SUMMARY
Bleeding and thrombotic disorders are major complications affecting patients with chronic kidney disease (CKD). Exposure of circulating platelets to uremic toxins and contact with artificial surfaces during dialysis induce platelet abnormalities and alter the platelet proteome. We hypothesized that these changes may be subsequent to changes in the composition and/or regulation of the platelet transcriptome. In this study, we investigated the circulating platelets of 10 CKD patients (i.e. 5 chronic hemodialysis patients and 5 stage 4 CKD uremic patients) and 5 age- and sex-matched healthy subjects. We observed an alteration of the platelet messenger RNA (mRNA) and microRNA transcriptome in CKD patients. Impaired in uremic platelets, the levels of some mRNAs and of most microRNAs appeared to be corrected by dialysis, which is consistent with a beneficial effect of dialysis and a mRNA regulatory role of platelet microRNAs. Reduced in platelets of uremic patients, phosphatidylcholine transfer protein (PCTP) and WD repeat-containing protein 1 (WDR1) were found to be regulated by microRNAs, the latter of which involving hsa-miR-19b, a microRNA increased in platelets of uremic patients and involved in platelet reactivity. These results suggest that an alteration of microRNA-based mRNA regulatory mechanisms may underlie the platelet response to uremia and entail the development of platelet-related complications in CKD.
Keywords: Chronic kidney disease, platelets, gene expression, mRNA, microRNA
INTRODUCTION
Cardiovascular disease (CVD) is a major cause of morbidity and mortality among patients suffering from chronic kidney disease (CKD) and is the leading cause of death in patients on chronic hemodialysis (1–3). The high prevalence of established traditional risk factors in CKD patients, such as lipid disorders, diabetes and hypertension, as well as non-traditional risk factors (e.g. oxidative stress, inflammation) and platelet abnormalities (4, 5), may contribute to accentuate the risks of CVD and thrombotic complications (6, 7). Patients with CKD suffer from complex hemostatic disorders. On one hand, uremic patients present abnormalities of primary hemostasis (i.e. platelet dysfunction and impaired platelet-vessel wall interactions) and increased risk of bleeding (8–10). On the other hand, several studies have shown that platelet reactivity is increased in CKD patients undergoing hemodialysis (11), which is associated with increased risk of thrombo-embolic events (12–14). CKD is thus characterized by a delicate balance in which deficient hemostasis paradoxically coexists with enhanced risk of thrombosis, and in which platelet abnormalities may play an important role.
Playing a critical role in the maintenance of hemostasis, platelets may directly contribute to cardiovascular complications, such as myocardial infarction and stroke, in CKD patients (8–10). Although devoid of a nucleus and lacking genomic DNA, circulating human platelets retain as much as 45% of the Refseq genes in the form of mRNAs (15), inherited mainly from their megakaryocyte progenitor cells (16). Several reports confirmed that platelet mRNAs can support de novo protein synthesis (17), including a recent study reporting the translation and secretion of the metalloproteinase inhibitor Timp 2 by activated platelets (18). Weyrich et al. (19) had previously demonstrated that synthesis of Bcl-3 by activated platelets participate in blood clot retraction, while Evangelista et al. (20) reported that de novo synthesis of cyclooxygenase-1 counteracts the suppression of platelet thromboxane biosynthesis by aspirin.
Proteomic analyses of platelets revealed that their protein expression profile is altered in CKD patients (21, 22). Although reticulated platelets sustain changes in terms of platelet volume, morphology and RNA content in subjects treated with hemodialysis (23, 24), whether the mRNA profile of platelets is altered in CKD and hemodialysis patients remains unknown. These observations prompted us to formulate and verify the hypothesis that the biochemical conditions (e.g., increased uremic toxins) prevailing in patients with CKD, leading or not to end-stage kidney failure and dialysis, might negatively affect the transcriptome of circulating platelets, which is constituted mainly of mRNAs and of non-coding microRNAs.
MATERIALS AND METHODS
Recruitment of the subjects
After obtaining informed consent, 10 CKD patients (n=5 chronic hemodialysis patients and n=5 stage 4 CKD patients, later referred as dialysis and uremic patients, respectively) were recruited to participate in this study, which was approved by our Institutional Human Ethics Committees. Five age- and sex-matched healthy subjects with normal renal function were recruited as control group. For all participants, data on demographics, medical history and current pharmacological treatment regimen were collected, as detailed in Table I. Approximately 400 ml of venous blood was collected from each subject using collection bag containing sodium citrate as anticoagulant. For dialysis patients, blood collection was performed immediately prior to dialysis treatment. Hematological and biochemical data were recorded for each subject and are summarized in Table II.
Table I.
Characteristics, causes of renal disease and pharmacological treatments of the subjects
| Subjects (n =5 in each group) | Healthy | Kidney failure | |
|---|---|---|---|
| Uremic | Dialysis | ||
| Age | 60 ±11 | 58 ±17 | 56 ±12 |
| Gender (Male:Female) | 4:1 | 4:1 | 3:2 |
| Smokers | - | 1 | - |
|
| |||
| Cause of renal disease | |||
| Polycystic kidney | - | 1 | 2 |
| Obstructive uropathy | - | 1 | - |
| Polynephritis | - | 1 | - |
| Diabetes | 1 | 2 | 2 |
| Hypertensive nephropathy | - | 1 | - |
| Amyloidosis | - | - | 1 |
| Lithium nephropathy | - | - | 1 |
|
| |||
| Treatments | |||
| Dialysis† | - | - | 5 |
| Anti hypertensives | - | 4 | 4 |
| Hypolipemiant | 1 | 3 | 3 |
| Anti-platelet agents | - | 2 | 4 |
| Hypoglycaemic | 1 | 2 | 2 |
Data are expressed as mean ± standard deviation (SD)
Dialysis treatment was complemented with the same pharmacological treatment for all the patients, which comprised Darbepoetin and Iron Sucrose (anemia treatment), 1-OH-Vitamin D and furosemide. Other agents: phosphate binders (calcium or non calcium), sodium polystyrene and allopurinol.
Table II.
Hematology and biochemistry profile of the subjects
| Subjects (n = 5 in each group) | Healthy | Chronic kidney disease (CKD) | Reference values | |
|---|---|---|---|---|
| Uremic | Dialysis | |||
| eGFR (ml/min/1.73 m2) | n.d. | 20.6 ± 5 | n.d. | 15–29† |
| Hematology | ||||
|
| ||||
| Red blood cells (1012/l) | 4.4 ± 0.4 | 4.2 ± 0.1 | 3.7 ± 0.2 * | 4.5–5.9 |
| Hematocrit | 0.41 ± 0.05 | 0.38 ± 0.03 | 0.36 ± 0.01 * | 0.42–0.50 |
| Hemoglobin (g/l) | 141 ± 15 | 129 ± 9 | 120 ± 4 * | 140–175 |
| White bood cells (109/l) | 6.4 ± 0.4 | 7.6 ± 0.5 | 7.0 ± 1.1 | 4.0–11.0 |
| Platelets (109/l) | 198 ± 54 | 237 ± 29 | 201 ± 35 | 150–400 |
| MPV (fl) | 8.6 ± 1.1 | 9.1 ± 0.8 | 8.4 ± 0.6 | 7.4–10.4 |
| Biochemistry | ||||
|
| ||||
| Urea (mmol/l) | 7.4 ± 1.7 | 18.6 ± 1.0 * | 20.2 ± 2.8 * | 2.4–4.1 |
| Creatinine (μmol/l) | 86 ± 22 | 289 ± 78 ** | 554 ± 187 * | 53–120 |
| Cholesterol (mmol/l) | 4.6 ± 1.3 | 4.2 ± 1.3 | 4.1 ± 0.7 | 4.2–6.2 |
| Triglycerides (mmol/l) | 2.2 ± 1.8 | 1.8 ± 0.8 | 1.8 ± 0.8 | 0.6–2.3 |
| HDL Cholesterol (mmol/l) | 1.4 ± 0.7 | 1.0 ± 0.1 | 1.2 ± 0.5 | 0.9–2.4 |
| Tot. Chol./HDL | 3.6 ± 1.2 | 4.1 ± 1.5 | 3.6 ± 1.1 | 0.0–5.0 |
Data are expressed as mean ± standard deviation (SD).
p<0.05;
p<0.01 versus healthy subjects (Mann-Whitney Test)
Reference values correspond to stage 4 chronic kidney disease (CKD).
n.d., not determined; eGFR, estimated glomerular filtration rate; MPV, mean platelet volume; HDL, high density lipoprotein; Tot. Chol., total cholesterol
Purification of blood platelets
Venous blood was centrifugated at 170 g for 15 min to obtain the platelet-rich plasma (PRP), which was cleared by another centrifugation at 600 g for 10 min and filtered through leukocyte depletion filters (Pall corporation). Platelets were collected by centrifugation at 1,500 g for 15 min and subjected to negative selection based on magnetic cell sorting using human CD45+ depletion kit (EasySep, Stemcell technologies). Approximately two thirds of purified platelets were lysed in TRIzol solution (Invitrogen) for RNA extraction, whereas the remaining platelets were flash-frozen in a dry-iced ethanol bath and stored at −80°C for subsequent enzymatic and Western blot experiments, as described previously (25).
RNA extraction and analysis
Platelet total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol. Purity of the platelet preparations was assessed by reverse-transcription (RT) polymerase chain reaction (PCR) amplification of the leukocyte marker CD45 and platelet marker GPIIIa, as previously described (25). For microarray analysis, 1.5 μg of total RNA from each subject were pooled together for each cohort. Pooled total RNA was purified further using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. The integrity of the total RNA samples was assessed by Bioanalyzer 2100 (Agilent Technology) prior to mRNA and microRNA profiling.
Microarray profiling and analysis of platelet mRNAs
mRNA profiling was performed through the CRCHUQ Genomics platform (CHUQ research center/CHUL) using Human Gene 1.0 ST DNA BioChip (Affymetrix), according to the manufacturer’s protocol. Analysis of mRNA profiles from Human Gene 1.0 ST microarray data was performed on protein-coding genes. Differentially expressed mRNAs were defined as mRNAs 2 fold more (or less) expressed among the uremic or dialysis patients as compared to healthy subjects.
Quantitative real-time PCR (qPCR) experiments
Expression of selected genes of interest was assessed by qPCR using QuantiTect SYBR green PCR kit (Qiagen) on a StepOnePlus Real-Time PCR system (Applied Biosystem). The ΔΔCt method (26) was used to perform the relative quantification of the target genes using GAPDH mRNA as the reference gene. Expression of microRNAs of interest was assessed by qPCR using the miSCRIPT PCR system (Qiagen). MiR-191 was used as the reference for relative quantification, since this microRNA displayed no variation in expression levels in our microarray data; similar observations were reported by Nagalla and colleagues upon analysis of platelet miR-191 from 17 healthy donors (27). All results were normalized to the average expression level obtained for the healthy subjects group, and arbitrarily set at 1.
Microarray profiling and analysis of platelet microRNAs
MicroRNA profiling was performed by Exiqon microRNA profiling service (Vedbaek, Denmark). The 3 samples (healthy, uremic and dialysis platelets total RNA pool) were labeled using the miRCURY™ Hy3/Hy5 Power labeling kit and hybridized on the miRCURY™ Locked Nucleic Acid (LNA) Array (5th generation arrays covering microRNA registered in miRBase 15.0 - Sanger Institute (28)). Differentially expressed microRNAs were defined as microRNA whose expression level was 2 fold higher or lower than that observed in healthy subject group. Predicted mRNA targets of differentially expressed microRNAs were determined using miRecords (http://mirecords.biolead.org) (29).
Reporter gene activity assays
HEK293 cells (2 × 105) were plated in 24-well plates 24 hours before cotransfection with a pGeneClip (Promega) construct expressing pre-miR-599 or pre-miR-19b-1 (2.5 to 250 ng DNA) and a psiCHECK (Promega) reporter construct, in which the 3′UTR of phosphatidylcholine transfer protein (PCTP) or WDR1 (100 ng DNA) was inserted downstream of the Rluc reporter gene, using Lipofectamine 2000 (Invitrogen). A pGeneClip-Neg vector (30), encoding a short hairpin RNA (shRNA) directed against a deleted region in Rluc mRNA, was used as a negative control. Forty-eight hours later, cells were harvested and protein extracts were prepared for measurement of Renilla luciferase (Rluc) and Firefly luciferase (Fluc) activities, essentially as described previously (30).
Statistical analyses
Results were expressed as mean ± standard deviation (SD). Statistical analyses were performed using InStat 3 software (GraphPad). For all analyses, a p < 0.05 was considered as statistically significant.
RESULTS
Recruitment and characteristics of the subjects
In this study, we characterized the mRNA and microRNA transcriptomes of circulating platelets from a total of 10 CKD patients: (i) 5 patients suffered from end-stage renal disease (stage 5) and were treated by chronic hemodialysis (dialysis patients) (Table I), whereas (ii) the other 5 patients suffered from stage 4 CKD (uremic patients), the last stage before renal replacement therapy (e.g. hemodialysis or renal transplantation), which corresponds to an eGFR ranging from 15 to 29 ml/min/1.73m2 (31). Table I shows chronic medication use in study participants. Although the dialysis patients were treated for anemia, their red blood cell count, hemoglobin content and hematocrit remained relatively lower than the other subjects (Table II). None of the subjects presented obvious platelet disorders, as platelet count and platelet mean volume were within a normal range.
Differential platelet mRNA profile of uremic and dialysis patients
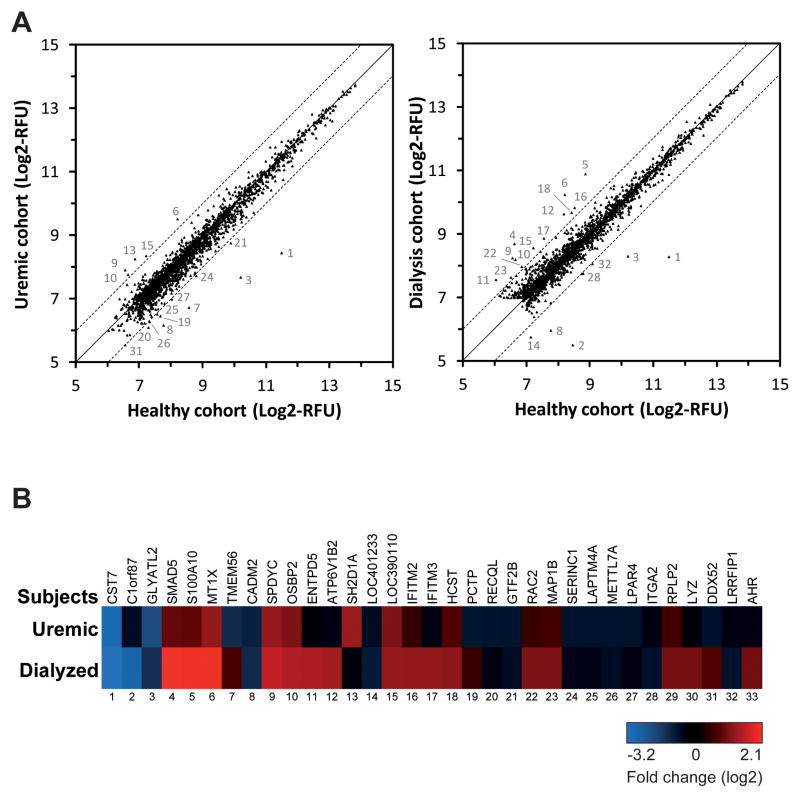
The previously reported alteration of the protein expression profile in circulating platelets of CKD patients (21, 22) prompted us to examine the impact of CKD on the platelet mRNA transcriptome. Microarray profiling of platelet mRNAs on pooled RNA samples revealed that the majority of platelet mRNAs is not markedly affected in uremic and dialysis patients, as compared to control healthy subjects. However, we did observe an altered expression (> 2 fold vs healthy subjects) of a total of 33 platelet mRNAs in CKD patients: (i) 17 platelet mRNAs were differentially expressed in uremic patients (Figure 1A, left panel), whereas (ii) in dialysis patients, 23 platelet mRNAs showed a differential expression (Figure 1A, right panel). Analyzing those differentially expressed platelet mRNAs among our uremic and dialysis patient cohorts (Figure 1B), 7 genes showed a similar deregulation in both cohorts of CKD patients, whereas 15 genes were altered specifically in dialysis patients.
Figure 1. Differential platelet mRNA profile of uremic and dialysis patients.
Platelet RNA samples were obtained from each subject, and equivalent amounts were pooled and analyzed by DNA-based microarray (Affymetrix, human Gene 1.0 chip). Platelet mRNA profile of the uremic and dialysis patients were compared to the healthy subjects. (A) Platelet mRNA expression level correlation between healthy and uremic (left panel) or dialysis (right panel) subjects. Data are expressed as log2-transformed relative fluorescence unit (RFU). The mRNAs that varied by more than 2-fold were numbered, as indicated in B. (B) Platelet mRNAs displaying more than 2-fold changes among the uremic and dialysis patients, as compared to the healthy subjects. Twenty-three (23) and 17 mRNAs are differentially expressed in platelets from dialysis and uremic patients respectively, as compared to healthy subjects. Seven (7) mRNAs are differentially expressed in both patient groups.
Validation of selected platelet mRNAs by qPCR analyses
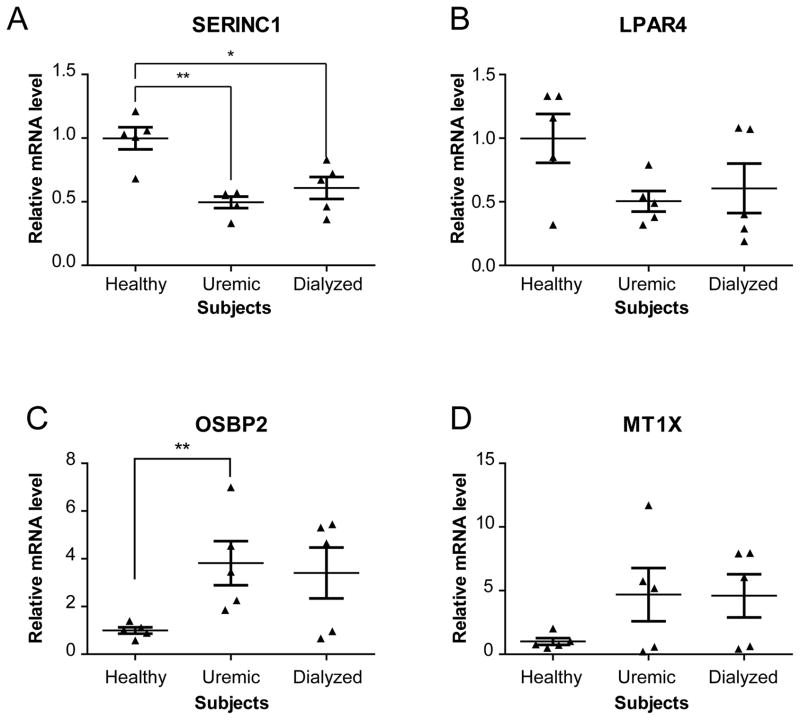
Next, we wished to confirm the differential expression observed for selected mRNAs, on an individual basis, by qPCR. Four genes were selected based on their different patterns of expression and potential relevance to platelet function. Assessment of their mRNA expression levels by qPCR accurately reflected those obtained by microarray profiling (Table III), thereby confirming the validity of our microarray approach using pooled RNA samples. As expected, the level of two of them, serine incorporator 1 (SERINC1) (Figure 2A) and lysophosphatidic acid receptor 4 (LPAR4) (Figure 2B), was reduced by ~50% in CKD patients, as compared to healthy subjects. In contrast, the mRNAs encoding for oxysterol binding protein 2 (OSBP2) and metallothionein 1X (MT1X) were upregulated in CKD patients (Figures 2C and 2D, respectively). Although in some cases statistical significance could not be reached, the differential mRNA expression assessed by microarray and qPCR displayed similar tendencies.
Table III.
Comparison of gene expression data obtained by microarray and qPCR
| Gene symbol Gene name |
Subjects | mRNA expression level (fold changes)
|
||
|---|---|---|---|---|
| Microarray | qPCR | |||
| SERINCl | Uremic | 0.48 | 0.49 ±0.10 ** | |
| serine incorporator 1 | Dialysis | 0.73 | 0.61 ±0.19 * | |
|
| ||||
| LPAR4 | Uremic | 0.49 | 0.50 ±0.18 | |
| lysophosphatidic acid receptor 4 | Dialysis | 0.83 | 0.61 ±0.43 | |
|
| ||||
| OSBP2 | Uremic | 2.17 | 3.81 ±2.06 ** | |
| oxysterol binding protein 2 | Dialysis | 2.93 | 3.40 ±2.39 | |
|
| ||||
| MT1X | Uremic | 2.48 | 4.69 ±4.68 | |
| metallothionein lX | Dialysis | 4.11 | 4.59 ±3.80 | |
qPCR data are provided as mean ± standard deviation.
p<0.05;
p<0.01 versus healthy subjects (Mann-Whitney Test)
Figure 2. qPCR analyses of selected platelet mRNA expression levels in uremic and dialysis patients.
(A–D) Serine incorporator 1 (SERINC1) (A), lysophosphatidic acid receptor 4 (LPAR4) (B), oxysterol binding protein 2 (OSBP2) (C) and metallothionein 1X (MT1X) (D) mRNAs were quantified by qPCR using the ΔΔCt method and GAPDH mRNA as a reference. Results were normalized to the average expression level of the healthy subjects and expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using the non-parametric Mann-Whitney Test. * p<0.05; ** p<0.01 versus the healthy group.
Alteration of the platelet microRNA profile in uremic patients
The conditions associated to CKD may affect the ability of platelets to synthesize or mediate the function of microRNAs, a family of small non-coding RNAs known as key regulators of mRNAs. To verify that possibility, we performed Dicer activity assays in vitro using protein extracts prepared from purified circulating platelets. We observed no significant changes in the ability of platelet Dicer to produce microRNAs among the uremic or dialysis patients, as compared to healthy subjects (Supplementary Figure S1A), although we noted a certain degree of interindividual variations. A similar observation was made when monitoring the expression level of the ribonuclease Dicer and of its cofactor TRBP (Supplementary Figure S1B), which are known to mediate platelet microRNA biogenesis.
Using a similar approach, we also assessed the functionality of microRNA effector complexes, composed of the ribonuclease Argonaute 2 (Ago2), by RISC activity assay in vitro. Our assays unveiled a platelet Ago2 activity that is relatively similar among all the subjects, which is in accordance with the relatively similar Ago2 expression level among healthy uremic and dialysis donors (Supplementary Figure S2). Altogether, our results suggest that the ability of platelets to synthesize or mediate the function of microRNAs is not affected by CKD.
Uremic toxins that accumulate in the blood of CKD patients may nevertheless exert systemic effects, including on platelet precursor megakaryocytic cells, and influence the microRNA content of circulating platelets. Examination of the microRNA profile of platelets isolated from CKD patients identified 21 microRNAs that were differentially expressed in uremic platelets, as compared to the healthy cohort (Figure 3A, left panel), thereby supporting an important alteration of the platelet microRNA profile in CKD.
Figure 3. Differential platelet microRNA profile of uremic and dialysis patients.
Platelet RNA samples were obtained from each subject, and equivalent amounts were pooled and analyzed by LNA-based microRNA microarray (Exiqon). The platelet microRNA profile of the uremic and dialysis patients were compared to the healthy subjects. (A) Platelet microRNA expression level correlation between healthy and uremic (left panel) or dialysis (right panel) subjects. Data are expressed as log2-transformed relative fluorescence unit (RFU). The microRNAs that varied by more than 2-fold were numbered, as indicated in B. (B) Platelet microRNAs displaying more than 2-fold changes among the uremic and dialysis patients, as compared to the healthy subjects. Whereas 21 platelet microRNAs are differentially expressed in platelets from uremic patients, as compared to healthy subjects, a single microRNA is altered by more than 2 fold in platelets of dialysis patients, namely miR-551b.
When analyzing the differentially expressed platelet microRNAs among our uremic and dialysis patient cohorts (Figure 3B), we observed that microRNAs of the same family, as exemplified by hsa-miR-33a and hsa-miR-33b, are regulated similarly. Notably, we noted a similar trend in the level of some microRNAs deriving from both strands of a precursor in uremic platelets. Originating from pre-miR-142, both hsa-miR-142-5p and 3p were upregulated. These tandem variations in mature microRNA expression levels are indicative of a microRNA pathway that is altered upstream of the pre-miRNA processing step in uremic patients. In contrast, hsa-miR-340 and -340* were altered in the opposite way, which is indicative of a differential strand selection process. Together, these observations suggest that microRNA biogenesis may be altered at multiple levels by uremia.
Restoration of the platelet microRNA profile upon dialysis
Importantly, only 1 microRNA (hsa-miR-551b) was found to be differentially expressed in the dialysis patients cohort, as compared to the healthy cohort (Figure 3C). Contrasting with the 21 microRNAs that are differentially expressed in platelets of uremic patients, these results suggest that hemodialysis may restore the microRNA expression profile of platelets in patients suffering from severe CKD.
Restoration in the level of some platelet mRNAs upon dialysis
Some of the microRNAs deregulated in platelets of uremic patients belong to the 20 most abundant platelet microRNAs in healthy subjects, such as hsa-miR-26a, hsa-miR-26b, hsa-miR-142-5p and 3p, and may likely influence platelet mRNAs. Incidentally, analyses of differentially expressed platelet microRNAs revealed that microRNAs sharing the same seed region, like hsa-miR-26b and hsa-miR-1297, are regulated similarly in uremic patients. Knowing that the microRNA seed region (i.e. nucleotides 2 to 8 from the 5′ end) plays a critical role in mRNA recognition (32), these observations are consistent with a possible link between microRNAs and mRNAs in platelets.
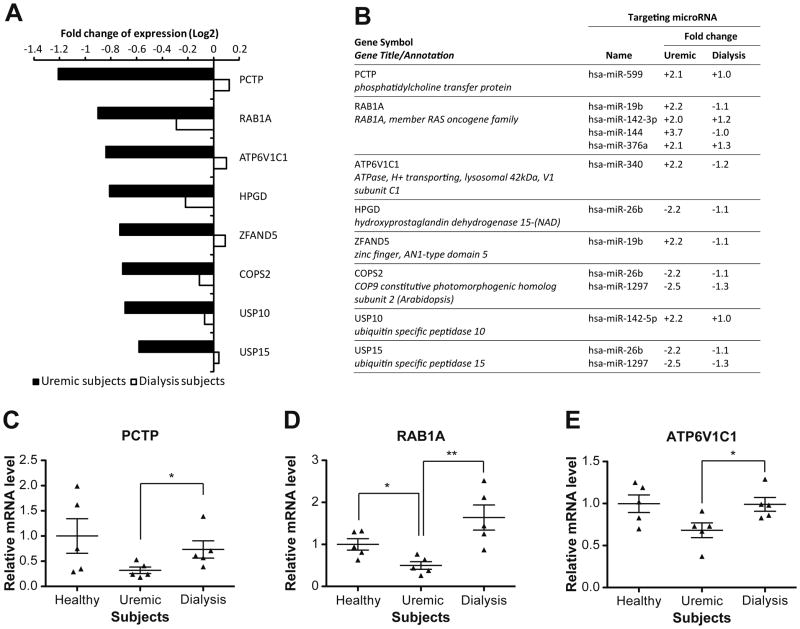
To verify that possibility, we analyzed our microarray data with bioinformatic microRNA target predictive tools in order to identify such platelet microRNA:mRNA pairs. Among a total of 81 platelet mRNAs, whose levels were corrected by dialysis, 8 mRNAs displayed, in their 3′UTR, binding site(s) for microRNAs that are differentially expressed in uremic patients (Figure 4A). The level of all 8 mRNAs was decreased in platelets of uremic patients. Changes in expression of the 3 mRNAs that were the most affected in the platelets of uremic patients, i.e. PCTP, RAB1A and ATP6V1C1 mRNAs, were validated by qPCR (Figure 4C). For 3 of these 8 mRNAs, we observed a concomitant decrease in the corresponding regulatory microRNAs, a direct correlation consistent with a mRNA stabilizing role of microRNAs in platelets. This is the case of the following pairs: HPGD mRNA:hsa-miR-26b, COPS2 mRNA:hsa-miR-26b/hsa-miR-1297 and USP15 mRNA:hsa-miR-26b/hsa-miR-1297 (Figure 4B).
Figure 4. The level of some platelet mRNAs is corrected by dialysis.
(A) Differential expression of mRNAs corrected by dialysis in platelets of CKD patients. Data are expressed as log2-transformed fold changes compared to the healthy subjects. (B) List of platelet mRNAs corrected by dialysis, together with their corresponding predicted regulatory microRNAs and differential expression in uremic and dialysis patients. Data are expressed as fold changes compared to the healthy subjects. PCTP (C), RAB1A (D) and ATP6V1C1 (E) mRNAs were quantified by qPCR using the ΔΔCt method and GAPDH mRNA as a reference. Results were normalized to the average expression level of the healthy subjects and expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using the non-parametric Mann-Whitney Test. * p<0.05; ** p<0.01 between depicted groups.
On the opposite, the remaining 5 mRNAs showed an inverted correlation with the corresponding microRNAs, which is expected for microRNAs that regulate platelet mRNAs through destabilization/degradation, as observed in the case of the PCTP mRNA:hsa-miR-599 pair (Figure 4B). The level of PCTP mRNA was decreased in platelets of uremic patients, whereas that of hsa-miR-599 was increased, which is consistent with a role for hsa-miR-599 in destabilizing PCTP mRNA. These changes in PCTP mRNA and hsa-miR-599 levels were corrected upon dialysis. Suggesting that the procedure helped restore their expression levels closer to those observed in healthy subjects, these data support a functional link between PCTP mRNA and hsa-miR-599.
MicroRNA regulation of platelet mRNAs in CKD
Computational analyses predicted the presence of several binding sites for microRNAs, including hsa-miR-599, in the 3′UTR of PCTP mRNA (Figure 5A). As shown in Figure 5B, we were able to document the regulatory control of hsa-miR-599 on the PCTP 3′UTR element in cultured HEK293 cells. These results support a role for hsa-miR-599 in regulating PCTP gene expression, which may explain, at least in part, their opposite variations in expression levels in platelets of uremic and dialysis patients.
Figure 5. Platelet PCTP mRNA, whose levels are corrected by dialysis, is regulated by hsa-miR-599.
(A) Upper panel, Schematic representation of human PCTP mRNA with putative microRNA binding sites predicted by at least 2 bioinformatic programs among miRanda, TargetScan and PicTar. MicroRNAs highlighted in black are expressed in platelets. Lower panel, Topology of the interaction between hsa-miR-599 and its putative binding site, as predicted using RNA hybrid, with its calculated minimum free energy (MFE) value. (B) Reporter gene activity assay. HEK293 cells were co-transfected with a pre-miR-599 expression vector and a reporter gene construct in which the sequence of PCTP 3′UTR was inserted downstream of the Rluc coding sequence. Rluc and Fluc activities were measured, and the values were normalized to those obtained with a non-relevant short hairpin expression vector (n = 4 experiments, in duplicate). ** p<0.01 (Student’s paired T test).
Since microRNAs may also mediate their function by interfering with mRNA translation without altering mRNA levels, and may have escaped our correlative analyses, we examined proteins whose expression was reported to be altered either in uremic or dialysis patients. Among these proteins, WD repeat domain 1 (WDR1 or AIP1) is reduced in uremic patients that display platelets defects (22). We observed no significant changes in WDR1 mRNA levels, either upon micro-array or qPCR analysis, in platelets (Figure 6C). Bioinformatic analyses revealed that WDR1 mRNA 3′UTR harbors several putative binding sites for microRNAs, including hsa-miR-19b (Figure 6A), whose levels are increased in platelets of uremic patients. MiR-19b expression, assessed by qPCR, confirmed that the expression of this microRNA is markedly increased in platelets of some uremic subjects (Figure 6D). Using a Rluc reporter gene system in cultured HEK293 cells, we confirmed the ability of hsa-miR-19b to regulate WDR1 mRNA through its 3′UTR element (Figure 6B). Together, these findings suggest that (i) both PCTP and WDR1 mRNAs may be under microRNA control in human platelets, (ii) the deregulation of the platelet mRNA transcriptome may be linked to that of microRNAs, and (iii) alteration of this microRNA-based mRNA regulatory mechanism may underlie the platelet response to uremia.
Figure 6. WDR1 protein expression, which is involved in uremic platelet defects, is subjected to microRNA regulation.
(A) Upper panel, schematic representation of human WDR1 mRNA with putative microRNA binding sites predicted by at least 2 bioinformatic programs among miRanda, TargetScan and PicTar. MicroRNAs highlighted in black are expressed in platelets. Lower panel, topology of the interaction between hsa-miR-19b and its putative binding site, as predicted using RNA hybrid, with its calculated minimum free energy (MFE) value. (B) Reporter gene activity assay. HEK293 cells were co-transfected with a pre-miR-19b expression vector and a reporter gene construct in which the sequence of WDR1 3′UTR was inserted downstream of the Rluc coding sequence. Rluc and Fluc activities were measured, and the values were normalized to those obtained with a non-relevant short hairpin expression vector (n = 4 experiments, in duplicate). ** p<0.01 (Student’s paired T test). WDR1 mRNA (C) and miR-19b (D) were quantitated by qPCR using GAPDH mRNA or miR-191 as a reference, respectively. Results were normalized to the average expression level of the healthy subjects and expressed as mean ± standard error of the mean (SEM).
DISCUSSION
Uremic toxins that accumulate in the blood of patients suffering from CKD may affect platelet function, induce hemostatic imbalances and mediate thrombotic disorders (1, 2, 4, 8–10). These effects have been related to an alteration of the platelet proteome that is observed in these patients (21, 22). In the present study, we observed that the platelet mRNA and microRNA transcriptome was altered in CKD patients and could be restored partially upon dialysis. Known to contribute to the platelet proteome, it may be the deregulation of the platelet transcriptome that underlies, and forms the molecular basis of, platelet-related disorders in CKD patients.
The level of metallothionein-encoding genes, such as MT1X, was increased in platelets of both uremic and dialysis patients, in which plasma zinc deficiency is common (33). Particularly abundant in platelets and involved in platelet reactivity and hemostasis (34), zinc imbalances are involved in oxidative stress (35), a state that induces expression of metallothionein genes (36). Elevated levels of platelet metallothionein genes may thus result from the altered zinc homeostasis and oxidative stress conditions that prevail in uremic and dialysis patients, and that may contribute to the relatively high prevalence of CVD in these patients (6, 37).
Additional genes deregulated in platelets of CKD patients could be linked to platelet-related disorders observed in uremia. For instance, the mRNA encoding for LPAR4, which is suspected to inhibit platelet reactivity to lysophophatidic acid (38), is reduced by ~50% in CKD patients. Lysophosphatidic acid accumulation in atherosclerotic lesions represents an important risk factor for the development of atherosclerosis and thrombosis (39), and the observed decrease in LPAR4 expression in CKD patients may explain the increased risk of cardiovascular events observed in these clinical cases. We also identified genes involved in lipid metabolism, such as glycine N-acyltransferase-like 2 (GLYATL2), phospholipase C eta 1 (PLCH1), phosphatidylcholine transfer protein (PCTP), OSBP2 and SERINC1, whose levels are deregulated in uremic conditions. This may partially explain the altered synthesis of bioactive lipids (40, 41) and membrane lipid composition (42, 43) observed in platelets of uremic patients.
The conditions associated to CKD did not affect the ability of platelets to synthesize or mediate the function of microRNAs. However, we observed an important alteration of the platelet microRNA profile in uremic patients, as a total of twenty-one (21), out of 247 microRNAs expressed in platelets, displayed more than 2-fold changes, as compared to healthy subjects. Considering that microRNA expression in mammalian cells can be regulated at multiple steps (gene transcription, processing, transport, strand selection/separation, microRNP assembly, stability) (44) and that uremia is usually part of a complex clinical portrait, the aim of identifying which the component(s) and/or step(s) of the platelet microRNA pathway is(are) altered in CKD patients, and how, appears to be challenging.
Correction of the level of most microRNAs and of some mRNAs, impaired in uremic platelets, upon dialysis is consistent with a mRNA regulatory role of platelet microRNAs. We were able to confirm the microRNA regulation of two mRNAs (PCTP and WDR1) that are reduced in platelets of uremic patients, the latter of which involving hsa-miR-19b. Using a proteomic approach, Marques and colleagues (22) demonstrated that WDR1 protein expression was reduced in platelets of uremic patients with low platelet reactivity and bleeding tendency. The fact that WDR1 mRNA levels were not altered in platelets of uremic or dialysis patients supports a role for hsa-miR-19b in repressing WDR1 mRNA translation, along a process independent of WDR1 mRNA levels (45). Interestingly, hsa-miR-19b is among a list of 7 microRNAs that were identified as good predictors of platelet hyperreactivity to epinephrine (27). Upregulated by >2 fold in platelets of uremic patients, hsa-miR-19b may thus be involved in the altered reactivity of uremic platelets and the bleeding disorders observed in these patients.
When attempting to correlate platelet microRNA and mRNA levels, the caveat has to be taken into account that microRNAs may silence platelet mRNAs through translational repression (46), without inducing their cleavage and degradation. Another major limitation is the plurality of microRNA targets which, together with the functional interplay of a myriad of microRNAs that may act in concert to regulate specific platelet mRNAs, makes reliable genome-wide assessment of functionally relevant platelet microRNA:mRNA pairs almost unattainable.
Our results suggest that the biochemical conditions prevailing in patients with CKD, leading or not to end-stage kidney failure and dialysis, may alter the mRNA and microRNA transcriptome of circulating platelets which, in turn, may affect platelet function and entail the development of platelet-related clinical complications, such as atherosclerosis and thrombosis. This study complements previous studies describing the relationship between platelet microRNAs and platelet reactivity (27, 47) or human diseases, including premature coronary artery disease (48). The molecular mechanisms underlying the deregulation of platelet mRNA and microRNA transcriptome in CKD patients, which may be multifaceted considering the complexity of the medical condition of these patients, warrants further investigations.
Supplementary Material
Acknowledgments
This work was supported by Catalyst Grant: Pilot Projects in Aging No. IAP-99000 from the Canadian Institutes of Health Research (to P.P. and F.M.).
P.P. is a Senior Scholar from the Fonds de la Recherche en Santé du Québec (FRSQ).
Footnotes
DECLARATION OF INTEREST STATEMENT
The authors report no conflicts of interest.
References
- 1.Collins AJ, Li S, Ma JZ, Herzog C. Cardiovascular disease in end-stage renal disease patients. Am J Kidney Dis. 2001 Oct;38(4 Suppl 1):S26–9. doi: 10.1053/ajkd.2001.27392. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. Chronic Kidney Disease and the Risk for Cardiovascular Disease, Renal Replacement, and Death in the United States Medicare Population, 1998 to 1999. Journal of the American Society of Nephrology. 2005 Feb 1;16(2):489–95. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 3.Sidhu MS, Dellsperger KC. Cardiovascular problems in dialysis patients: impact on survival. Adv Perit Dial. 26:47–52. [PubMed] [Google Scholar]
- 4.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000 Jul;58(1):353–62. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 5.Xue JL, Frazier ET, Herzog CA, Collins AJ. Association of heart disease with diabetes and hypertension in patients with ESRD. Am J Kidney Dis. 2005 Feb;45(2):316–23. doi: 10.1053/j.ajkd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Libetta C, Sepe V, Esposito P, Galli F, Canton AD. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clinical Biochemistry. doi: 10.1016/j.clinbiochem.2011.06.988. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- 7.Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif. 31(1–3):189–96. doi: 10.1159/000321845. [DOI] [PubMed] [Google Scholar]
- 8.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004 Oct;30(5):579–89. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 9.Rios DR, Carvalho MG, Lwaleed BA, Simoese Silva AC, Borges KB, Dusse LM. Hemostatic changes in patients with end stage renal disease undergoing hemodialysis. Clin Chim Acta. Feb;411(3–4):135–9. doi: 10.1016/j.cca.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006 Jul-Aug;19(4):317–22. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal A, Kabbani SS, Rimmer JM, Gennari FJ, Taatjes DJ, Sobel BE, et al. Biphasic effects of hemodialysis on platelet reactivity in patients with end-stage renal disease: a potential contributor to cardiovascular risk. Am J Kidney Dis. 2002 Aug;40(2):315–22. doi: 10.1053/ajkd.2002.34510. [DOI] [PubMed] [Google Scholar]
- 12.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Holoch PA, Sobel BE, et al. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001 Jul 10;104(2):181–6. doi: 10.1161/01.cir.104.2.181. [DOI] [PubMed] [Google Scholar]
- 13.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991 Aug;84(2):613–7. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 14.Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990 May 31;322(22):1549–54. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 15.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood [Research Support, NIH, Extramural] 2011 Oct 6;118(14):e101–11. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth GJ, Hickey MJ, Chung DW, Hickstein DD. Circulating human blood platelets retain appreciable amounts of poly (A)+ RNA. Biochem Biophys Res Commun. 1989 Apr 28;160(2):705–10. doi: 10.1016/0006-291x(89)92490-x. [DOI] [PubMed] [Google Scholar]
- 17.Schubert P, Devine DV. De novo protein synthesis in mature platelets: a consideration for transfusion medicine. Vox Sang. 2010 Aug 1;99(2):112–22. doi: 10.1111/j.1423-0410.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 18.Cecchetti L, Tolley ND, Michetti N, Bury L, Weyrich AS, Gresele P. Megakaryocytes differentially sort mRNAs for matrix metalloproteinases and their inhibitors into platelets: a mechanism for regulating synthetic events. Blood. 2011 Aug 18;118(7):1903–11. doi: 10.1182/blood-2010-12-324517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007 Mar 1;109(5):1975–83. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evangelista V, Manarini S, Di Santo A, Capone ML, Ricciotti E, Di Francesco L, et al. De novo synthesis of cyclooxygenase-1 counteracts the suppression of platelet thromboxane biosynthesis by aspirin. Circ Res. 2006 Mar 17;98(5):593–5. doi: 10.1161/01.RES.0000214553.37930.3e. [DOI] [PubMed] [Google Scholar]
- 21.Walkowiak B, Kaminska M, Okroj W, Tanski W, Sobol A, Zbrog Z, et al. The blood platelet proteome is changed in UREMIC patients. Platelets. 2007 Aug;18(5):386–8. doi: 10.1080/09537100601095871. [DOI] [PubMed] [Google Scholar]
- 22.Marques M, Sacristan D, Mateos-Caceres PJ, Herrero J, Arribas MJ, Gonzalez-Armengol JJ, et al. Different protein expression in normal and dysfunctional platelets from uremic patients. J Nephrol. Jan-Feb;23(1):90–101. [PubMed] [Google Scholar]
- 23.Tassies D, Reverter JC, Cases A, Escolar G, Villamor N, Lopez-Pedret J, et al. Reticulated platelets in uremic patients: effect of hemodialysis and continuous ambulatory peritoneal dialysis. Am J Hematol. 1995 Nov;50(3):161–6. doi: 10.1002/ajh.2830500303. [DOI] [PubMed] [Google Scholar]
- 24.Schoorl M, Bartels PCM. Changes in platelet volume, morphology and RNA content in subjects treated with haemodialysis. Scandinavian Journal of Clinical & Laboratory Investigation. 2008;68(4):335–42. doi: 10.1080/00365510701744481. [DOI] [PubMed] [Google Scholar]
- 25.Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009 Sep;16(9):961–6. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011 May 12;117(19):5189–97. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004 Jan 1;32(Database issue):D109–11. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009 Jan;37(Database issue):D105–10. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plante I, Davidovic L, Ouellet DL, Gobeil LA, Tremblay S, Khandjian EW, et al. Dicer-derived microRNAs are utilized by the fragile X mental retardation protein for assembly on target RNAs. J Biomed Biotechnol. 2006;2006(4):64347. doi: 10.1155/JBB/2006/64347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 32.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009 Jan 23;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanholder R, Cornelis R, Dhondt A, Lameire N. The role of trace elements in uraemic toxicity. Nephrol Dial Transplant. 2002;17 (Suppl 2):2–8. doi: 10.1093/ndt/17.suppl_2.2. [DOI] [PubMed] [Google Scholar]
- 34.Tubek S, Grzanka P, Tubek I. Role of Zinc in Hemostasis: A Review. Biological Trace Element Research. 2008;121(1):1–8. doi: 10.1007/s12011-007-8038-y. [DOI] [PubMed] [Google Scholar]
- 35.Foster M, Samman S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal. Nov 15;13(10):1549–73. doi: 10.1089/ars.2010.3111. [DOI] [PubMed] [Google Scholar]
- 36.Kadota Y, Suzuki S, Ideta S, Fukinbara Y, Kawakami T, Imai H, et al. Enhanced metallothionein gene expression induced by mitochondrial oxidative stress is reduced in phospholipid hydroperoxide glutathione peroxidase-overexpressed cells. European Journal of Pharmacology. 626(2–3):166–70. doi: 10.1016/j.ejphar.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 37.Fujii H, Nakai K, Fukagawa M. Role of Oxidative Stress and Indoxyl Sulfate in Progression of Cardiovascular Disease in Chronic Kidney Disease. Therapeutic Apheresis and Dialysis. 15(2):125–8. doi: 10.1111/j.1744-9987.2010.00883.x. [DOI] [PubMed] [Google Scholar]
- 38.Pamuklar Z, Lee JS, Cheng H-Y, Panchatcharam M, Steinhubl S, Morris AJ, et al. Individual Heterogeneity in Platelet Response to Lysophosphatidic Acid. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008 Mar 1;28(3):555–61. doi: 10.1161/ATVBAHA.107.151837. [DOI] [PubMed] [Google Scholar]
- 39.Cui MZ. Lysophosphatidic acid effects on atherosclerosis and thrombosis. Clin Lipidol. Aug;6(4):413–26. doi: 10.2217/clp.11.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Minno G, Martinez J, McKean M-L, De La Rosa J, Burke JF, Murphy S. Platelet dysfunction in uremia. Multifaceted defect partially corrected by dialysis. The American Journal of Medicine. 1985;79(5):552–9. doi: 10.1016/0002-9343(85)90051-8. [DOI] [PubMed] [Google Scholar]
- 41.Vecino AM, Teruel JL, Navarro JL, Cesar JsM. Phospholipase A2 activity in platelets of patients with uremia. Platelets. 2002;13(7):415–8. doi: 10.1080/0953710021000024000. [DOI] [PubMed] [Google Scholar]
- 42.Bonomini M, Dottori S, Amoroso L, Arduini A, Sirolli V. Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. Journal of Thrombosis and Haemostasis. 2004;2(8):1275–81. doi: 10.1111/j.1538-7836.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 43.Vecino A, Navarro-Antolin J, Teruel J, Navarro J, Cesar J. Lipid composition of platelets in patients with uremia. Nephron. 1998;78(3):271–3. doi: 10.1159/000044934. [DOI] [PubMed] [Google Scholar]
- 44.Saj A, Lai EC. Control of microRNA biogenesis and transcription by cell signaling pathways. Current Opinion in Genetics & Development. 21(4):504–10. doi: 10.1016/j.gde.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 12(2):99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 46.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010 Aug 12;466(7308):835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kondkar AA, Bray MS, Leal SM, Nagalla S, Liu DJ, Jin Y, et al. VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA. Journal of Thrombosis and Haemostasis. 8(2):369–78. doi: 10.1111/j.1538-7836.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sondermeijer BM, Bakker A, Halliani A, de Ronde MWJ, Marquart AA, Tijsen AJ, et al. Platelets in Patients with Premature Coronary Artery Disease Exhibit Upregulation of miRNA340* and miRNA624*. PLoS One. 6(10):e25946. doi: 10.1371/journal.pone.0025946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.