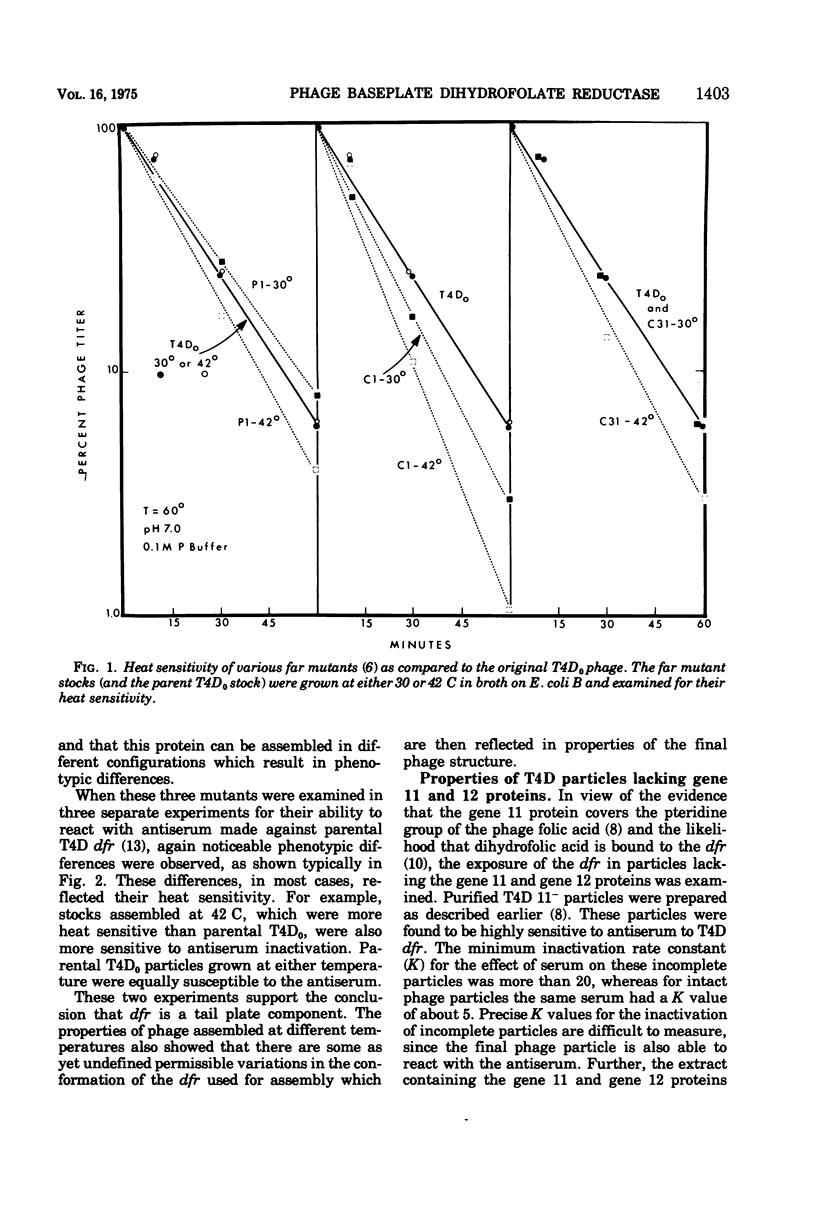
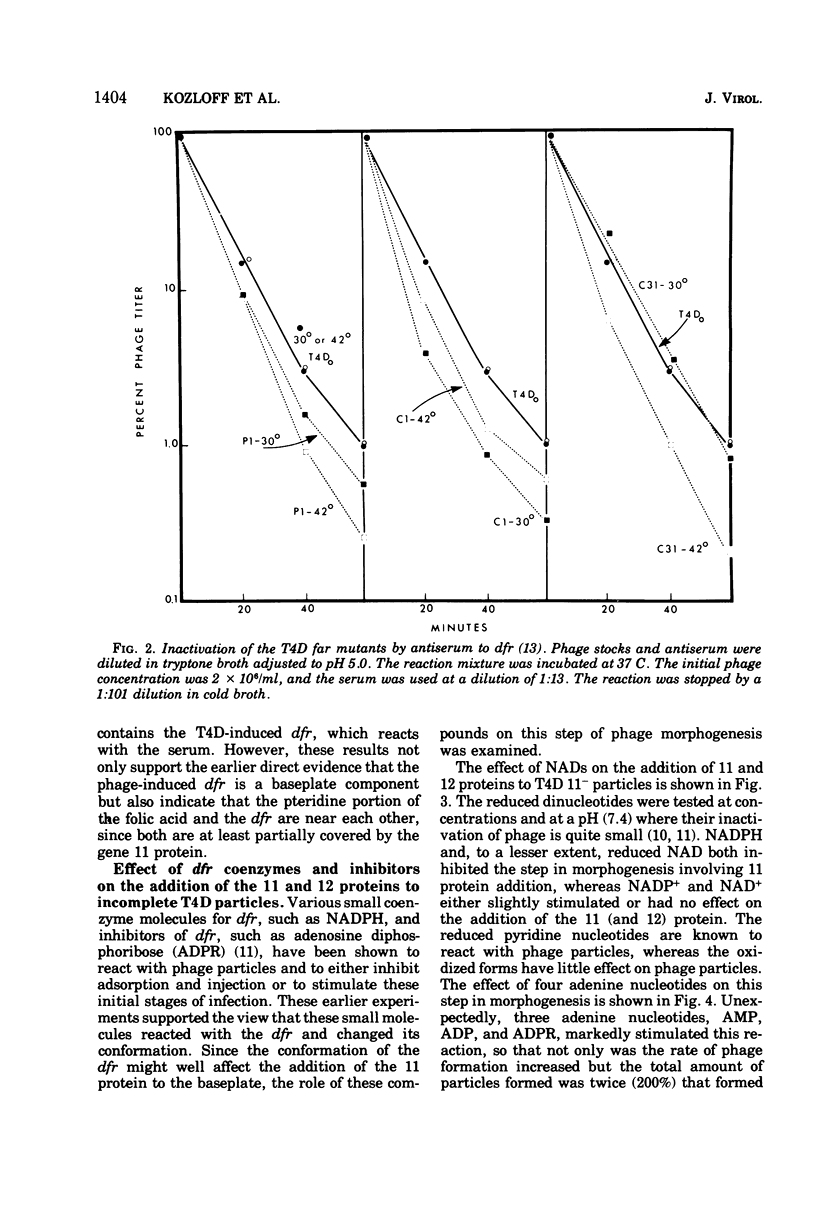
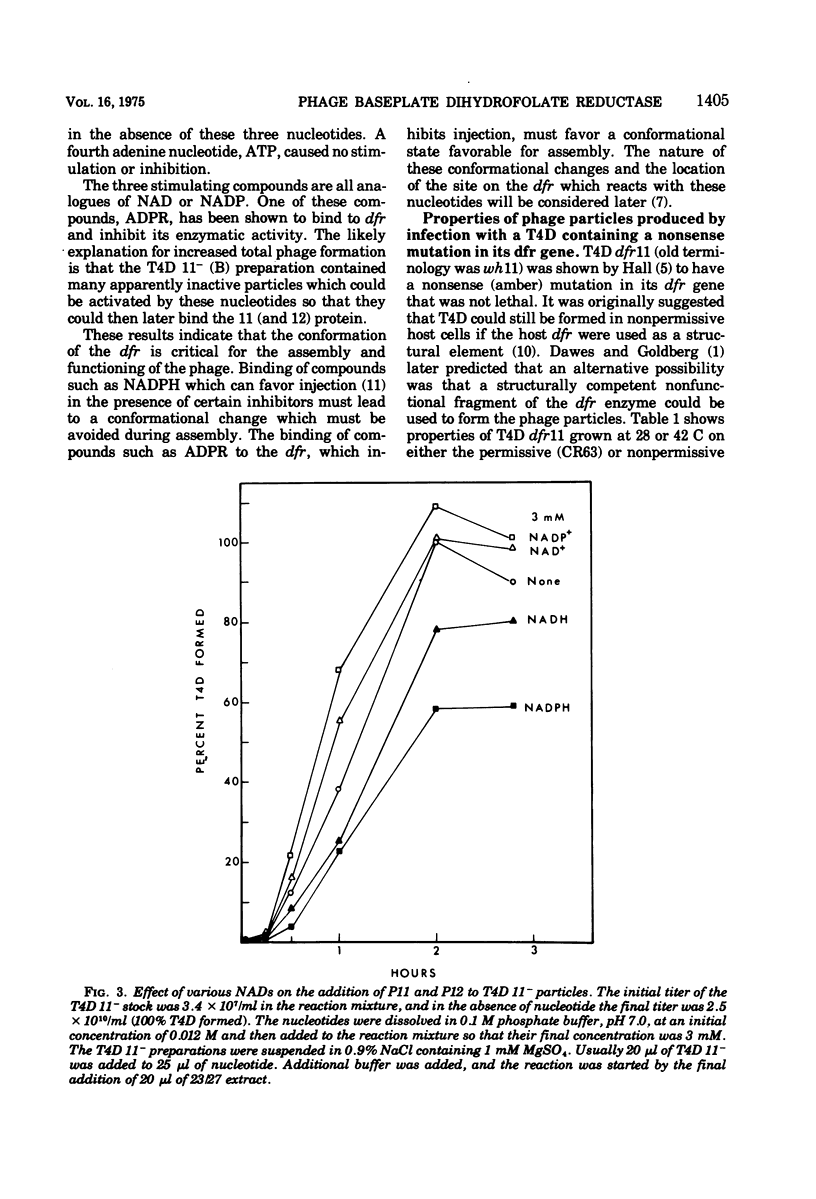
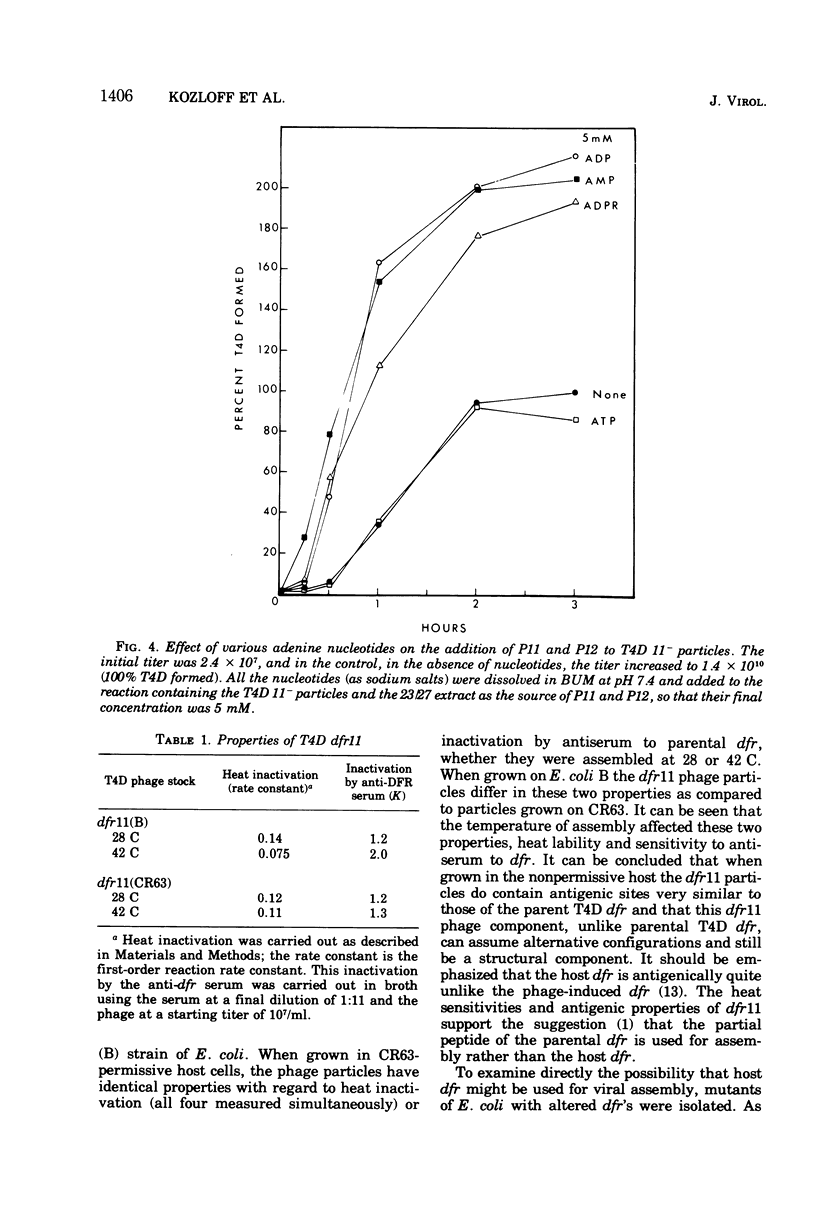
Abstract
The location of T4D phage-induced dihydrofolate reductase (dfr) has been determined in intact and incomplete phage particles. It has been found that phage mutants inducing a temperature-sensitive dfr (dfrts) procude heat-labile phage particles. The structural dfr produced by these ts mutants was shown to assume different configurations depending on the temperature at which the phage is assembled. Morphogenesis of incomplete phage particles lacking the gene 11 protein on their baseplates was found to be inhibited by reagents binding to dfr, such as antibodies to dfr. Further, cofactor molecules for dfr, such as reduced nicotinamide adenine dinucleotide phosphate and reduced nicotinamide adenine dinucleotide, also inhibited the step in morphogenesis involving the addition of gene 11 product. On the other hand, inhibitors of dfr, such as adenosine dephosphoribose, stimulated the addition of the gene 11 protein. It has been concluded that the phage-induced dfr is a baseplate component which is partially covered by the gene 11 protein. The properties of phage particles produced after infection of the nonpermissive host with the one known T4D mutant containing a nonsense mutation in its dfr gene suggested that these progeny particles contained a partial polypeptide, which was large enough to serve as a structural element.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawes J., Goldberg E. B. Functions of baseplate components in bacteriophage T4 infection. I. Dihydrofolate reductase and dihydropteroylhexaglutamate. Virology. 1973 Oct;55(2):380–390. doi: 10.1016/0042-6822(73)90178-5. [DOI] [PubMed] [Google Scholar]
- Dawes J., Goldberg E. B. Functions of baseplate components in bacteriophage T4 infection. II. Products of genes 5, 6, 7, 8, and 10. Virology. 1973 Oct;55(2):391–396. doi: 10.1016/0042-6822(73)90179-7. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Mathews C. K. T4 bacteriophage-specific dihydrofolate reductase: purification to homogeneity by affinity chromatography. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1164–1170. doi: 10.1016/0006-291x(71)90585-7. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Isolation and characterization of mutants of bacteriophage T4 resistant to folate analogs. Virology. 1973 Jun;53(2):413–426. doi: 10.1016/0042-6822(73)90221-3. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Crosby L. K., Lute M. Bacteriophage T4 baseplate components. III. Location and properties of the bacteriophage structural thymidylate synthetase. J Virol. 1975 Dec;16(6):1409–1419. doi: 10.1128/jvi.16.6.1409-1419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K. Bacteriophage T4 baseplate components. I. Binding and location of the folic acid. J Virol. 1975 Dec;16(6):1391–1400. doi: 10.1128/jvi.16.6.1391-1400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K., Rao N., Chapman V. A., DeLong S. S. Bacteriophage tail components. I. Pteroyl polyglutamates in T-even bacteriophages. J Virol. 1970 Jun;5(6):726–739. doi: 10.1128/jvi.5.6.726-739.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Verses C., Lute M., Crosby L. K. Bacteriophage tail components. II. Dihydrofolate reductase in T4D bacteriophage. J Virol. 1970 Jun;5(6):740–753. doi: 10.1128/jvi.5.6.740-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male C. J., Kozloff L. M. Function of T4D structural dihydrofolate reductase in bacteriophage infection. J Virol. 1973 Jun;11(6):840–847. doi: 10.1128/jvi.11.6.840-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K., Crosby L. K., Kozloff L. M. Inactivation of T4D bacteriophage by antiserum against bacteriophage dihydrofolate reductase. J Virol. 1973 Jul;12(1):74–78. doi: 10.1128/jvi.12.1.74-78.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Identity of genes coding for soluble and structural dihydrofolate reductases in bacteriophage T4. J Virol. 1971 Apr;7(4):531–533. doi: 10.1128/jvi.7.4.531-533.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To C. M., Kellenberger E., Eisenstark A. Disassembly of T-even bacteriophage into structural parts and subunits. J Mol Biol. 1969 Dec 28;46(3):493–511. doi: 10.1016/0022-2836(69)90192-2. [DOI] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Uchida H. Organization and function of bacteriophage T4 tail. I. Isolation of heat-sensitive T4 tail mutants. Virology. 1973 Mar;52(1):234–245. doi: 10.1016/0042-6822(73)90412-1. [DOI] [PubMed] [Google Scholar]



