Introduction
Currently, haematologists working both in haemotherapy and clinical haematology have no guidelines for the management and treatment of patients with post-transfusional iron overload. To address this issue, the Spanish Society of Haematology and Haemotherapy (SEHH) and the Spanish Society of Blood Transfusion (SETS) selected several of their members to develop “Guidelines on Haemovigilance of Post-Transfusional Iron Overload”.
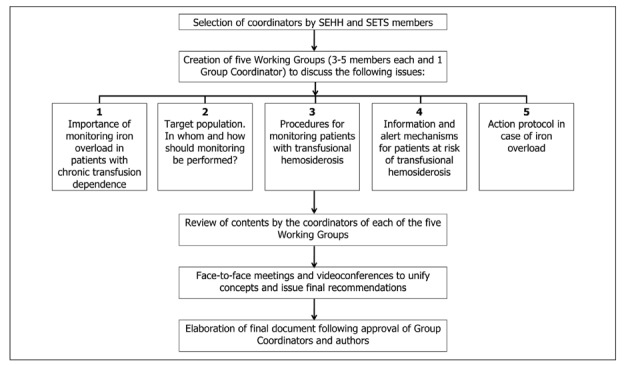
The first step consisted in a systematic MEDLINE search that included articles published from 1980 to October 2010 using the search term limits “iron overload”, “iron chelation therapy”, “thalassemia”, “myelodysplastic syndromes”, and “magnetic resonance imaging”. Then, in a face-to-face working meeting held in November 2009, the contents and sections of the guidelines were established, the group coordinators and members were selected, the working method to be followed was defined, and the deadlines were set.
The members and group coordinators consisted of a team of experts appointed by the SEHH and the SETS, who were to work in one of five working groups. Overall, each of the five working groups consisted of three to five members (one member could work in more than one working group), and one group coordinator who was responsible for establishing a dialogue between members, and setting deadlines.
The purpose of the five working groups was to: (i) define the importance of monitoring iron overload in patients with chronic transfusion dependence; (ii) characterise the target population, i.e. which patients should be monitored and how this monitoring should be carried out; (iii) describe the procedures for monitoring transfusional haemosiderosis, including assessment of ferritin levels, transferrin saturation index, units of packed red blood cell units transfused, and general measures for the care of patients; (iv) define which information mechanisms and alert systems should be put into practice, what information must be recorded in a patient’s transfusion history and how this information can be made accessible to treating physicians and generate a computerised warning system for patients at risk of transfusional haemosiderosis based on the patient’s transfusion history; and (v) describe an action protocol in the case of iron overload (Figure 1).
Figure 1.
Process of elaboration of this guideline.
Once each working group had developed the contents of the five main topics, the information was first sent to the appropriate group coordinator to be revised and approved, and then to the rest of the authors for their approval. Before June 2010, two additional face-to-face working meetings and several videoconferences were held in order to unify concepts and issue final recommendations.
The contents of these guidelines are, therefore, divided into five different sections. The purpose is to provide the best recommendations from currently available scientific evidence on the issues raised, thus filling the void in the field of post-transfusional iron overload. Because the clinical benefit of many strategies discussed herein is widely accepted, despite not being supported by high-quality randomised trials, the group of experts agreed that the use of levels of evidence was not compulsory in the present guidelines.
1. Relevance of iron overload control in patients with chronic transfusion dependence
The human body has complex and effective methods to regulate iron absorption by the intestine, as well as the transport of this metal through plasma and finally its storage and release; however, there are not effective physiological methods to regulate its excretion. Iron overload is a serious inevitable consequence of chronic transfusion support and it is directly related to the number of transfusions received1. One unit of packed red blood cells contains approximately 200–250 mg of iron. After approximately 10–20 consecutive transfusions, iron is deposited in the tissues where it may cause toxicity. In addition, the transfusion of 20 or more units of packed red blood cells increases the risk of developing secondary haemochromatosis. As iron overload progresses, the saturation capacity of transferrin is exceeded, and a non-transferrin-bound iron fraction is detected in plasma; this fraction is very toxic as it promotes the formation of free hydroxyl radicals and facilitates iron uptake by the tissues. The free hydroxyl radicals induce lipid peroxidation which causes cell death and fibrosis2.
1.1. Factors determining iron overload
In patients with anaemia and ineffective erythropoiesis, an increase in intestinal iron absorption occurs even before transfusions are given because of decreased hepcidin levels caused by erythroid hyperplasia (erythropoietic stimulus). The consequence of this process is iron overload prior to transfusion. Thus, it is not rare to find a high initial ferritin level, maybe ≥1,000 μg/L, in such patients. For instance, it was observed that patients with myelodysplastic syndromes, who subsequently required transfusions, had mean initial ferritin levels of 589 μg/L, with 13% of them eventually reaching values greater than 1,000 μg/L3. Therefore, patients with low- or intermediate-risk myelodysplastic syndromes, thalassaemia intermedia, and chronic haemolytic anaemia may have iron overload even before receiving any transfusion. Because of this, several chelation guidelines recommend measuring basic parameters to evaluate iron level from the time of diagnosis, regardless of whether or not the patients have received transfusions4.
1.2. Main organs affected by iron deposition
The organs most affected by iron overload are the heart, the liver, and the endocrine glands5. The most serious adverse effect of iron overload in the heart is sudden death from heart failure. Thirty years ago, children with β-thalassaemia major who did not receive chelation therapy developed left ventricular hypertrophy and cardiac conduction disturbances before the age of 10, and ventricular arrhythmias and refractory myocardial failure at approximately 15 years of age. Two clinical trials found that systemic iron load is the main prognostic factor for determining the clinical course of these patients6,7: patients whose ferritin levels were below 2,500 μg/L during the follow-up period had a heart disease-free survival rate of 91% after 15 years of follow-up, versus a heart disease-free survival rate of 21% in patients with ferritin measurements greater than 2,500 μg/L7. In addition, the survival rate at 25 years of age of patients with levels greater than 15 mg Fe/g of dry liver was 32% whereas no deaths occurred in patients with levels below 15 mg Fe/g of dry liver6.
The liver is the organ with the greatest capacity to store iron and, because of this, in general it is the first organ affected. Iron deposition in the liver may cause rapid development of portal fibrosis and cirrhosis, with portal fibrosis having even been demonstrated in children under 3 years old7. However, the initial signs of iron deposition in the liver (hepatomegaly or elevated liver enzymes) may be absent or, in some cases, mild. Additionally, the concentration of liver iron is an expression of iron deposition in tissues. Normal levels of liver iron in a healthy individual range from 0.6–1.2 mg Fe/g of dry liver. Levels between 3.2–7.0 mg Fe/g of dry liver indicate a moderate level of iron overload, with little clinical impact. Levels between 7–15 mg Fe/g of dry liver promote the development of liver fibrosis and diabetes mellitus, while levels greater than 15 mg Fe/g of dry liver increase the risk of heart disease and premature death. Patients with β-thalassaemia major receiving repeated transfusions may acquire levels greater than 15 mg Fe/g of dry liver very early, even before 5 years of age7.
Lastly, the most common endocrine disorders that result from liver iron overload are hypogonadotropic hypogonadism, which may result in limited pubertal growth, and disorders in development and sexual maturation, growth hormone deficiency, diabetes mellitus due to pancreatic β-cell damage and insulin resistance. Other disorders seen are hypothyroidism, hypoparathyroidism and reduced adrenal androgen secretion7.
1.3. Evaluation of the impact of iron overload in patients requiring chronic transfusion
In thalassaemia the negative impact of iron overload on survival has been fully established, but this is not the case for other diseases requiring chronic transfusion support (Table I). In patients with myelodysplastic syndromes or acute leukaemia and in haematopoietic stem cell transplant recipients, the evidence of the negative impact of haemosiderosis is only based on observational studies. It is, therefore, necessary to conduct controlled studies to confirm such a negative impact. In addition, prospective randomised studies are required to ascertain the efficacy and safety of chelation therapies in these populations, as well as to establish their capacity to prolong survival.
Table I.
Diseases associated with chronic transfusion support.
| Adult and paediatric patients | Diseases |
|---|---|
| Haematological diseases | Myelodysplastic syndrome |
| Acute leukaemia | |
| Lymphoma | |
| Acquired bone marrow aplasia | |
| Multiple myeloma | |
| Non-haematological diseases | Malignancies under chemotherapy |
| Stem cell transplantation | |
| Ineffective erythropoiesis and congenital haemolytic anaemias | Thalassaemias and haemoglobinopathies |
| Dyserythropoietic anaemias | |
| Myelodysplastic syndromes | |
| Hereditary spherocytosis and other membrane disorders | |
| Pyruvate-kinase deficiency and other enzyme disorders | |
| Congenital aplastic anaemias | Blackfan-Diamond anaemia |
| Fanconi’s and other anaemias |
The literature review allowed us to draw the following conclusions:
Transfusion dependence is an independent prognostic factor for survival in myelodysplastic syndromes, irrespective of morphology and cytogenetics8. It has been shown that both overall survival and leukaemia-free survival are lower in patients with myelodysplastic syndromes requiring more than 2 units of packed red blood cells per month. Evidence of the impact of transfusion dependence on survival for these patients prompted the incorporation of the variable “transfusion dependence” into previously established conventional criteria when the new prognostic index for myelodysplastic syndromes, the WPSS, was designed8. In addition, recent studies indicate that transfusion intensity, and not the cumulative transfusion burden, is the transfusion-related variable that determines the prognosis of patients with transfusion-dependent myelodysplastic syndromes9. In patients with refractory anaemia, serum ferritin level is associated with survival, independently of transfusion dependence10. Delea et al. reported that transfusion therapy may increase the risk of typical complications of iron overload in these patients compared with the control population11
In hematopoietic stem cell transplantation, iron overload is a consequence of previous transfusions as well as of transfusions received during the transplant procedure12. The iron overload following hematopoietic stem cell transplantation has been associated with a higher frequency of early and late transplant complications, including sinusoidal obstruction syndrome, fungal and bacterial infections and liver function test abnormalities13–15. However, no cases of fibrosis, liver cirrhosis, or severe cardiac dysfunction have been reported, except in patients with thalassaemia undergoing haematopoietic stem cell transplantation16.
In patients with myelodysplastic syndromes and acute leukaemia who have undergone myeloablative conditioning, increased ferritin levels and pre-transplantation transfusion dependency have been associated with shorter disease-free survival13,15.
- In the paediatric population, patients with iron overload are mainly those with thalassaemia17,18, sickle cell anaemia and other constitutional anaemias. However, other populations, such as patients with malignant haematological diseases, patients with acquired aplastic anaemia unresponsive to immunosuppressive therapy who lack a donor to undergo haematopoietic stem cell transplantation, and haematopoietic stem cell transplant recipients, are also at risk of iron overload because they may receive multiple transfusions of red blood cells during the course of their disease.
- 4a. It is well known that iron overload is the main cause of morbidity and mortality in patients with thalassaemia major17–19 and that this overload is secondary to multiple transfusions and a paradoxical increase in intestinal iron absorption, causing hoarding of hepatic and extrahepatic iron and cell damage. The direct impact of adequate iron chelation on the survival of these patients is well established.
- 4b. Patients with sickle cell anaemia develop liver complications from iron overload, but the frequency of extrahepatic complications, such as myocardial damage, is lower. This may be because of lower plasma levels of non-transferrin-bound iron. Thus, the pathophysiological mechanism of iron overload in sickle cell anaemia appears to be different from that in thalassaemia20.
- 4c. There is very little information on transfusional iron overload in patients with constitutional anaemias who do not have thalassaemia or sickle cell anaemia, but the patients who develop such overload are those with other congenital haemoglobinopathies, congenital dyserythropoietic anaemias, constitutional aplastic anaemias (Blackfan-Diamond anaemia, Fanconi’s anaemia, etc.), haemolytic anaemias due to congenital erythrocyte membrane disorders (hereditary spherocytosis, etc.) and haemolytic anaemias due to a congenital enzyme disorder (pyruvate kinase deficiency, etc.) who receive repeated transfusions5,18.
- 4d. Although there is evidence of iron overload associated with transfusion in adult patients with myelodysplastic syndromes or acute leukaemia and stem cell transplant recipients14,21–23, there is a lack of information regarding children except for those undergoing haematopoietic stem cell transplantation for the treatment of thalassaemia. In this situation, transplant patients who become transfusion-free after transplantation benefit from post-transplant iron chelation to decrease iron overload24.
2. Candidate population for chelation
From our point of view, patients on transfusion support who meet the following criteria should be considered candidates for chelation therapy: (i) age greater than 2 years; (ii) an expected survival beyond 1 year; (iii) recipient, in 1 year, of more than 20 units of packed red blood cells if adult or 10 units of packed red blood cells if a paediatric patient; and (iv) documented iron overload (ferritin levels greater than 1,000 μg/L at two or more consecutive measurements within 15 days, or a liver iron concentration greater than 7 mg Fe/g of dry tissue, measured by liver biopsy or by magnetic resonance imaging [MRI]).
3. Procedures for monitoring transfusional haemosiderosis
Monitoring the level of iron deposition is a key factor in patients with transfusional iron overload. The purpose of this monitoring is to maintain ferritin levels lower than 1,000 μg/L. It should not be forgotten that iron is involved in several critical metabolic processes and the goal of chelation should not, therefore, be to deplete iron deposits completely, but to achieve a balance between the effectiveness and toxicity of the chelator, with levels that are not harmful to the body.
The first issue that should be considered is “when should monitoring of possible iron overload be started” (Table II). The second issue to be taken into account when monitoring patients with transfusional haemosiderosis is “what are the most appropriate methods for measuring iron overload”. Finally, the third issue that should be clarified is “how often should iron overload be measured”.
Table II.
Recommendations for monitoring iron overload.
| Patients to be monitored: |
| Children who have received >10 units of packed red blood cells |
| Adults who have received >20 units of packed red blood cells |
| Patients with chronic haemolytic anaemia (starting from diagnosis) |
| Myelodysplastic syndromes (starting from diagnosis) |
|
|
| Frequency of monitoring: |
| Serum ferritin: every 3 months in transfused patients |
| Liver iron (MRI) and heart iron (MRI T2*): once a year |
MRI: magnetic resonance imaging.
3.1. When should monitoring of iron overload be started?
Several chelation guidelines recommend measuring basic parameters to evaluate iron levels, starting at the time of diagnosis, regardless of whether or not the patient has received transfusions4. A general recommendation included in various national and international guidelines, as well as an observation in several studies, is that when patients begin to be transfused, administration of more than 20–25 units of packed red blood cells causes iron overload25–29. In children, the limit from which iron deposits should be monitored is lower, at 10 units of packed red blood cells (Table II).
3.2. What are the most appropriate methods for measuring iron overload?
There are different techniques for monitoring iron overload including transferrin saturation index, measurement of serum ferritin, measurement of liver iron level by biopsy or MRI and, finally, measurement of iron overload in the heart by calculating the ejection fraction or by MRI T2*. All these methods have limitations, and because of this, their combined use is advisable (Table III).
Table III.
Methods used to assess iron overload.
| Transferrin saturation index55 |
| Assessment of serum ferritin15 |
| Measurement of liver iron deposits: |
| Biopsy12 |
| MRI56 |
| Study of cardiac iron overload: |
| Heart function (ejection fraction)57 |
| MRI5,58 |
MRI: Magnetic resonance imaging.
3.2.1. Transferrin saturation index
This is a simple and highly useful clinical method for diagnosis which is even more sensitive than the measurement of serum ferritin. Thus, it is considered an essential parameter for initial diagnosis of hereditary haemochromatosis, although in the case of post-transfusional secondary haemochromatosis its utility decreases after the patient has been transfused30. A transferrin saturation index greater than 50 should alert the physician to the risk of possible iron overload.
3.2.2. Measurement of serum ferritin
A correlation between serum ferritin levels and body iron deposits has been observed, so serial ferritin measurements are very useful for monitoring iron overload. A good correlation has been shown between ferritin levels and liver iron concentration; therefore, long-term ferritin changes usually reflect changes in iron deposits in the body31. In addition, sequential monitoring of serum ferritin levels has prognostic value. In patients with thalassaemia, ferritin levels lower than 2,500 μg/L are correlated with an increased heart disease-free survival, while levels greater than 2,500 μg/L suggest a poor course, with increased mortality from cardiomyopathy7. Additionally, measurement of serum ferritin levels has the advantage of being a simple, low-cost, easy-to-perform, and non-invasive procedure. This assay is, therefore, the most commonly used indirect method for assessing iron overload. However, this method is not free of disadvantages. Because ferritin is an acute phase reactant, its short-term values may fluctuate in response to infections, inflammation, hepatitis, haemolysis, or vitamin C deficiency. Consequently, it is recommended that ferritin levels are measured at intervals of at least 2 weeks in cases of febrile episodes. The guidelines on thalassaemia treatment from the Thalassaemia International Federation also recommend concomitant measurement of C-reactive protein in these cases32.
3.2.3. Measurement of iron deposits in the liver
The liver is the main organ for iron storage, and liver iron concentration is closely correlated with iron balance in the body. Thus, high levels of liver iron (more than 15 mg Fe/g of dry liver) are predictive of cardiac risk, cardiomyopathy, and early death from heart lesions6.
Measurement of liver iron concentration by liver biopsy is considered the best method for assessing the deposits in the body because it is a direct measurement of liver iron, and its standardisation has been widely validated. Moreover, this technique provides additional information on liver histology, although it has certain risks and drawbacks, including the fact that it is an invasive technique and that it may be contraindicated in patients with thrombocytopenia. In addition, it requires a sample greater than 1 mg of liver tissue, which in patients with liver fibrosis, in whom deposits may be heterogeneously distributed, substantially increases the coefficient of variation.
At present, measurement of liver iron concentration by non-invasive methods, such as MRI, has replaced liver biopsy because it has been found that the results of the two techniques have a good correlation33,34. MRI of the liver has high sensitivity and specificity, with values considered thresholds in the management of patients with iron overload secondary to transfusion. Additionally, it offers a series of advantages over liver biopsy, including the fact that it can measure iron at different points of the liver parenchyma and that it can be performed regularly as it is a non-invasive technique. In contrast, it has the disadvantage of being an indirect method, which requires prior validation and appropriate MRI equipment.
3.2.4. Measurement of iron deposits in the heart
A high percentage of patients with transfusion iron overload die from cardiomyopathy. Measurement of iron deposits in this target organ is, therefore very important, particularly since there is not always a close correlation between liver and heart iron. Indeed, moderate increases in liver iron are sometimes associated with a marked increase in heart iron. This is because the heart can take up labile plasma iron directly, which is the toxic fraction of non-transferrin-bound iron, and cause cardiomyopathy. It is, therefore, currently recommended to monitor not only iron deposits in the liver, but also in the heart. Measurement of heart iron by MRI T2* is a rapid, effective, non-invasive and reproducible technique. It has been shown that when T2* relaxation time decreases below 20 ms, there is a pathological accumulation of iron in the myocardium and a progressive reduction of the ejection fraction. This methodology is already being validated in many hospitals with state-of-the-art MRI equipment35–37. Combining the sensitivity of echocardiography with the study of ejection fraction is of less use, since there may be high iron levels in the heart, with a near normal ejection fraction38.
The clinical diagnostic value of cardiac MRI T2* has recently been established. Patients with cardiac MRI T2* values of less than 10 ms are at increased risk of heart failure, while patients with cardiac MRI T2* values of less than 5 ms are at greater risk of arrhythmias. It has also been reported that cardiac MRI T2* is a better predictor of possible heart failure and arrhythmia than serum ferritin and liver MRI39. The main disadvantage of this procedure is that it is not readily available at all hospitals, being confined to highly specialised centres.
It can be concluded that serial measurements of serum ferritin currently remain a key method for the systematic monitoring of iron overload and chelation therapy. However, it is recommended that this method is combined with MRI to calculate the total amount of iron in the body, and with MRI T2* to detect iron deposits in the heart (Table II).
3.3. How often should iron overload be measured
The appropriate management of iron overload requires regular assessment of iron deposition, particularly if patients continue to receive transfusion therapy (Table II). Regarding the frequency at which this monitoring should be performed, the following should be taken into account: (i) in patients who are transfused regularly (every month or even every 2–3 weeks), ferritin should be measured every 3 months1; (ii) iron deposits in the liver (MRI) and heart (MRI T2*) should be measured at least once a year, if possible. This monitoring should continue to be performed regularly if the patient remains on a transfusion regimen; (iii) previously transfused patients, even those no longer receiving transfusions, should continue to be monitored regularly until the their ferritin levels are lower than 500 μg/L; and (iv) patients with thalassaemia intermedia or other types of dyserythropoiesis who are not regularly transfused should be monitoring lifelong.
4. Alert on the risk of transfusion haemosiderosis based on a patient’s transfusion history
In 2002, the European Parliament and the Council Directive (European Parliament and Council Directive 2002/98/EC of 27 January, 2003) established standards of quality and safety for the collection and handling of human blood in order to ensure a high level of health protection40. This Directive was subsequently adapted to the Spanish legal framework in a Royal Decree of the Ministry of Health and Consumer Affairs (RD 1088/2005, of 16 September)41. A Ministerial Order (Order SCO/322/2007, of 9 February) was subsequently issued on the traceability and reporting of adverse reactions and serious adverse effects of blood and blood components, including transfusional haemosiderosis as a potential adverse effect of transfusion42. As with other related serious adverse effects, transfusion departments should establish mechanisms that guarantee its early detection, and appropriate monitoring, control and reporting to the relevant health authorities.
Monitoring and evaluation of patients receiving multiple transfusions should be a common practice in transfusion departments in order to detect the population at risk of iron overload5,11,18. At present, a system to control and monitor patients receiving multiple transfusions has not been fully established by transfusion departments. Furthermore, no communication channels have been established with the care units to inform the physician who is caring for the patient.
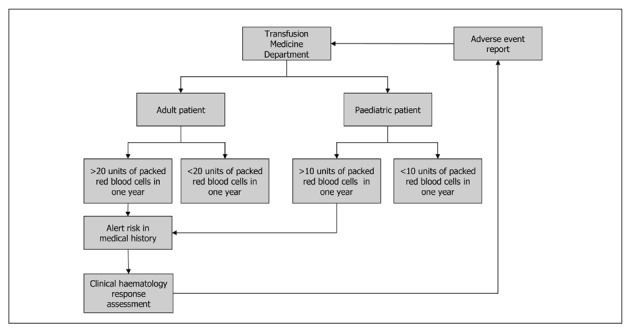
In order to establish an adequate, functional haemovigilance programme that alerts transfusion departments to the risk of transfusion haemosiderosis, the following should be taken into account (Figure 2):
- Information management systems in clinical services and transfusion departments should be connected. The prescriber of the transfusion should know, at the time of the prescription, the following variables related to the patient:
- 1.1 requirements for specific blood components (patients requiring irradiated blood components, cytomegalovirus-seronegative patients, phenotyping for certain red blood cell antigens, etc.).
- 1.2 History of adverse reactions that may have occurred during previous transfusions, if any.
- 1.3 Number of blood components received over a given period.
- Computer management systems in transfusion departments should be adapted in order to:
- 2.1 Identify patients receiving a given number of units of packed red blood cells within a defined period (adult patients more than 20 units or paediatric patients more than 10 units in 1 year);
- 2.2 Be able to transmit this information to a patient’s electronic clinical records.
- 2.3 Issue an alert to the physician caring for the patient. After evaluation of the iron status, the physician will report back to the transfusion service in the case of secondary haemosiderosis.
Figure 2.
Algorithm to alert transfusion departments of the risk of transfusion haemosiderosis.
5. Action protocol in the case of iron overload
In the event of iron overload, the first requirement is to define which patients should receive chelation therapy, analysing both the inclusion and the exclusion criteria for such therapy. Other important aspects to be defined are the appropriate time to start treatment, the type of chelator to be used, and its route of administration. Finally, the efficacy and safety of the chelating agent, both in the short- and in the long-term, must be evaluated, in conjunction with careful monitoring to minimise the adverse effects of the chelator and to accurately define dose adjustments or even cessation of treatment, either because of lack of efficacy of the chelator or because of adverse effects secondary to chelation43.
5.1. Types of currently available chelating agents
The basic criteria for selecting a chelator are its efficacy and long-term safety, together with good treatment compliance. Chelator drugs take up toxic iron and remove it through the urine and/or faeces25,43–45. The essential characteristics of a good chelator include: (i) a high and specific affinity for ferric iron (Fe3+); (ii) good penetration into cells and tissues, without subsequent redistribution throughout the body; (iii) elimination of labile plasma iron or the toxic iron fraction over 24 hours, thus allowing a negative iron balance to be achieved; (iv) oral administration and long half-life in plasma (these two aspects will contribute to a good adherence to therapy); (v) good efficacy and very long-term safety, since chelation therapy must often be continued throughout the entire life of the patient; and (vi) an adequate cost-effectiveness ratio.
There are currently three iron chelators on the market: deferoxamine, deferiprone and deferasirox (Table IV).
Table IV.
Characteristics of currently available chelator agents.
|
Active substance
|
|||
|---|---|---|---|
| Deferoxamine | Deferiprone | Deferasirox | |
| Approved indication | Fe overload in TM >6 years | Fe overload in TM >10 years1 | Transfusion overload1 |
| Dose | 25–50 mg/kg/day | 75 mg/kg/day | 10–30 mg/kg/day |
| Route of administration | SC infusion 8–10 h/day | Oral/8 h | Oral/24 h |
| NTBI (24 h) | Persists | Signs detected | Negative |
| Compliance | Poor | Good | Good |
| Main adverse effects | Local problems | Agranulocytosis | Renal impairment |
| GI toxicity | |||
| Clinical experience in TM | 30–40 years | 12 years | 5 years |
Legend
Fe: iron; SC: subcutaneous; NTBI: non-transferrin-bound iron; GI: gastrointestinal; TM: thalassaemia major.
When deferoxamine is contraindicated or inadequate.
5.1.1. Deferoxamine
Deferoxamine has been shown to be an effective chelator through the experience gained in patients with thalassaemia major over the past 40 years, which makes it the standard drug for comparative studies to evaluate new chelators45. Due to its chemical structure and high molecular weight, it has poor oral bioavailability. In addition, given its rapid clearance from plasma, deferoxamine must be given as a slow subcutaneous infusion at doses of 25–50 mg/kg/day over 8–12 hours, 5 to 7 days a week46. This hinders compliance with treatment as well as tolerance. The drug dose, as with the other two oral chelators, depends on the severity of iron overload and transfusion requirements43.
From a pharmacological viewpoint, deferoxamine has two disadvantages. The first is that it has poor intracellular penetration. The second is that it does not provide 24-hour chelator coverage. Its adverse effects include local problems at the administration site, growth disorders, loss of hearing capacity, vision disturbances, gastrointestinal symptoms and risk of Yersinia enterocolitica infection. The drug has been approved by both the American and European Health Agencies (the U.S. Food and Drug Administration [FDA] and the European Medicines Agency [EMEA]) for the treatment of all patients with iron overload aged 3 years and older.
5.1.2. Deferiprone
Deferiprone is an oral chelator approved by the EMEA for second-line treatment of patients with thalassaemia major aged 10 years or older in whom deferoxamine is contraindicated or inadequate45. The recommended dose is 75 mg/kg/day orally, as three divided doses every 8 hours. There are several studies comparing deferoxamine and deferiprone47–51. Unlike deferoxamine, deferiprone does not allow a negative iron balance to be obtained, but it has been shown to be useful in chelating myocardial iron more effectively, thereby achieving an improved ejection fraction and an increase in T2*50. It is very useful alone or in combination with deferoxamine in clear cases of heart iron overload. Reported side effects include agranulocytosis (0.5%) and neutropenia (5%), as well as gastrointestinal disorders in 35% of patients, arthralgia and joint disease, changes in transaminases and zinc deficiency52. Its use is not approved for the treatment of iron overload in myelodysplastic syndromes.
5.1.3. Deferasirox
Deferasirox is an orally administered chelator with a slow plasma clearance and a long half-life of 11–19 hours, which allows single daily dosing. At a dose of 20–30 mg/kg/day it can obtain a negative iron balance with a reduction in liver deposits, as measured by the liver iron concentration, and a reduction in heart deposits with an increase in T2*. Deferasirox therapy offers the advantage over previous chelators of achieving a persistent elimination of the toxic labile plasma iron fraction over 24 hours, and it has been approved by the FDA and EMEA as first-line treatment for patients with thalassaemia aged 6 years and older, for second-line treatment of children from 2 years of age and for transfusion patients with iron overload of any aetiology53.
Deferasirox is a well-tolerated treatment; its side effects include gastrointestinal disorders (26%), which are more common in children43, transient and moderate elevations of creatinine (36%), which disappear upon reducing or discontinuing the drug, skin rash (8%), elevation of transaminases (2%) and, very rarely, cataracts and impaired hearing. Treatment should not be started if the patient’s creatinine exceeds twice the normal baseline value, or if the creatinine clearance is less than 60 mL/min.
Deferasirox offers advantages over previously described treatments, because its use is associated with increased compliance, a lower number of discontinuations and an improved quality of life. Clinical experience with this drug is now considerable (5 years), although limited compared to the extensive experience with deferoxamine43,53,54.
Dosing and dose adjustments of deferasirox largely depend on changes in iron overload and adverse effects. If ferritin levels fall below 500 μg/L, treatment should be temporarily interrupted. When ferritin levels rise again above 500 μg/L, the drug may be restarted at a dose of 5 mg/kg less than the previous dose used. In thalassaemia major, the goal is to maintain serum ferritin levels at approximately 1000 μg/L or lower using deferasirox doses of 20–30 mg/kg/day. For dosing and dose adjustments based on adverse effects, we recommend adhering to the clinical guidelines on chelation in patients with myelodysplastic syndromes43.
5.2. Control of chelation therapy
For a safe control of adverse effects, it is recommended that patients undergo various tests before starting chelation therapy, and also during its administration (Table V).
Table V.
Control of chelation therapy.
| Prior to starting chelation therapy | After 15 days | After 1 month | Monthly | Every 3 months | Once a year | |
|---|---|---|---|---|---|---|
| Complete blood test | + | + | + | + | ||
| Iron metabolism study: | ||||||
| - Ferritin | ||||||
| - Transferrin | ||||||
| - Transferrin saturation index | + | + | ||||
| - Sideraemia | ||||||
| Liver function: | ||||||
| - AST, ALT | + | + | + | + | ||
| - Creatinine | + | +1 | +1 | +1 | +2, 3 | |
| - Creatinine clearance | + | +1 | +1 | +1 | +2, 3 | |
| - Proteinuria | + | +1 | +1 | +1 | +2, 3 | |
| - Zinc | +3 | +3 | ||||
| Liver MRI (LIC) | + | + | ||||
| Heart MRI (T2*) | + | + | ||||
| ECG and LV function by echocardiography or nuclear medicine | + | + | ||||
| Blood glucose | + | + | ||||
| Hormonal tests: | ||||||
| - TSH, Free T4 | ||||||
| - FSH, LH, testosterone (boys) or oestradiol (girls) in peripubertal or pubertal patients | + | + | ||||
| - IGF-1 and cortisol | ||||||
| Growth assessment in children | +2 | +2 | ||||
| Hearing tests | +1, 2 | +1, 2 | ||||
| Ophthalmologic test | +1, 2 | +1, 2 |
Legend
deferasirox;
deferoxamine;
deferiprone.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; LIC: liver iron concentration; MRI: magnetic resonance imaging; ECG: electrocardiogram; LV: left ventricle; TSH: thyroid-stimulating hormone; T4: thyroxine; FSH: follicle-stimulating hormone; LH: luteinising hormone; IGF-1: insulin-like growth factor-1.
Acknowledgments
The authors would like to thank Novartis Oncology, which facilitated the meetings necessary to evaluate and discuss all the data presented in these guidelines.
Footnotes
Funding
The development of these guidelines was funded in part by Novartis Oncology, which provided grants for the cost of holding the consensus meetings, but did not participate in the discussions nor did it review the experts’ recommendations prior to their publication.
Authorship and Disclosures
Ángel Remacha, Cristina Sanz, Enric Contreras, Cristina Díaz de Heredia, Joan Ramón Grifols, Montserrat Lozano, Guillermo Martín Nuñez, Ramón Salinas, Mercedes Corral and Ana Villegas contributed equally to this manuscript. The Authors reported no potential conflicts of interest.
References
- 1.Cazzola M, Malcovati L. Myelodysplastic syndromes - coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536–8. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AR, Porter JB. Consequences of iron-mediated toxicity. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 3.Remacha AF, Arrizabalaga B, Del Canizo C, et al. Iron overload and chelation therapy in patients with low-risk myelodysplastic syndromes with transfusion requirements. Ann Hematol. 2010;89:147–54. doi: 10.1007/s00277-009-0794-7. [DOI] [PubMed] [Google Scholar]
- 4.Gatterman MI, Hansen DT. Development of chiropractic nomenclature through consensus. J Manipulative Physiol Ther. 1994;17:302–9. [PubMed] [Google Scholar]
- 5.Shander A, Sazama K. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion. 2010;50:1144–55. doi: 10.1111/j.1537-2995.2009.02551.x. [DOI] [PubMed] [Google Scholar]
- 6.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 8.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 9.Pereira A, Nomdedeu M, Aguilar JL, et al. Transfusion intensity, not the cumulative red blood cell transfusion burden, determines the prognosis of patients with myelodysplastic syndrome on chronic transfusion support. Am J Hematol. 2011;86:245–50. doi: 10.1002/ajh.21959. [DOI] [PubMed] [Google Scholar]
- 10.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 11.Delea TE, Hagiwara M, Phatak PD. Retrospective study of the association between transfusion frequency and potential complications of iron overload in patients with myelodysplastic syndrome and other acquired hematopoietic disorders. Curr Med Res Opin. 2009;25:139–47. doi: 10.1185/03007990802565867. [DOI] [PubMed] [Google Scholar]
- 12.Koreth J, Antin JH. Iron overload in hematologic malignancies and outcome of allogeneic hematopoietic stem cell transplantation. Haematologica. 2010;95:364–6. doi: 10.3324/haematol.2009.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–84. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altes A, Remacha AF, Sarda P, et al. Early clinical impact of iron overload in stem cell transplantation. A prospective study. Ann Hematol. 2007;86:443–7. doi: 10.1007/s00277-007-0266-x. [DOI] [PubMed] [Google Scholar]
- 15.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–8. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahindra A, Sobecks R, Rybicki L, et al. Elevated pretransplant serum ferritin is associated with inferior survival following nonmyeloablative allogeneic transplantation. Bone Marrow Transplant. 2009;44:767–8. doi: 10.1038/bmt.2009.77. [DOI] [PubMed] [Google Scholar]
- 17.Borgna-Pignatti C. The life of patients with thalassemia major. Haematologica. 2010;95:345–8. doi: 10.3324/haematol.2009.017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009;97:185–97. doi: 10.1111/j.1423-0410.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 19.Porter JB. Optimizing iron chelation strategies in beta-thalassaemia major. Blood Rev. 2009;23(Suppl 1):S3–7. doi: 10.1016/S0268-960X(09)70003-7. [DOI] [PubMed] [Google Scholar]
- 20.Inati A. Recent advances in improving the management of sickle cell disease. Blood Rev. 2009;23(Suppl 1):S9–13. doi: 10.1016/S0268-960X(09)70004-9. [DOI] [PubMed] [Google Scholar]
- 21.de Witte T. The role of iron in patients after bone marrow transplantation. Blood Rev. 2008;22(Suppl 2):S22–8. doi: 10.1016/S0268-960X(08)70005-5. [DOI] [PubMed] [Google Scholar]
- 22.Litzow MR, Tarima S, Perez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850–7. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997–1003. doi: 10.1038/bmt.2008.99. [DOI] [PubMed] [Google Scholar]
- 24.Batlle M, Vallejo C, Vázquez L, et al. Efficacy and safety of deferasirox for the treatment of iron chelation following allogeneic stem cell transplantation: preliminary results of CILC670AES04 trial. 37th Annual Meeting of the European Group for Blood and Marrow Transplantation; Available at: http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=123469&XNSPRACHE_ID=2&XNKONGRESS_ID=138&XNMASKEN_ID=900. Accessed on 15/06/2011. [Google Scholar]
- 25.Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83:858–61. doi: 10.1002/ajh.21269. [DOI] [PubMed] [Google Scholar]
- 26.Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 27.Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78:487–94. doi: 10.1111/j.1600-0609.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 28.Wells RA, Leber B, Buckstein R, et al. Iron overload in myelodysplastic syndromes: a Canadian consensus guideline. Leuk Res. 2008;32:1338–53. doi: 10.1016/j.leukres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Zamora C, Díaz M, Cid J. XXI Congreso de la SETS. Tarragona; Spain: 2010. Características clínicas de la población con dependencia transfusional crónica en España. Resultados del Estudio 20CH. [Google Scholar]
- 30.Jensen PD. Evaluation of iron overload. Br J Haematol. 2004;124:697–711. doi: 10.1111/j.1365-2141.2004.04838.x. [DOI] [PubMed] [Google Scholar]
- 31.Taher A, Cappellini MD, Vichinsky E, et al. Efficacy and safety of deferasirox doses of >30 mg/kg per d in patients with transfusion-dependent anaemia and iron overload. Br J Haematol. 2009;147:752–9. doi: 10.1111/j.1365-2141.2009.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cappellini MD, Cohen A, Eleftheriou A, et al. Guidelines for the Clinical Management of Thalassaemia. 2nd Edition. ( http://www.thalassaemia.org.cy/pdf/Guidelines_2nd_revised_edition_EN.pdf). In: Thalassemia International Federation, 2008. Accessed on 15/11/2011. [PubMed]
- 33.Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–31. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 34.St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 35.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 36.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–55. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 37.Pennell DJ. T2* magnetic resonance and myocardial iron in thalassemia. Ann N Y Acad Sci. 2005;1054:373–8. doi: 10.1196/annals.1345.045. [DOI] [PubMed] [Google Scholar]
- 38.Davis BA, O’Sullivan C, Jarritt PH, et al. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–9. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 39.Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–8. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Directiva 2002/98/CE del Parlamento Europeo y del Consejo de 27 de enero de 2003 por la que se establecen normas de calidad y de seguridad para la extracción, verificación, tratamiento, almacenamiento y distribución de sangre humana y sus componentes y por la que se modifica la Directiva 2001/83/CE (http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:033:0030:0040:ES:PDF). Accessed on 15/11/2011.
- 41.Real Decreto 1088/2005, de 16 de septiembre, por el que se establecen los requisitos técnicos y condiciones mínimas de la hemodonación y de los centros y servicios de transfusión (http://www.boe.es/boe/dias/2005/09/20/pdfs/A31288-31304.pdf). Accessed on 15/11/2011.
- 42.Orden SCO/322/2007, de 9 de febrero, por la que se establecen los requisitos de trazabilidad y de notificación de reacciones y efectos adversos graves de la sangre y de los componentes sanguíneos (http://www.boe.es/boe/dias/2007/02/17/pdfs/A07010-07016.pdf). Accessed on 15/11/2011.
- 43.Arrizabalaga B, del Cañizo C, Remacha A, et al. Guía clínica de quelación del paciente con síndrome mielodisplásico. Hematologia. 2008;93:1–10. [Google Scholar]
- 44.Bronspiegel-Weintrob N, Olivieri NF, Tyler B, et al. Effect of age at the start of iron chelation therapy on gonadal function in beta-thalassemia major. N Engl J Med. 1990;323:713–9. doi: 10.1056/NEJM199009133231104. [DOI] [PubMed] [Google Scholar]
- 45.Hershko C. Oral iron chelators: new opportunities and new dilemmas. Haematologica. 2006;91:1307–12. [PubMed] [Google Scholar]
- 46.Angelucci E, Barosi G, Camaschella C, et al. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders. Haematologica. 2008;93:741–52. doi: 10.3324/haematol.12413. [DOI] [PubMed] [Google Scholar]
- 47.Anderson LJ, Wonke B, Prescott E, et al. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 48.Maggio A, D’Amico G, Morabito A, et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells Mol Dis. 2002;28:196–208. doi: 10.1006/bcmd.2002.0510. [DOI] [PubMed] [Google Scholar]
- 49.Olivier NF, Brittenham GM. Final result of the randomized trial of deferiprone (L1) and deferoxamine (DFO); Blood (ASH Annual Meeting Abstracts); 1997. Abstract 264. [Google Scholar]
- 50.Pennell DJ, Berdoukas V, Karagiorga M, et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 51.Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–84. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 52.Hoffbrand AV, Cohen A, Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102:17–24. doi: 10.1182/blood-2002-06-1867. [DOI] [PubMed] [Google Scholar]
- 53.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 54.Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80:168–76. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanz G, Nomdedeu B, Such E, et al. Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome. 50th ASH Annual Meeting of the American Society of Hematology; San Francisco, California, EEUU. 2008. Abstract 640. [Google Scholar]
- 56.Rose C, Brechignac S, Vassilief D, et al. Positive impact of iron chelation therapy (CT) on survival in regularly transfused MDS patients. A prospective analysis by the GFM. Blood (ASH Annual Meeting Abstracts) 2007;110:249. [Google Scholar]
- 57.Porter JB. Concepts and goals in the management of transfusional iron overload. Am J Hematol. 2007;82:1136–9. doi: 10.1002/ajh.21100. [DOI] [PubMed] [Google Scholar]
- 58.List AF, Baer MR, Steensma DP, et al. Two-year analysis of efficacy and safety of deferasirox (Exjade®) treatment in myelodysplastic syndrome patients enrolled in the US03 study. Blood (ASH Annual Meeting Abstracts) 2009;114:3829. [Google Scholar]


