Abstract
Background and objectives
Many patients with chronic anaemia require blood transfusions as part of their treatment regimen. As a result, iron overload will inevitably develop if not adequately managed by iron chelation therapy. There are many guidelines relating to transfusion and chelation practices for patients with transfusion-dependent anaemia; however, there is a lack of information on how treatment practices differ around the world. The objective of this manuscript is to highlight key features of current transfusion and chelation management, including similarities and differences across various anaemias and between geographical regions worldwide.
Materials and methods
Data collected at study entry to the multicentre Evaluation of Patients’ Iron Chelation with Exjade (EPIC) study, which recruited 1,744 patients with a variety of transfusion-dependent anaemias across 23 countries from three geographic regions, were assessed. These analyses compared transfusion and chelation treatment prior to the start of study treatment, together with iron burden assessed at study entry by serum ferritin, liver iron concentration and labile plasma iron levels.
Results and conclusions
Data show that transfusion and iron chelation practices differ between anaemias and between geographical regions; this may be linked to availability and accessibility of transfusion and chelation therapy, patients’ compliance, physicians’ attitudes, costs and use of treatment guidelines. Approximately 60% of these transfusion-dependent patients were severely iron overloaded with a serum ferritin level over 2,500 ng/mL, indicating that the risks of iron burden may have been underestimated and current iron chelation therapy, if considered, may not have been adequate to control iron burden.
Keywords: transfusion practice, chelation practice, iron overload
Introduction
Blood transfusion therapy is the cornerstone of management of many patients with chronic anaemias from hereditary to acquired conditions. Indeed, regular transfusions are the standard of care for the treatment of β-thalassaemia major (TM). For sickle cell disease (SCD), transfusions provide effective treatment and prevention of many complications, including a decreased risk of stroke1,2, and for myelodysplastic syndromes (MDS), transfusions are an integral part of supportive care3. However, iron overload is an inevitable serious complication of chronic blood transfusions and can lead to significant morbidity and mortality if left untreated4,5. Iron overload necessitates iron chelation therapy for patients with transfusion-requiring anaemias, including TM, severe thalassaemia intermedia (TI) such as HbE/β-thalassaemia, SCD, inherited and acquired bone marrow dysfunction from congenital dyserythropoietic anaemia, Diamond-Blackfan anaemia, aplastic anaemia (AA), MDS and others. Although these disorders can be grouped together as “transfusion-dependent”, it is important to note that the clinical onset of anaemia, the level of haemoglobin reduction and related severity and transfusion requirements remain widely variable across diseases. There are several management practice guidelines available for these disorders, which include recommendations on transfusion and iron chelation treatment regimens6–14. However, current “real-world” practice approaches within these populations of patients may not reflect the ideal treatment circumstances that can occur in leading Western medical centres. Moreover, such information has not been widely compared across geographical regions.
The multicentre Evaluation of Patients’ Iron Chelation with Exjade (EPIC) study was the first prospective study to demonstrate that fixed starting doses of deferasirox, based on ongoing iron intake from blood transfusions, with dose titrations according to serum ferritin trends and safety markers, could provide effective chelation as assessed by reduced serum ferritin levels15. The study was conducted across 23 countries and recruited 1,744 patients with various transfusion-dependent anaemias. Data collected at study entry therefore provide a substantial dataset from which an insight can be gained into actual transfusion and chelation treatment practices across anaemias and geographical regions. It is of interest to explore the extent to which guidelines and recommendations for transfusion and iron chelation management of transfusion-dependent anaemias have been adopted throughout the world. Different local issues on treatment accessibility and limitations might vary across geographical regions and may play significant roles in actual management and practices. Any future recommendations for adapting guidelines to local treatment approaches should take these regional issues into account.
The objective of this descriptive assessment is to highlight key features of transfusion and chelation management, comparing and contrasting these features across various transfusion-dependent anaemias and geographical regions.
Materials and methods
Patients’ recruitment into the EPIC study
A detailed overview of the design and methodology of the EPIC study (clinicaltrials.gov identifier: NCT00171821) has been published previously15. Briefly, key inclusion criteria included patients (aged ≥2 years) with transfusional iron overload as shown by a serum ferritin level of ≥1,000 ng/mL or <1,000 ng/mL but with a history of multiple transfusions (>20 transfusions or 100 mL/kg of red blood cells) and R2 magnetic resonance imaging (MRI)-confirmed liver iron concentration (LIC) ≥2 mg of Fe/g dry weight (dw). Underlying anaemia was reported as per the investigator’s clinical knowledge. Patients with a life expectancy of <1 year were excluded from the study. Patients previously receiving deferiprone (DFP) discontinued treatment at least 28 days before entering the study (washout period) but could switch to deferoxamine (DFO) during this time. Patients were permitted DFO until 1 day immediately prior to study entry. Patients (or parents/guardians) provided written, informed consent before entering the study. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Assessments
In this assessment, the patients’ characteristics at enrolment were reported according to the following underlying anaemias - TM, TI, MDS, SCD and AA - and geographical regions - Europe (Austria, Belgium, Denmark, France, Germany, Greece, Italy, The Netherlands, Spain, Switzerland and United Kingdom), Middle East/Africa (Egypt, Israel, Lebanon, South Africa and Turkey) and Asia-Pacific (Australia, China, Hong Kong, Malaysia, South Korea, Taiwan and Thailand). Serum ferritin levels at study entry were taken as the average of the available measurements (1 or 2 depending on chelation history) within 28 days prior to the start of study drug treatment with deferasirox.
Patients enrolled at centres with appropriate MRI scanners/expertise were invited to participate in a MRI substudy assessing LIC, for which separate informed consent forms were provided. R2 scans were performed using FerriScan (Resonance Health, Perth, Australia) according to standardised procedures. The magnetic resonance scanners were calibrated according to the providers’ specification, and accuracy was verified by a central laboratory (Inner Vision Biometrics, a subsidiary of Resonance Health, Perth, Australia). Axial images of the liver were acquired at all imaging sites, and all raw image data were transmitted electronically to a central laboratory for analysis using a previously described technique16.
Labile plasma iron (LPI) levels at study entry were analysed at a central laboratory according to a previously described method, using an assay that measures iron-specific redox cycling capacity in the presence of low ascorbate concentrations17. Levels were compared against a normal LPI level of ≤0.4 μmol/L18. Haemoglobin levels at study entry were measured locally at the investigational sites in addition to the assessment carried out by the central laboratory.
Statistical analyses
For descriptive measures of normally distributed variables the mean±standard deviation (SD) are presented. Qualitative data are presented as numbers (n) and % fractions.
Results
Patients’ characterisation
Patients were enrolled into the EPIC study between April 2005 and May 2007. Overall, 1,744 patients were enrolled, of whom 1,558 are included in the analyses presented here (Table I); patients with other rare anaemias (predominantly red cell aplasia and haemolytic anaemias), and malignant diseases were not included in these analyses because of the low numbers of patients divided by geographical region. The characteristics of the patients at study entry, divided by underlying anaemia and geographical region, are shown in Table I.
Table I.
Characteristics of the patients included in these analyses at the time of these enrolment into the EPIC study.
| Characteristic | All regions (n=1558) | Europe (n=680) | Middle East/Africa (n=275) | Asia-Pacific (n=603) |
|---|---|---|---|---|
| Number of patients | ||||
| TM | 937 | 281 | 240 | 416 |
| TI | 84 | 21 | 7 | 56 |
| MDS | 341 | 293 | 3 | 45 |
| AA | 116 | 29 | 5 | 82 |
| SCD | 80 | 56 | 20 | 4 |
| Mean age (range), years | ||||
| TM | 18.4 (2–72) | 24.9 (2–72) | 14.5 (2–43) | 16.2 (2–65) |
| TI | 19.2 (4–70) | 34.7 (5–70) | 12.4 (4–28) | 14.3 (4–45) |
| MDS | 67.9 (11–89) | 68.8 (11–89) | 62.7 (33–78) | 62.4 (18–86) |
| AA | 33.3 (2–79) | 36.1 (2–79) | 18.2 (3–42) | 33.2 (7–67) |
| SCD | 23.9 (4–60) | 26.9 (9–51) | 12.7 (4–41) | 36.5 (22–60) |
| Male:female, n (% male) | ||||
| TM | 450:487 (48) | 126:155 (45) | 124:116 (52) | 200:216 (48) |
| TI | 46:38 (55) | 15:6 (71) | 2:5 (29) | 29:27 (52) |
| MDS | 204:137 (60) | 178:115 (61) | 2:1 (67) | 24:21 (53) |
| AA | 67:49 (58) | 16:13 (55) | 2:3 (40) | 49:33 (60) |
| SCD | 39:41 (49) | 26:30 (46) | 9:11 (45) | 4:0 (100) |
| Race (Caucasian:Oriental:Other), n | ||||
| TM | 441:452:44 | 234:9:38 | 168:69:3 | 39:374:3 |
| TI | 26:54:4 | 18:1:2 | 6:1:0 | 2:52:2 |
| MDS | 309:30:2 | 290:1:2 | 0:3:0 | 19:26:0 |
| AA | 32:80:4 | 29:0:0 | 0:1:4 | 3:79:0 |
| SCD | 18:15:47 | 8:2:46 | 7:13:0 | 3:0:1 |
| History of hepatitis B and/or C, n (%) | ||||
| TM | 258 (27.5) | 122 (43.4) | 59 (24.6) | 77 (18.5) |
| TI | 8 (9.5) | 3 (14.3) | 1 (14.3) | 4 (7.1) |
| MDS | 11 (3.2) | 10 (3.4) | 0 (0) | 1 (2.2) |
| AA | 8 (6.9) | 2 (6.9) | 0 (0) | 6 (7.3) |
| SCD | 19 (23.8) | 12 (21.4) | 6 (30.0) | 1 (25.0) |
| Splenectomy, n (%) | ||||
| TM | 333 (35.5) | 101 (35.9) | 110 (45.8) | 122 (29.3) |
| TI | 28 (33.3) | 17 (81.0) | 2 (28.6) | 9 (16.1) |
| MDS | 13 (3.8) | 10 (3.4) | 0 (0) | 3 (6.7) |
| AA | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SCD | 22 (27.5) | 13 (23.2) | 9 (45.0) | 0 (0) |
Legend
TM: thalassaemia major; TI: thalassaemia intermedia; MDS: myelodysplastic syndromes; AA: aplastic anaemia; SCD: sickle cell disease.
The patients’ mean age at enrolment was consistently lower in the Middle East/Africa region across all anaemias, apart from that of patients with MDS; the patients’ mean age was generally highest in Europe for all anaemias apart from SCD, for which it was higher in the Asia-Pacific region, although the number of patients was small (n=4). The proportion of patients with a history of hepatitis B and/or C was generally higher in Europe than in the Asia-Pacific and the Middle East/Africa regions.
Overall, patients with TM (35.5%), transfusion-dependent TI (33.3%) and SCD (27.5%) had a higher incidence of splenectomy than patients with MDS (3.8%) and AA (0%). However, this practice varied between different geographical regions; for example 45.8% of patients with TM in the Middle East/Africa region were splenectomised compared with 35.9% and 29.3% of patients with TM in Europe and the Asia-Pacific region, respectively. Furthermore, a higher proportion of patients with transfusion-dependent TI were splenectomised in Europe (81.0%) and a higher proportion with SCD were splenectomised in the Middle East/Africa region (45.0%) compared with other regions (Table I).
Transfusion history
The transfusion history of patients, divided by type of anaemia and geographical region, is shown in Figure 1 and Table II. Figure 1 illustrates the percentage of the patients’ lifetime receiving transfusions; Table II presents the mean number of transfusion sessions and blood volume administered in the year prior to study entry (data for SCD patients are not given as some patients received exchange transfusions).
Figure 1.
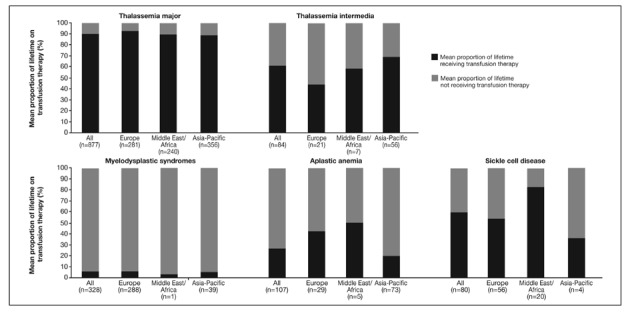
Mean proportion of lifetime on transfusion therapy* for patients enrolled in the EPIC study included in these analyses.
*Determined by the number of years the subject had already received transfusion therapy divided by age at screening; regular or intermittent transfusions were not differentiated in the protocol.
Data from Taiwan were excluded because of inadequate data recording as a result of patients being referred to investigator sites for enrolment in the study.
Table II.
Transfusion history in the year prior to entry into the EPIC study for patients included in these analyses.
| All regions | Europe | Middle East/Africa | Asia-Pacific | |
|---|---|---|---|---|
| Mean ± SD number of transfusion sessions in the year prior to study entry, n | ||||
| TM | 17.5±8.8 (n=935) | 22.7±10.6 (n=279) | 15.8±8.3 (n=240) | 14.9±5.5 (n=416) |
| TI | 13.5±7.1 (n=82) | 12.5±11.6 (n=19) | 10.9±4.5 (n=7) | 14.2±5.2 (n=56) |
| MDS | 24.3±17.7 (n=335) | 25.7±18.3 (n=288) | 12.0±0.0 (n=2) | 16.0±10.2 (n=45) |
| AA | 12.5±13.0 (n=114) | 18.0±20.7 (n=28) | 12.0±7.4 (n=5) | 10.7±8.8 (n=81) |
| Mean ± SD volume blood transfused in the year prior to study entry, mL/kg | ||||
| TM | 189.8±139.3 (n=917) | 190.9±210.3 (n=263) | 141.4±76.9 (n=240) | 217.2±97.2 (n=414) |
| TI | 155.4±87.0 (n=80) | 63.2±73.5 (n=17) | 97.2±56.6 (n=7) | 190.7±68.1 (n=56) |
| MDS | 116.4±123.1 (n=302) | 117.5±128.2 (n=255) | 83.8±25.3 (n=2) | 111.3±93.2 (n=45) |
| AA | 115.8±179.4 (n=112) | 129.6±138.1 (n=26) | 113.6±35.7 (n=5) | 111.4±196.4 (n=81) |
Legend: numbers in brackets are the total numbers of patients with the specific diagnosis within the geographical region. Data for SCD patients are not given in this table as some patients received exchange transfusions.
The proportion of the patients’ lifetime on transfusion therapy varied according to the type of anaemia they had, although the trend across all geographical regions was similar (Figure 1). Patients with TM received transfusion therapy for most of their lifetime (∼90%), which was consistent across geographical regions. Patients with SCD also received transfusion therapy for a considerable proportion of their lifetime (∼60%); however, this proportion was considerably higher in the Middle East/Africa region (∼80%) than in Europe and the Asia-Pacific region (∼50% and ∼35%, respectively). An early age of diagnosis and the established benefit of early and regular transfusion for patients with TM and SCD may be reasons for the large proportion of these patients’ lifetime on transfusion; this trend may also be linked to the higher frequency of patients reported to have a history of hepatitis B and/or C (27.5% and 23.8%, respectively) in these groups (Table I).
Transfusion-dependent patients with TI enrolled in EPIC had received transfusion therapy for a larger proportion of their lifetime in the Asia-Pacific region than in Europe; this may be partially because patients with the more severe phenotype HbE/β-thalassaemia are most prevalent in the Asia-Pacific region. However, their transfusion load was lower than that of TM patients across different regions. The proportion of lifetime receiving transfusions for patients with MDS was very low compared with that of patients with other types of anaemia, and was similar across geographical regions.
The mean number of transfusions in the year prior to study entry in TM patients was lower in those from the Asia-Pacific region than in those from other regions, although the mean volume of blood transfused was higher in the Asia-Pacific region for patients with TM and TI than in other regions (Table II). This suggests different standards relating to the volume of blood contained in each unit provided in the Asia-Pacific region compared with other parts of the world.
Chelation history
Whether patients received chelation therapy prior to entry into the EPIC study for this analysis is shown in Figure 2. The lightest grey sections represent the proportion of patients who were chelation-naïve, the other shaded sections show the type of chelator given to those patients who were receiving chelation therapy.
Figure 2.
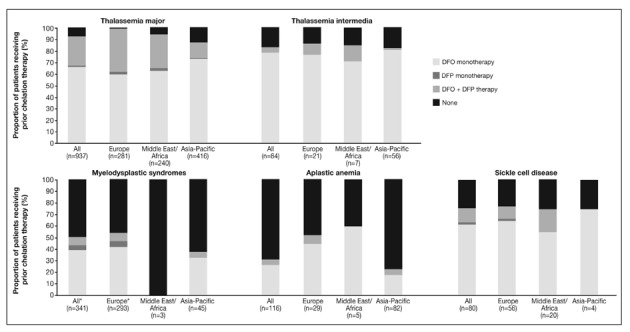
Prior chelation therapy of patients enrolled in the EPIC study included in these analyses.
*One MDS patient in Europe was classified as receiving “other” chelation therapy.
Monotherapy with DFO was the most widely used prior iron chelation therapy; other therapies, including DFP monotherapy, and DFO + DFP therapy (both therapies received either as monotherapy or in combination), were more common in Europe and the Middle East/Africa regions than in the Asia-Pacific region, where there may have been limited access to DFP during the last decade. When DFP was prescribed, this appears to have been rarely as monotherapy and far more commonly in combination with DFO across all types of anaemia.
Overall, as expected for diseases in which chelation therapy is more clinically established, the proportion of previously chelated patients was higher among TM and TI patients than among those with other anaemias. Of patients with MDS and AA, 48.4% and 68.1% respectively, were chelation-naïve despite having significant iron overload. In general, chelation-naïve patients were more common in the Asia-Pacific region than in the other regions. Only one patient (aged 2 years; 0.4%) with TM in Europe (overall mean age 24.9 years [n=281]) was chelation-naïve, compared with 5.4% of patients in the Middle East/Africa region (overall mean age 14.5 years [n=240]; chelationnaïve mean age 5.5 years [n=13]) and 12.5% in the Asia-Pacific region (overall mean age 16.2 years [n=416]; chelation-naïve mean age 6.8 years [n=52]).
Among previously chelated patients, the proportion of their lifetime receiving this therapy is shown in Figure 3A. Overall, as expected, across all regions the proportion of lifetime receiving chelation therapy (Figure 3A) was smaller than the proportions receiving transfusion therapy (Figure 1). Across all regions, the mean difference between the proportion of lifetime receiving transfusions and chelation therapy was greatest amongst TM patients (32.8%; AA=8.8%, MDS=2.6%, SCD=20.4% and TI=20.5%). This indicates that TM patients, despite heavy transfusion regimens, are waiting a considerable period before iron chelation therapy is initiated. For TM patients, the proportion of lifetime receiving chelation therapy was 55.5% in the Asia-Pacific region compared with >60% in the other regions (Figure 3A). Plots of the percentage of lifetime receiving transfusion vs the percentage of lifetime receiving chelation therapy for TM patients confirm that, compared with European patients, there are more patients in the Asia-Pacific region who spend less of their lifetime receiving chelation therapy (more patients are clustered in the top left quadrant: low % lifetime on chelation, high % lifetime on transfusion; Figure 3B).
Figure 3A.
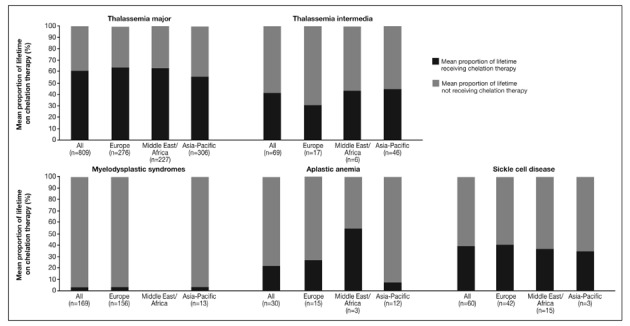
Mean proportion of lifetime on chelation therapy* for patients enrolled in the EPIC study included in these analyses.
*Determined by the number of years the subject had already received chelation therapy divided by age at screening; regular or intermittent transfusions were not differentiated in the protocol.
Figure 3B.
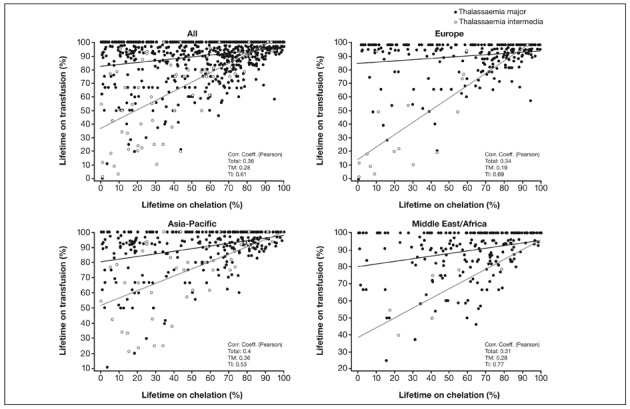
Percentage of lifetime on transfusion vs percentage of lifetime on chelation for thalassaemia major and thalassaemia intermedia.
Data from Taiwan were excluded due to inadequate data recording as a result of patients being referred to investigator sites for enrolment in the study.
Another difference is in SCD patients in the Middle East/Africa region, where although the number of patients was small (n=20), the proportion of lifetime receiving transfusion was high at 82% but the proportion of lifetime receiving chelation therapy was only 37%.
Iron burden
To allow a comparison of the iron burden of these patients, levels of serum ferritin, LIC, haemoglobin and LPI at study entry are shown in Figures 4, 5 and 6.
Figure 4.
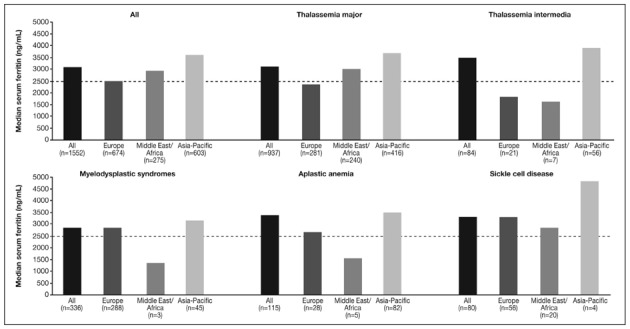
Serum ferritin levels at study entry of patients enrolled in the EPIC study included in these analyses.
Dashed line represents a serum ferritin level threshold of 2,500 ng/mL.
Figure 5.
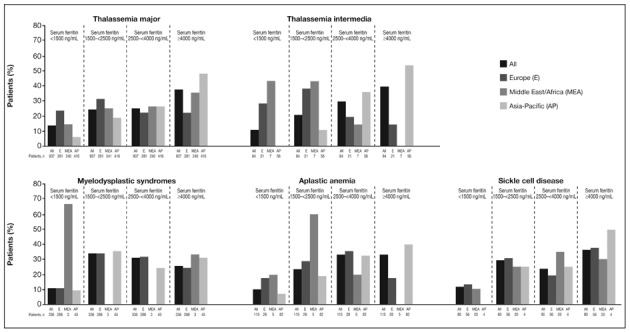
Serum ferritin level categories at study entry of patients enrolled in the EPIC study included in these analyses.
Figure 6.
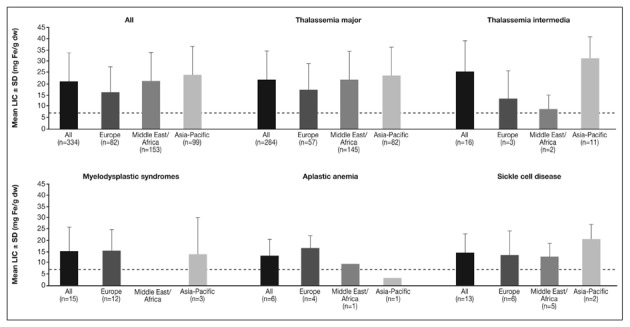
LIC at study entry of a subset of patients enrolled in the EPIC study included in these analyses.
Dashed line represents a LIC threshold of 7 mg Fe/g dw.
The overall median serum ferritin level was above 2,500 ng/mL across all regions. Levels were highest in the Asia-Pacific region, with levels substantially above 2,500 ng/mL in patients with all types of anaemia (Figure 4). The median serum ferritin levels were below 2,500 ng/mL for patients with TM and TI in Europe, and for patients with TI, MDS and AA in the Middle East/Africa region; however, the numbers of patients with the latter underlying anaemias were low in the Middle East/Africa region.
Figure 4 shows the median serum ferritin levels, while Figure 5 shows serum ferritin levels categorised by range for each underlying anaemia and geographical region. A substantial proportion of patients across anaemias and regions (25.3–39.3%) had a serum ferritin level of ≥4,000 ng/mL; patients within this serum ferritin level category were particularly common in the Asia-Pacific region across all anaemias (31.1–53.6%; Figure 5). The number of TM patients in the ≥4,000 ng/mL category was also greater in the Middle East/Africa region than in Europe.
In addition to serum ferritin values at study entry, LIC data were available from a subset of patients enrolled in the EPIC trial (n=334, Figure 5). Overall, mean LIC was >15 mg Fe/g dw across all regions (Figure 6). With the exception of the AA patient from the Asia-Pacific region, the mean LIC was >7 mg Fe/g dw across all regions and across all underlying types of anemia. The overall pattern of LIC across anaemias and regions reflects that seen for serum ferritin values, with LIC generally being highest across most anaemias in the Asia-Pacific region.
LPI data were available for 763 patients (49%). Overall, mean LPI level was above 0.4 μmol/L18 in patients with TM and MDS (Figure 7). In the Middle East/Africa region, mean LPI levels in patients with TM was considerably higher than in patients with TM from other regions. Patients with SCD and TI had the lowest LPI levels, which were within the normal range across all regions. As previously noted, patients with AA also tended to have low LPI levels19.
Figure 7.
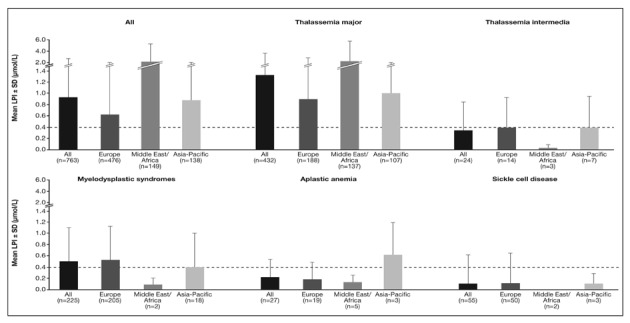
LPI levels in a subset of patients enrolled in the EPIC study included in these analyses.
Dashed line represents a LPI level threshold of 0.4 μmol/L.
Discussion
The large population of patients with various types of transfusion-dependent anaemia recruited into the multinational EPIC study has provided a unique opportunity to compare transfusion and chelation practices, in addition to iron burden, across various anaemias and geographical regions.
Irrespectively of the underlying anaemia, one of the most important observations is that serum ferritin levels were >2,500 ng/mL in a large proportion of patients (∼60%); a threshold known to be associated with negative outcomes20. Although patients with TM had received prior chelation for a larger proportion of their lives than patients with other anaemias, this did not translate into reduced overall serum ferritin levels, possibly due to sub-optimal chelation regimens with respect to their higher transfusional iron intake; this could be related to under-dosing or compliance issues with previous, primarily DFO, chelation therapy21. Mean LIC was also above 15 mg Fe/g dw in all regions, a value associated with increased hepatic and extra-hepatic risk including cardiac disease and early death4,22.
Transfusion-dependent patients with “TI” in this study had received transfusions for a considerable proportion of their lifetime and had a relatively heavy transfusion burden. This reflects the relatively imprecise use of the term “TI”. Many patients with milder β-thalassaemia genotypes (such as HbE/β-thalassaemia) start life independent of transfusions but later become transfusion-dependent. Such patients may continue to be classified as TI despite receiving treatment more typical of TM. It may be more reasonable in the future for clinicians to classify these as “thalassaemia major” or “severe TI” patients based on their current transfusion schedule.
The proportion of lifetime receiving transfusions in patients with MDS was comparatively small. This may be expected given the advanced age of patients when diagnosed with these conditions; the mean age (67.9 years) of MDS patients reported here was higher than that of the other anaemias. Nevertheless, patients with MDS were heavily iron overloaded, demonstrating how quickly iron overload can develop and the possible impact of increased gastrointestinal absorption due to ineffective erythropoiesis. Patients with AA also received transfusion therapy for a smaller proportion of their lifetime than patients with other types of anaemia, with a relatively light transfusion burden; however, overall serum ferritin levels still exceeded 2,500 ng/mL.
Transfusion-dependent patients with SCD had been receiving transfusion therapy for approximately 60% of their lifetime, and whether receiving transfusions or exchange transfusions, were heavily iron-overloaded. Elevated iron levels in SCD patients highlights how iron overload can also be a cumulative process that can develop over many years.
A relatively small proportion of patients with MDS and AA had received prior chelation therapy, suggesting that the risks of iron overload and possible need for iron chelation therapy continue to be underestimated. In these disorders, other cytopenias such as thrombocytopenia and neutropenia are prevalent beside anaemia and reticulocytopenia, which also demand transfusion. At the time of enrolment, DFO was the only other chelator approved for use in MDS and AA patients; however patients with thrombocytopenia may be prohibited from undergoing regular subcutaneous infusions with DFO because of possible local bleeding. For patients with anaemias related to bone marrow disorders, the availability of deferasirox provides clinicians with an option that might allow for better management of iron overload.
Regional differences in transfusion and chelation practices
Variations in transfusion and chelation practices between geographical regions noted here are likely to reflect variations in regional treatment practices, access to treatment, cost/patient reimbursement and treatment guidelines.
Europe
The percentage of TM patients with a history of hepatitis B and/or C was higher in Europe than in other regions. TM patients in Europe were generally older (mean 24.9 years) than in other regions (Middle East/Africa: 14.5 years; Asia-Pacific: 16.2 years) and, therefore, may have been at greater risk of receiving infected blood products over these additional ∼10 years; more recent coverage of vaccinations and rigorous blood donor screening implemented in the last decade in the Asia-Pacific region may have contributed to the lower rate of hepatitis in these patients. Among all the regions, the number of transfusion sessions for TM patients in the year prior to study entry was highest in Europe; the volume of blood transfused in Europe was higher than in the Middle East/Africa region but slightly lower than in the Asia-Pacific region. This may be explained by logistical factors, whereby physicians in Asia might provide more blood per session and extend the period between transfusion sessions. The proportion of lifetime that TM patients had received transfusions was similar in all regions. Serum ferritin levels and LIC in patients with TM were, however, lower in European patients, a result that may reflect that these patients had received chelation treatment for a larger proportion of their lifetime than had patients in the Asia-Pacific region, for example. Indeed the accessibility or awareness of the need for effective iron chelation therapy in Europe may be higher than in other regions.
European patients with MDS received more transfusions in the year prior to study entry than did patients in other regions, perhaps reflecting the current practice of initiating transfusions at a higher median threshold of haemoglobin (8–10 g/dL compared with 6–9 g/dL in the Asia-Pacific region)23.
Middle East/Africa
The large percentage of TM patients from the Middle East/Africa region with high overall serum ferritin mirrors the high baseline levels (3,356 ng/mL [n=237]) for Middle Eastern patients seen in another deferasirox study (ESCALATOR).24
Raised LPI appears predominantly in highly transfused patients such as those with TM; the LPI in patients with TM from this region was considerably higher than that in patients from other regions. This was possibly indicative of inadequate prior chelation regimens since the proportions of lifetime that TM patients in the Middle East/Africa regions received transfusion and chelation therapy (∼90% and ∼60% of lifetime, respectively) were similar to those in other regions. Suboptimal prior chelation therapy may be partially attributed to logistic reasons, in that in many countries in the Middle East iron chelators are not provided free of charge or are not available on a sustainable basis. A subset of TM patients from the ESCALATOR trial also showed high baseline LPI (0.98 μmol/L), despite the patients having received prior DFO and DFP therapy25; this finding is further indicative of inadequate prior chelation regimens.
The proportion of lifetime receiving transfusion therapy in patients with SCD was considerably higher in the Middle East/Africa region while the proportion of lifetime receiving chelation therapy was similar to that in other regions (although the numbers of patients were small), which contributed to iron overload in these patients (Figures 1, 3A, 3B, 4, 5). Age may have affected this finding, as 10 of the 20 SCD patients in the Middle East/Africa region were aged 6–12 years, whereas in Europe the majority of the SCD patients (42/56) were aged 16–50 years.
Asia-Pacific
Compared with patients in Europe, a larger proportion of patients in the Asia-Pacific region had serum ferritin levels over 4,000 ng/mL. Patients with TM in this region had a similar proportion of lifetime on transfusion therapy to those in Europe but the proportion of lifetime on chelation therapy in TM patients was lower (48.9%; mean age 16.2 years versus 63.6%; mean age 24.9 years), which is likely to have contributed to the high serum ferritin levels. The high proportion of patients with serum ferritin levels >4,000 ng/mL from the Asia-Pacific region may have influenced the overall serum ferritin levels of patients with TM enrolled in the EPIC study, which was higher than that seen in other deferasirox trials not recruiting from the Asia-Pacific region (e.g., Cappellini et al. 2006, median serum ferritin: 2,143 ng/mL [n=586])26. High serum ferritin in paediatric patients from Thailand has previously been reported with approximately 40% of β-thalassaemia patients having serum ferritin levels >2,500 ng/mL27.
In Taiwan, transfusion therapy is usually given every 2–4 weeks with the aim of maintaining haemoglobin levels >10 g/dL28; the Thalassemia International Federation recommend levels of >9–10.5 g/dL12. This practice may increase transfusion requirements and, therefore, the rate of iron loading13. However, it is important to note that a greater number of patients with TM and TI in the Asia-Pacific region were not adequately chelated, with a considerable proportion being chelation-naïve. In Thailand, many children have severe thalassaemia and are under-treated, with pre-transfusion haemoglobin below 7 g/dL, but very few receive adequate chelation therapy27.
In the Asia-Pacific region, serum ferritin levels in patients with MDS, unlike the levels in patients with other diagnoses, were not considerably higher than in Europe. This may be linked to the more restrictive way MDS patients are transfused in this region compared to other regions, supported by a lower number of transfusions in the year prior to study entry in this region compared with Europe (Table II).
In the last 10 years, only a few Asia-Pacific countries (e.g., Taiwan, Australia and Hong Kong) have been providing free DFO for their patients. Before 2007, iron chelation therapy was not included in Thailand’s healthcare reimbursement programme; as a result more than half of patients with TM and TI were under-dosed with DFO (<40 mg/kg/day). There was a similar situation in other countries, including Malaysia and China. However, despite some patients having more access to a standard dose of DFO, difficult administration and frequent injection site reactions have been disadvantageous and have restricted the regular use of DFO; this has caused significant poor compliance in the Asia-Pacific region among patients with TM and TI28.
In this region, where the incidence of AA is higher than in Western countries29, patients generally received transfusion therapy for less of their lifetime and a lower number of transfusions than patients in Europe and the Middle East/Africa region. However, iron overload was still evident, and there were a high number of chelation-naïve patients. These data support recent findings that many patients with AA had iron overload but iron chelation with DFO was not actively administered until complications related to iron overload had appeared30. Poor compliance to DFO once administered may also contribute to iron-overload in this population.
Comparison of treatment practices and guidelines
In light of the data reported here, it is interesting to report on the many guidelines for transfusions and iron chelation therapy. For patients with TM, the Thalassemia International Federation12 and Italian Society of Haematology practice guidelines6 recommend iron chelation therapy for patients who have had 10–20 transfusions or have a serum ferritin level of >1,000 ng/mL. In practice, this requires the introduction of chelation therapy within 2 years of commencing regular transfusions. The difference between the proportion of lifetime receiving transfusion and chelation for TM patients (32.8%) reported here implies that TM patients are often waiting a longer period than recommended before initiating chelation therapy. In addition, if these guidelines were followed worldwide, all patients with TM recruited into the EPIC study should have been receiving chelation therapy, whereas many were not.
Many MDS treatment guidelines recommend transfusions as supportive care. Additionally, most guidelines recommend iron chelation therapy in patients with MDS who have lower risk, are transfusion-dependent, have a serum ferritin level >1,000 ng/mL7,8,10,23,31 (>2,500 ng/mL according to NCCN guidelines10) and have a life expectancy of at least 1 year8,31. This assessment shows that the treatment guidelines are not widely adhered to as a high proportion of patients with MDS were chelation-naïve. This may partially be explained by the ongoing debate over the benefit of iron chelation therapy in MDS given the advanced age of these patients and their relatively short life expectancy, which may limit the extent to which iron chelation therapy is used in many cases.
For patients with SCD, National Institutes of Health14 and UK11 guidelines recommend that iron chelation therapy is given to patients with ≥20 transfusions in their lifetime or a LIC ≥7 mg Fe/g dw. Similarly, in the UK AA guidelines9, iron chelation therapy is recommended if serum ferritin levels are >1,000 ng/mL. Nevertheless, a considerable proportion of patients with SCD and AA enrolled into the EPIC study were chelation-naïve. Thus, although guidelines exist for many anaemias worldwide, they are not fully adhered to.
Limitations and conclusions
There are several limitations to these analyses. Participating centres in the EPIC trial may not necessarily provide a good representation of actual practice; participating physicians may have preferentially included patients with relatively high serum ferritin levels, potentially selecting patients who had compliance issues with prior iron chelation therapy. Most of the centres in developing countries were affiliated with medical schools or university hospitals where a higher standard of care may be expected compared with that of general hospitals. In addition, patient demographics at study entry were not comparable between regions, confounding geographical comparisons for clinical practice.
Patients with SCD recruited into the EPIC study in particular may not be representative of “typical” SCD patients as they had received transfusions for a considerable proportion of their lifetime. It is also important to note that patients with TI recruited into the EPIC study do not necessarily reflect a “typical” TI population as, in order to meet the enrolment criteria for the trial, they were regularly transfused. As there are currently no standard guidelines for the management of TI, further study into the treatment practices in this important disorder are warranted. Revisiting the international standard on criteria and definition of thalassaemia diagnosis and severity grading may also be warranted, particularly in the Asia-Pacific region where most patients with TI were enrolled.
Other limitations include that data are generalised to geographical regions with some regions being represented by only a few countries, such as those in the Middle East. Country-specific treatment practices may differ, particularly within the highly diverse Asia-Pacific and Middle East/Africa regions, and these analyses do not capture this. Even within Europe, countries have different approaches to drug funding, which may lead to variations in the management of transfusional iron overload that are overlooked in this study. There are small numbers of patients per region for some anaemias such as AA, MDS and SCD; these data should be interpreted with caution as treatment practices may reflect the attitude of only a few physicians and centres. Age, pre-transfusion haemoglobin levels and weight are also confounding factors.
Overall, these analyses indicate that transfusion and iron chelation practices differ between geographical regions, possibly linked to regional variations in specific disease characteristics (severity, transfusion requirement), treatment practices (e.g., haemoglobin level at which transfusion is initiated), availability and accessibility of transfusion and chelation therapy, patients’ compliance, physicians’ attitudes and adherence to treatment guidelines. It is clear that a considerable proportion of transfusion-dependent patients with various types of anaemia are severely iron overloaded with a serum ferritin level over 2,500 ng/mL, indicating that previous approaches to iron chelation therapy may not be optimal. Increased clinical awareness of iron overload and chelation therapy among clinicians and patients with various anaemias are warranted to improve standards of clinical practice worldwide. Improvements in clinical practice will also be dependent on healthcare policy makers providing access to chelation therapy, particularly in developing countries.
Acknowledgments
This study was sponsored by Novartis Pharma AG. Vip Viprakasit is supported by a Thailand Research Fund, BIOTEC and National Research University Grant, Thailand. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Michelle Utton-Mishra, PhD, for the medical editorial assistance with this manuscript.
Vip Viprakasit, Norbert Gattermann, Jong Wook Lee, John B. Porter, Ali T. Taher and Maria Domenica Cappellini served as investigators in the EPIC trial, enrolled patients, contributed to interpreting the study entry data, critically reviewed the results and approved the final submitted manuscript. Dany Habr and Gabor Domokos coordinated the execution of the EPIC trial, contributed to the analysis, interpretation, and reporting of the study entry data, critically reviewed the results and approved the final submitted manuscript. Nicolas Martin served as the study statistician critically reviewed the results and approved the final submitted manuscript.
Appendix. Remaining investigators who participated in the EPIC study
L. Agaoglu, Istanbul University, Medical Faculty, Capa, Istanbul, Turkey; G. Alimena, Az. Policlinico Umberto I, Roma, Italy; D. Alonso, Hospital Universitario Virgen del Rocio, Sevilla, Spain; S. Ame, Hôpital Hautepierre, Strasbourg, France; E. Angelucci, Ospedale Oncologico A. Businco, Cagliari, Italy; B. Arrizabalaga, Hospital de Cruzes, Barakaldo, Spain; M. Athanasiou-Metaxa, Aristotle University of Thessaloniki, Thessaloniki, Greece; B. Augustson, Sir Charles Gairdner Hospital, Perth, Australia; Y. Aydinok, Ege University, Medical Faculty, Bornova, Izmir, Turkey; A. Baba, Hospital University Sains Malaysia, Kota Bahru, Malaysia; M. Baccarani, Az. Ospedaliera di Bologna, Malpighi, Bologna, Italy; J. Beck, Klinikum der Universität Mainz, Mainz, Germany; P. Beris, Hôpitaux Universitaires de Genève, Geneva, Switzerland; O. Beyne-Rauzy, Hôpital Purpan, Toulouse, France; H. Birgens, Amtssygehuset i Herlev, Herlev, Denmark; D. Bordessoule, CHU Limoges, Limoges, France; C. Borgna-Pignatti, Az. Osp. Universitaria Sant’Anna, Ferrara, Italy; A. Bosly, Cliniques Universitaires U.C.L., Godinne, Belgium; K. Bouabdallah, CHU Bordeaux, Bordeaux, France; D. Bowden, Monash Medical Centre, Melbourne, Australia; D. Bowen, Leeds General Infirmary, Leeds, UK; D. Bron, Institut Jules Bordet, Brussels, Belgium; M. Capra, Ospedale Civico G. di Cristina M. Ascoli, Palermo, Italy; G. Cartron, Clinique Victor Hugo, Le Mans, France; M. Cazzola, Policlinico S. Matteo IRCCS, Pavia, Italy; C. Chalkias, General Hospital of Larissa, Larissa, Greece; L. L. Chan, University Malaya Medical Centre, Kuala Lumpur, Malaysia; S. Chancharunee, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; C. Chapman, Leicester Royal Infirmary, Leicester, UK; P. Charoenkwan, Chiang Mai University, Chiang Mai, Thailand; E. Chasapopoulou, University Hospital of Thessaloniki AHEPA, Thessaloniki, Greece; S. Cheze, CHR Clemenceau, Caen, France; A. Chuansumrit, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; P. Cianciulli, Ospedale S. Eugenio, Roma, Italy; C. Dauriac, CHRU Rennes, Hôpital Pontchaillou, Rennes, France; M. Delforge, UZ Gasthuisberg, Leuven, Belgium; G. Dölken, Ernst-Moritz-Arndt-Universität Greifswald, Greifswald, Germany; H. Dombret, Hôpital Saint Louis, Paris, France; J. Duyster, Klinikum rechts der Isar der TU München, München, Germany; T. Economopoulos, Athens University Medical School, Xaidari, Greece; G. Ehninger, Universitätsklinikum Dresden, Dresden, Germany; M. Elalfy, Ain Shams University, Cairo, Egypt; A. El-Beshlawy, Cairo University, Cairo, Egypt; L. Enggaard, Hillerod Sygehus, Hillerod, Denmark; P. Fenaux, Hôpital Avicenne, Bobigny, France; G. Fillet, Center Hospitalier Universitaire Sart Tilman, Liège, Belgium; A. Filosa, Az. Osp. A. Cardarelli, Napoli, Italy; G. Forni, Ospedale Galliera, Genova, Italy; R. Galanello, Ospedale Regionale Microcitemie, Cagliari, Italy; A. Ganser, Kliniken der Med. Hochschule Hannover, Hannover, Germany; G. Gastl, Uni-Klinik Innsbruck, Innsbruck, Austria; S. Giraudier, Hôpital Henri Mondor, Créteil, France; A. Goldfarb, Hadassah Medical Centre, Jerusalem, Israel; A. Grigg, Royal Melbourne Hospital, Melbourne, Australia; A. Guerci-Bresler, CHU Nancy, Vandoeuvre Les Nancy, France; F. Gumruk, Hacettepe University, Medical Faculty, Sihhiye, Ankara, Turkey; S. Y. Ha, Queen Mary Hospital, Hong Kong, Hong Kong; D. Haase, Universitätsklinikum Göttingen, Göttingen, Germany; B. Heinrich, Gemeinschaftspraxis Brudler/Heinrich, Ausburg, Germany; M. Hertzberg, Westmead Hospital, Sydney, Australia; J. Ho, Royal Prince Alfred Hospital, Sydney, Australia; H-C. Hsu, Taipei Veterans General Hospital, Taipei, Taiwan; S. Huang, Guang Zhou Zhong Shan No.2 Hospital, Guangzhou, China; M. Hunault-Berger, CHU d’Angers, Angers, France; B. Inusa, Evelina Childrens Hospital, London, UK; D. Jaulmes, Hôpital Saint Antoine-Paris, Paris, France; J. Jensen, Arhus Sygehus, Arhus, Denmark; A. Kattamis, First Department of Pediatrics, University of Athens, Athens, Greece; Y. Kilinc, Cukurova University, Medical Faculty, Balcali, Adana, Turkey; K-H. Kim, Samsung Medical Centre, Seoul, South Korea; S. Kinsey, St James’ University Hospital, Leeds, UK; L. Kjeldsen, Rigshospitalet, Copenhagen, Denmark; A. Koren, Ha’emek Medical Center, Afula, Israel; M. E. Lai, Ospedale Regionale Microcitemie, Cagliari, Italy; Y. Lai, No.1 hospital of Nanjing Medical University, Nanjing, China; K-H. Lee, Asan Medical Center, Seoul, South Korea; S-H. Lee, Royal Adelaide Hospital, Adelaide, Australia; L. Legros, Hôpital de l’Archet, CHU de Nice, Nice, France; C. Li, Guang Zhou Nang Fang Hospital, Guangzhou, China; C-K. Li, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong; Q. Li, Shanghai No.1 Hospital, Shanghai, China; K-H. Lin, National Taiwan University Hospital, Taipei, Taiwan; W. Linkesch, Med. Univ. Klinik Graz, Graz, Austria; M. Lübbert, Universitätsklinikum, Freiburg, Germany; D. Lutz, A.Ö. Krankenhaus der Elisabethinen, Linz, Austria; A. J. Mohamed Thalha, Hospital Universiti Kebangsaan, Kuala Lumpur, Malaysia; G. Mufti, King’s College Hospital, London, UK; P. Muus, UMC St. Radboud, Nijmegen, The Netherlands; F. Nobile, Az. Osp. Bianchi Melacrino Morelli, Reggio Calabria, Italy; N. Papadopoulos, Aristotle University of Thessaloniki, Thessaloniki, Greece; S. Perrotta, l Policlinico ll Università di Napoli, Napoli, Italy; M. Petrini, Az. Osp. Ospedali Riuniti S. Chiara, Pisa, Italy; M. Pfeilstöcker, Hanusch-Krankenhaus, Wien, Austria; A. Piga, Ospedale Regina Margherita, Torino, Italy; J. Poole, Johannesburg Hospital, Johannesburg, South Africa; E. Pungolino, Az, Osp. Niguarda Ca’ Granda-Università degli Studi di Milano, Milan, Italy; G. Quarta, Ospedale A. Perrino, Brindisi, Italy; C. Ravoet, H.H. Jolimont Lobbes (Jolimont), La Louviere, Belgium; A. F. Remacha, Hospital de la Santa Creu i San Pau, Barcelona, Spain; C. Rose, Hôpital Saint-Vincent de Paul (Groupe Francophone des Myélodysplasies), Lille, France; L. Roy, Centre Jean Bernard, Poitiers, France; G. Saglio, Az. Sanitaria Ospedale S. Luigi Gonzaga, Orbassano, Italy; G. Sanz, Hospital La Fe, Valencia, Spain; M. Schmid, Universität Ulm, Ulm, Germany; M. Schmugge, Kinderspital Zürich, Zürich, Switzerland; H. Schots, Universitair Ziekenhuis Gent, Gent, Belgium; G. Secchi, Ospedale Civile, Azienda USL 1, Sassari, Italy; J. F. Seymour, Peter MacCallum Cancer Center, Melbourne, Australia; F. Shah, Whittington Hospital, London, UK; H. Shah, General Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; Z. Shen, Shang Hai Rui Jin Hospital, Shanghai, China; B. Slama, Centre Hospitalier General, Avignon, France; P. Sutcharitchan, Chulalongkorn University, Bangkok, Thailand; H. Tamary, Schneider Children’s Medical Center, Petach Tikva, Israel; K. Taylor, Mater Hospital, Brisbane, Australia; H-J. Tesch, Onkologische Gemeinschaftspraxis, Frankfurt, Germany; S. L. Thein, King’s College London School of Medicine, King’s College Hospital, London, UK; J. Troncy, Hôpital Edouard Herriot, Lyon, France; D. Vassilieff, Hôpital Cochin, Paris, France; A. Villegas, Hospital Clinico Universitario San Carlos, Madrid, Spain; L. Wainwright, Chris Hani Baragwanath Hospital, Johannesburg, South Africa; B. Wassmann, Johann Wolfgang Goethe Universität Frankfurt, Frankfurt, Germany; M. Wettervald, CHU Dunkerque, Dunkerque, France; A. Will, Manchester Children’s Hospital, Pendlebury, UK; B. Wörmann, Städt. Klinikum Braunschweig, Braunschweig, Germany; J. Wright, Royal Hallamshire Hospital, Sheffield, UK; S-P. Yeh, China Medical University Hospital (Taichung), Taichung, Taiwan; S-S. Yoon, Seoul National University Hospital, Seoul, South Korea; N. C. Zoumbos, Patras University Medical School, Patras IRO, Greece; and S. Zweegman, VUMC, Amsterdam, The Netherlands.
Sources of support and conflict of interests
Vip Viprakasit reports receiving research grant support and lecture fees from Novartis Pharmaceuticals and research grant support from GPO-L-ONE, Thailand; FerroKin Biosciences, and National Research University (NRU), Thailand. Norbert Gattermann reports receiving honoraria from Novartis Pharmaceuticals and participating in advisory boards for deferasirox clinical trials, receiving honoraria and research support from Celgene GmbH, Germany, and participating in advisory boards for azacitidine clinical trials. John B. Porter reports receiving research funding from Novartis Pharmaceuticals, being a member of an advisory committee and participating in a Novartis speaker’s bureau. Ali T. Taher reports receiving honoraria and research funding from Novartis. Maria Domenica Cappellini is a member of the Novartis’ Speakers Bureau. Dany Habr, Gabor Domokos and Nicolas Martin are full-time employees of Novartis Pharmaceuticals. Jong Wook Lee has no relevant conflicts of interest to disclose.
References
- 1.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 2.Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–78. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 3.Hellström-Lindberg E. Management of anemia associated with myelodysplastic syndrome. Semin Hematol. 2005;42:S10–S13. doi: 10.1053/j.seminhematol.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 5.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: a report from the multi-center study of iron overload. Am J Hematol. 2007;82:255–65. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 6.Angelucci E, Barosi G, Camaschella C, et al. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders. Haematologica. 2008;93:741–52. doi: 10.3324/haematol.12413. [DOI] [PubMed] [Google Scholar]
- 7.Arrizabalaga B, del Cañizo C, Remacha A, et al. Guía clínica de quelación del paciente con síndrome mielodisplásico [Clinical guide to chelation therapy for patients with myelodysplastic syndrome (Spanish Guidelines)] Haematologica. 2008;93(Suppl 1):3–10. [Google Scholar]
- 8.Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83:858–61. doi: 10.1002/ajh.21269. [DOI] [PubMed] [Google Scholar]
- 9.Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology v.2: Myelodysplastic Syndromes. 2010. Available at: http://www.nccn.org/professionals/physician_gls/pdf/mds.pdf. Accessed on 25/01/2012.
- 11.Sickle Cell Society Standards for the clinical care of adults with sickle cell disease in the UK. 2008. Available at: http://www.sicklecellsociety.org/app/webroot/files/files/CareBook.pdf. Accessed on 25/01/2012.
- 12.Thalassaemia International Federation Guidelines for the clinical management of thalassaemia. 2nd Revised Edition. Avaliable at http://www.thalassaemia.org.cy/pdf/Guidelines_2nd_revised_edition_EN.pdf 2008. Accessed on 25/01/2012. [PubMed]
- 13.Vichinsky E, Levine L. Standards of Care Guidelines for Thalassemia. Children’s Hospital & Research Center Oakland; 2008. [Google Scholar]
- 14.National Institutes of Health NHLBI The management of sickle cell disease. NIH Publication No 02-2117. 2002. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Accessed on 25/01/2012.
- 15.Cappellini MD, Porter JB, El-Beshlawy A, et al. Tailoring iron chelation by iron intake and serum ferritin trends: the prospective multicenter EPIC study of deferasirox in 1744 patients with various transfusion-dependent anemias. Haematologica. 2010;95:557–66. doi: 10.3324/haematol.2009.014696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Pierre TG, Clark PR, Chua-anusorn W. Single spin-echo proton transverse relaxometry of iron-loaded liver. NMR Biomed. 2004;17:446–58. doi: 10.1002/nbm.905. [DOI] [PubMed] [Google Scholar]
- 17.Esposito BP, Breuer W, Sirankapracha P, et al. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–7. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 18.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–87. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee J-W, Yoon S-S, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: A subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448–54. doi: 10.1182/blood-2010-01-261289. [DOI] [PubMed] [Google Scholar]
- 20.Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous β-thalassemia. N Engl J Med. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 21.Abetz L, Baladi J-F, Jones P, Rofail D. The impact of iron overload and its treatment on quality of life: results from a literature review. Health Qual Life Outcomes. 2006;4:73. doi: 10.1186/1477-7525-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telfer PT, Prestcott E, Holden S, et al. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971–7. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 23.Gattermann N, Porter J, Lopes LF, Seymour J. Consensus statement on iron overload in myelodysplastic syndromes. Hematol Oncol Clin North Am. 2005;19(Suppl 1):18–25. [Google Scholar]
- 24.Taher A, El-Beshlawy A, Elalfy MS, et al. Efficacy and safety of deferasirox, an oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia: the ESCALATOR study. Eur J Haematol. 2009;82:458–65. doi: 10.1111/j.1600-0609.2009.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daar S, Pathare A, Nick H, et al. Reduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with β-thalassaemia. Eur J Haematol. 2009;82:454–7. doi: 10.1111/j.1600-0609.2008.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 27.Riewpaiboon A, Nuchprayoon I, Torcharus K, et al. Economic burden of beta-thalassemia/Hb E and beta-thalassemia major in Thai children. BMC Research Notes. 2010;3:29. doi: 10.1186/1756-0500-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viprakasit V, Chan LL, Chong QT, et al. Iron chelation therapy in the management of thalassemia: the Asian perspectives. Int J Hematol. 2009;90:435–45. doi: 10.1007/s12185-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 29.Storb R. Aplastic anemia. J Intraven Nurs. 1997;20:317–22. [PubMed] [Google Scholar]
- 30.Lee JW. Iron chelation therapy in the myelodysplastic syndromes and aplastic anemia: a review of experience in South Korea. Int J Hematol. 2008;88:16–23. doi: 10.1007/s12185-008-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T, Tomonaga M, Miyazaki Y, et al. Japanese epidemiological survey with consensus statement on Japanese guidelines for treatment of iron overload in bone marrow failure syndromes. Int J Hematol. 2008;88:30–5. doi: 10.1007/s12185-008-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


