Abstract
Objective
Calcific aortic valve disease (CAVD) is a major public health problem with no effective treatment available other than surgery. We previously showed that mature heart valves calcify in response to retinoic acid (RA) treatment through downregulation of the SRY-transcription factor Sox9. In this study, we investigated the effects of excess vitamin A and its metabolite RA on heart valve structure and function in vivo, and examined the molecular mechanisms of RA signaling during the calcification process in vitro.
Methods and Results
Using a combination of approaches, we defined CAVD pathogenesis in mice fed 200 IU/g and 20 IU/g of retinyl palmitate for 12 months at molecular, cellular and functional levels. We show that mice fed excess vitamin A develop aortic valve stenosis and leaflet calcification associated with increased expression of osteogenic genes and decreased expression of cartilaginous markers. Using a pharmacological approach, we show that RA-mediated Sox9 repression and calcification is regulated by classical RA signaling and requires both RAR and RXR receptors.
Conclusions
Our studies demonstrate that excess vitamin A dietary intake promotes heart valve calcification in vivo. Therefore suggesting that hypervitaminosis A could serve as a new risk factor of CAVD in the human population.
Introduction
Calcific aortic valve disease (CAVD) is the most prevalent valvular disorder and a major contributor to adult cardiac pathology, affecting nearly 30% of adults over the age of 65.1 Healthy mature aortic valve cusps are composed of three stratified layers of extracellular matrix (ECM) and several proteins within these layers share molecular characteristics with cartilage including expression of type II collagen (col2a1).2 In contrast, calcified valves are characterized by alterations in ECM stratification, formation of calcific nodules, and increased expression of osteogenic genes including Spp1 and Runx2.3–5 Collectively, these abnormal changes alter the biomechanics of the valve leading to less supple and more stiffened, or stenotic valve cusps.2 At present, valve replacement surgery is the only effective treatment for aortic stenosis and CAVD, and no medical therapies exist to prevent disease progression.6 Despite the clinical significance, the underlying mechanisms that promote abnormal bone-like processes in the valves remain largely unknown. Over the past several years a number of clinical, genetic and anatomical risk factors for CAVD have been identified1, 7, 8 with influence from the environment and habitual lifestyles. Understanding the contribution of these risk factors to the pathogenesis of CAVD has been greatly improved by studies utilizing mouse models9–14 including hypercholestrolemic diets previously shown to promote aortic valve disease and CAVD onset in vivo.10, 15
Retinoic acid (RA) is the active metabolite of vitamin A (retinol) that is supplied to the body from dietary sources largely in the form of carotenoids.16 Once in the circulation and target tissues, active retinoids function as signaling molecules to specify cell identities and control gene expression.17 RA signals through specific nuclear receptors including the retinoic acid receptor (RAR), and retinoid X receptor (RXR) families consisting of three isoforms: α, β, and γ.18 Signaling through these receptors is involved in many key cellular processes including cell differentiation, cell cycle control, cell growth and cellular responses to injury; and has been implicated in the pathogenesis of obesity, diabetes, and cardiovascular disease.19 The most fundamental mechanism of action of retinoids is through transcriptional regulation of RA responsive nuclear receptors, which is dependent on the formation of dimers. RXR can signal as a homodimer, or act as a heterodimeric partner for other nuclear receptors such as RAR, or vitamin D receptors (VDR). These complexes then bind to specific response elements including RA response elements (RAREs) located in enhancer regions of target genes to modulate positive and negative function.18
In the heart, genetic and pharmacological approaches to add excess or remove RA in the embryo have revealed essential roles during cardiogenesis. Alterations in RA signaling in several animal model systems recapitulate phenotypes associated with human congenital heart defects including DiGeorge Syndrome,20, 21 transposition of the great arteries,22 ventricular septal defects, persistent truncus arteriosus, double outlet right ventricle,23 hypoplastic ventricular chambers24 and tetralogy of fallot.25 Defects of the outflow tract are largely attributed to the established roles that RA signaling plays in cardiac neural crest and second heart field cell lineages.26–28 While hypoplastic ventricular phenotypes observed in RA mutant mice24, 29 have been reported to be the result of altered signaling in non-myocyte cell lineages30, including cardiac progenitors31, 32 and epicardially derived cells.33–35 In addition to these structures, endocardial cushion abnormalities in the outflow tract and atrioventricular regions are apparent in mice depleted of RXR alpha23 and RALDH236, therefore suggesting a role in early valve formation. Due to premature lethality of these mice, the role of RA on valve maturation could not examined, however previous work from our lab suggests that high RA signaling promotes abnormal changes in the composition of connective tissue within the mature valve leaflets leading to calcification in vitro.37 We further showed that RA-induced calcification is mediated through repression of Sox9, an SRY transcription factor previously shown to promote cartilage-like phenotypes and prevent bone-like processes in healthy valves, similar to its role in the skeletal system.38
The importance of tightly regulated RA signaling on heart development in the embryo has been extensively examined, however the effects of altered retinoid activity on cardiac structure and function after birth remain largely unknown. Based on our previous observations identifying a role for RA in regulating heart valve connective tissue homeostasis, the goal of this current study was to determine the effects of excess vitamin A and RA signaling on valve structure and function in vivo, and examine the molecular mechanisms of RA signaling on Sox9 during this process. Our findings show that increased dietary intake of retinyl palmitate in mice at levels previously shown to induce hypervitaminosis A, leads to aortic valve stenosis associated with leaflet calcification. Using an established explant system, we describe that RA-mediated calcification via repression of Sox9 requires both RAR and RXR function in vitro. This decrease in Sox9 is associated with increased expression of the osteogenic target gene Spp1 and downregulation of the cartilaginous collagen type, Col2a1.39 Therefore suggesting that in mature heart valves, high RA signaling as a result of excess dietary vitamin A intake, represses Sox9 and promotes formation of bone-like calcific nodules at the expense of healthy cartilaginous connective tissue. Together, these observations could provide insights into a previously unappreciated risk factor of CAVD related to increased intake of vitamin A derivatives in the human population.
Materials and Methods
Wild type C57BL/6J mice were fed either regular chow mix containing 20 IU/g of retinol as retinyl palmitate (Harlan, TD.93160) or a modified excess vitamin A chow containing 200 IU/g retinyl palmitate (Harlan, TD.110146) for a period of 12 months and subjected to echocardiography as described.40 Following functional analysis, RNA was extracted from aortic valves or whole hearts and cDNA generated for quantitative real-time PCR as described.37 Alternatively, whole hearts were fixed and sectioned for histological staining as described.40 For in vitro studies, post natal mouse or embryonic day (E)10 chick mitral and aortic valve explants were treated with ATRA (Sigma, 1µM), 9-cis-RA (Enzo, 1µM), LE540 (100µM), PA452 (100µM), AM580 (Tocris Bioscience, 1µM), Adapalene (Tocris Bioscience, 10µM), or DMSO (0.001% final concentration) for 48 hours. Explants were then mounted onto glass slides and fixed for histological staining, or RNA was extracted for microarray analysis using an Affymetrix Mouse GeneST Array. For a full description of materials and methods see supplementary material.
Results
Long term excess dietary vitamin A promotes aortic valve stenosis and calcification
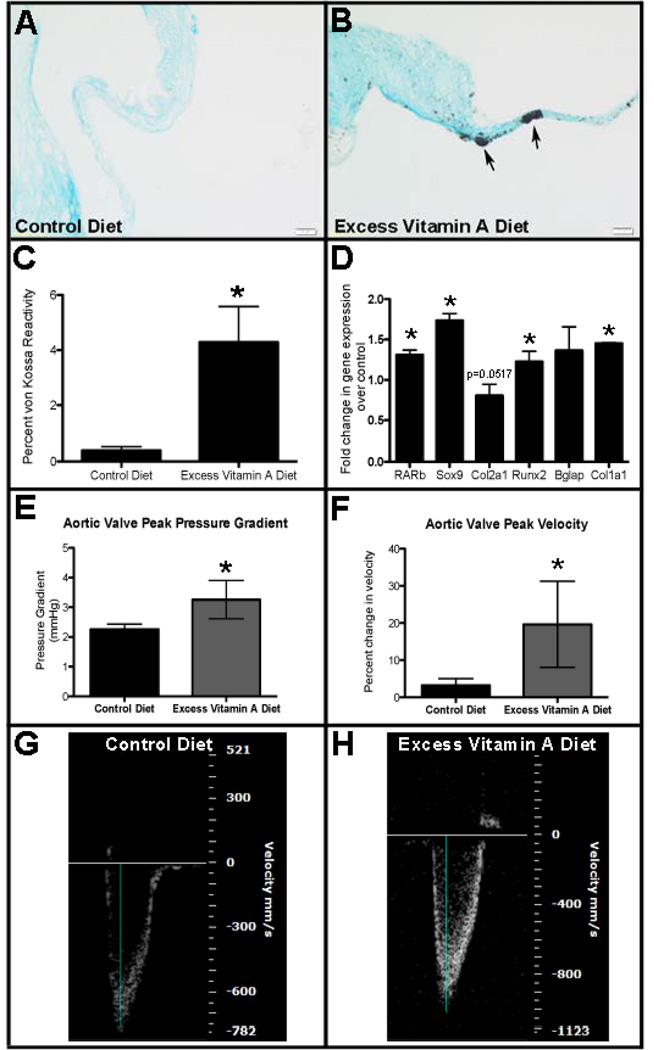
Our previous studies have shown that short-term (48 hours) all-trans retinoic acid (ATRA) treatment of murine heart valve explants in vitro promotes calcific phenotypes.37 To expand these studies and determine the long-term effects of excess retinol exposure in vivo, C57BL/6J wild type mice were fed either a control diet containing 20IU/g of retinol as retinyl palmitate from the time of weaning (4 weeks), or a modified diet containing excess vitamin A (200 IU/g retinyl palmitate). Mice fed these levels of dietary vitamin A intake have previously been shown to serve as models for hypervitaminosis A.41–44 Following 12 months of dietary intake, mice were subjected to histological, molecular and functional analysis. To examine calcium deposition in aortic valve leaflets, von Kossa staining was performed on tissue sections collected from 13 month old mice fed control (Figure 1A) or the excess vitamin A (Figure 1B) diet. As shown in Figure 1C, mice fed excess vitamin A show significantly greater von Kossa reactivity (0.39%±0.04) compared to controls (4.29%±0.41), indicative of calcification. At the molecular level, increased RAR-beta (Rarb) expression confirms activation of RAR/RXR-mediated signaling45 in the excess vitamin A experimental group. In addition to changes in RARb, expression of Sox9, Col2a1, Runx2, bone gamma-carboxyglutamic acid-containing protein (Bglap) and type I collagen (Col1a1) are all increased in animals following long-term exposure to excess dietary vitamin A (Figure 1D), consistent with ongoing calcification processes.3, 5, 46 Functional analysis by echocardiography reveals significant increases in aortic valve peak pressure gradient (Figure 1E) and velocity (Figures 1F–H) during ventricular systole in mice fed the excess vitamin A diet (n=6), suggesting aortic valve stenosis. These data suggest that long-term exposure to excess dietary vitamin A in mice recapitulates CAVD phenotypes at cellular, molecular and functional levels.
Figure 1. Excess dietary vitamin A promotes heart valve calcification phenotypes in mice.
C57BL/6J wild type mice were fed either a control diet containing 20 IU/g retinyl palmitate or an excess vitamin A diet containing 200 IU/g over a period of 12 months. (A, B) von Kossa reactivity indicates calcific nodule formation (arrows) in aortic valves from mice fed control (A), or an excess vitamin A (B) diet. (C) Quantification of von Kossa as a percentage of total area defined by Alcian blue staining (n=3). (D) qPCR to show fold changes in gene expression in aortic valves from mice fed an excess vitamin A diet compared to controls (n=3). (E–H) Echocardiography and Doppler flow analysis to show aortic valve peak pressure gradient (E) and peak velocity (F) in mice fed excess vitamin A. (G, H) Representative Doppler profiles of aortic outflow velocity for mice subjected to control (G) and excess vitamin A (H) diets (n=6). Blue line indicates measurements taken. * p<0.05 over control.
RA-mediated calcification requires activity of both RAR and RXR
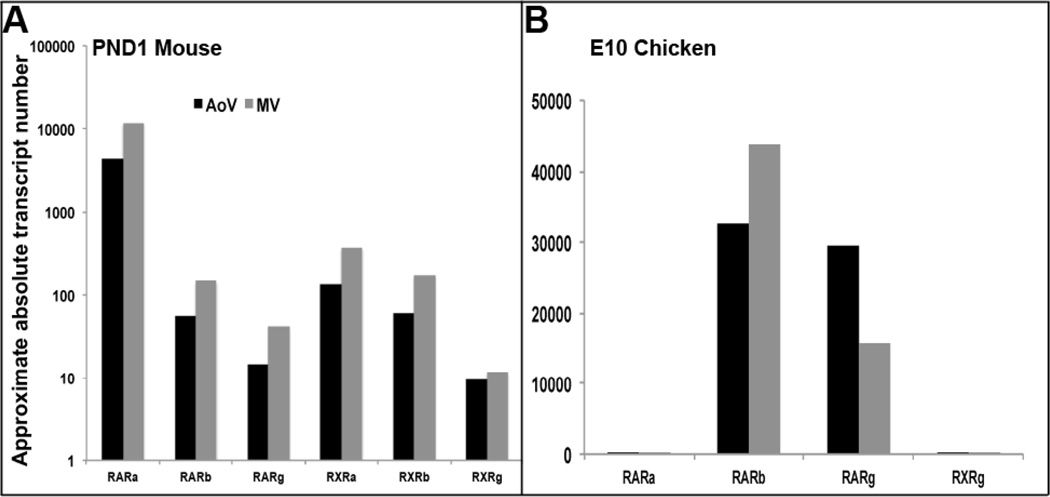
Findings from in vivo studies in mice (Figure 1) and our previously published in vitro work37 support the hypothesis that increased RA signaling promotes heart valve calcification. To further delineate this and determine the molecular mechanisms required for this process, the approximate absolute transcript number of the RAR and RXR isoforms were examined in postnatal mouse aortic and mitral valves, as well as comparable stages in the chicken (embryonic (E) day 10) (Figure 2). In mouse valves, comparable levels of RA receptor isoforms are identified in mitral and aortic valves, with RARalpha (RARa) being most highly expressed (Figure 2A). In contrast, RARb and RARgamma (RARg) are most predominant in chicken valves (Figure 2B). As RXRa and RXRb isoforms have not been reported in the avian system they were not included in this analysis.
Figure 2. RARb,g isoforms are highly expressed in mouse and chicken aortic and mitral valves.
Quantitative real-time PCR to show approximate absolute transcript numbers of various RAR and RXR isoforms in postnatal mouse (A), and embryonic day 10 chicken (B) aortic (AoV) and mitral (MV) valves.
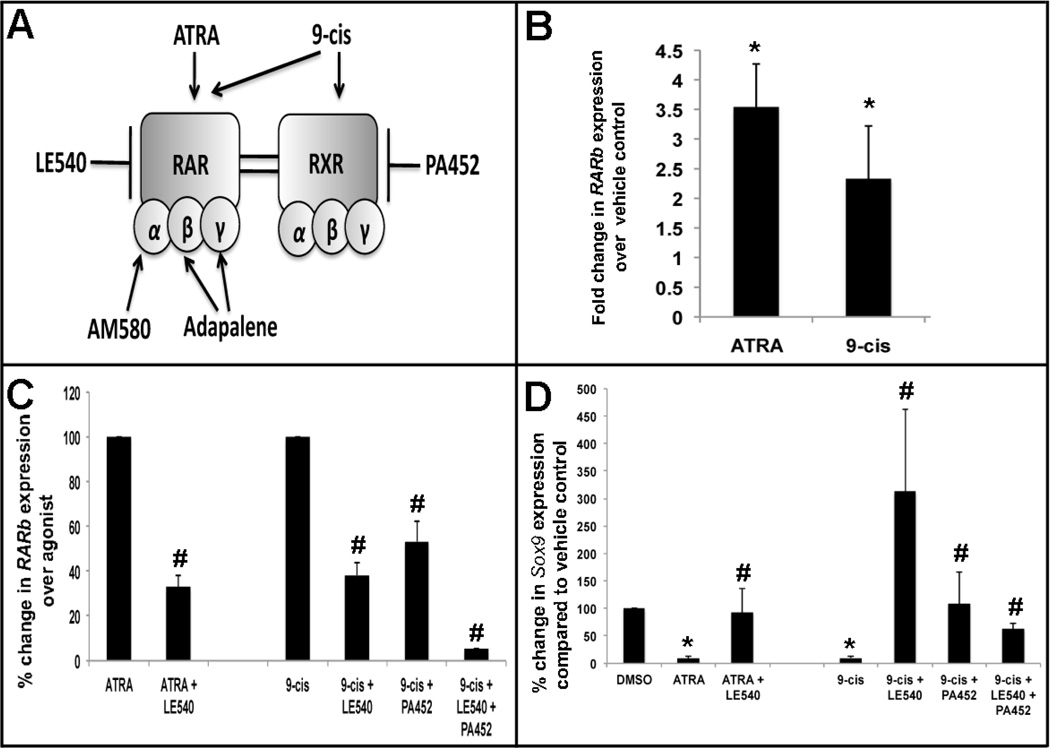
To determine if RA functions via classical RA receptor signaling to promote calcification, avian and murine aortic and mitral valve explants were treated with various pharmacological RAR and RXR agonists and antagonists (Figure 3A). At moderate concentrations, it has been shown that ATRA is an elective agonist for RARs, while 9-cis activates both RARs and RXRs (Figure 3A).17 As for antagonists, LE540 is as an established pan-antagonist for RARs, and PA452 is known to inhibit RXR function (Figure 3A).47 To determine the efficiency of agonist and antagonist action on RAR and RXR activity, qPCR was used to determine mRNA levels of the RAR/RXR-mediated RA target gene, RARb.45 As expected, ATRA and 9-cis significantly increase RARb expression over vehicle controls (Figure 3B). To examine relative levels of inhibition by the antagonists in this system, agonist activity was set at 100%. Using this approach, we observe that LE540 treatment inhibits ATRA-, and 9-cis-mediated RAR activity to 32.95%±4.93 and 38.28%±5.72, respectively (Figure 3C). For PA452, co-treatment reduces RXR activity to 53.32%±9.27, while treatment with both LE540 and PA452 inhibits activity to 5.11%±0.05 (Figure 3D). These studies confirm the intended pharmacological activation and inhibition of RAR and RXR.
Figure 3. ATRA-mediated heart valve calcification requires RAR and RXR activity.
cE10 mitral valve explants were treated for 48 hours with indicated agonists, with or without a 4 hour antagonist pre-treatment. (A) Diagram depicting selectivity of RAR and RXR agonists and antagonists. (B–C) qPCR to show changes in RARβ expression (B, C) as a fold change with agonist treatments (B), and as a percent change following pre-treatment with RAR (LE540) or RXR (PA452) pan-antagonists compared to ATRA and 9-cis agonists alone. (D) qPCR analysis to show percentage changes in Sox9 expression in agonist treatment alone, and pre-treatment with antagonists. * p<0.05 over vehicle; # p<0.05 over agonist.
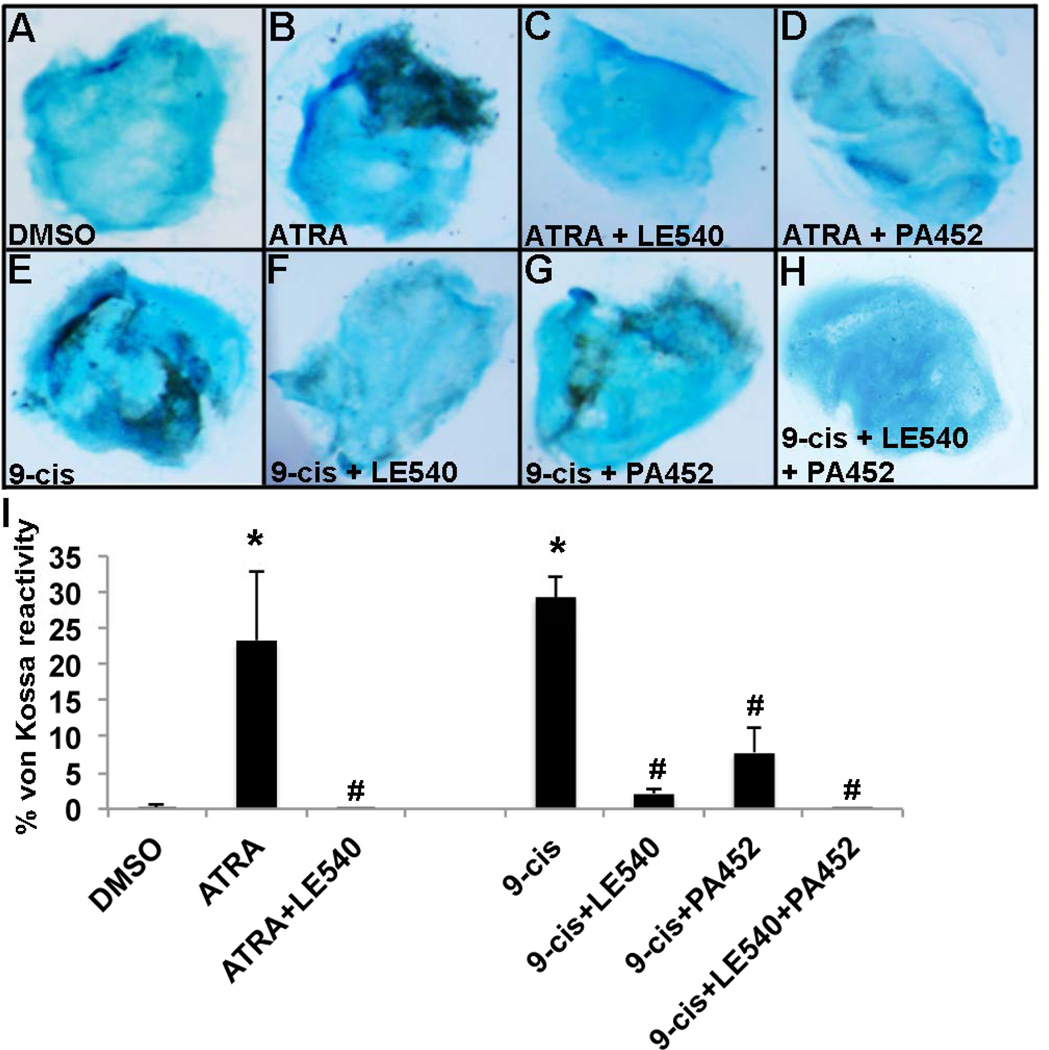
As our previous studies have shown that ATRA treatment promotes calcification via repression of Sox937, we next examined changes in Sox9 expression in response to agonist and antagonist treatments. As shown in Figure 3D, both ATRA and 9-cis significantly repress Sox9 expression in avian E10 aortic valve explants, and this repression is alleviated to levels comparable with vehicle controls when co-treated with LE540. Similarly with 9-cis, LE540 or PA452 treatments significantly prevent 9-cis-mediated Sox9 repression, although treatment with both antagonists is most effective (Figure 3D). Similar observations were observed using siRNA to target RAR, RXR or both RAR and RXR knockdown in C3H10T1/2 cells treated with ATRA, compared to DMSO and non-targeting siRNA controls (Supplementary Figure 1). These data suggest that both RAR and RXR are required to mediate Sox9 repression and calcification by activated RA signaling. Consistent with reduced Sox9 expression in Figure 3, von Kossa reactivity indicative of calcification is significantly increased with ATRA and 9-cis treatments of avian mitral valve explants and this phenotype is lost when co-treated with LE540, PA452, or both antagonists (Figure 4).
Figure 4. Retinoic acid treatment promotes calcific nodule formation in cE10 mitral valve explants.
Compared to vehicle controls (A), von Kossa reactivity is increased following treatment with ATRA (B) and 9-cis RA (E). von Kossa reactivity is attenuated when co-treated with pharmacological antagonists (C,D,F,G,H). (I) Quantification of von Kossa reactivity normalized to area, as indicated by Alcian blue counterstain. * p<0.05 over vehicle; # p<0.05 over agonist.
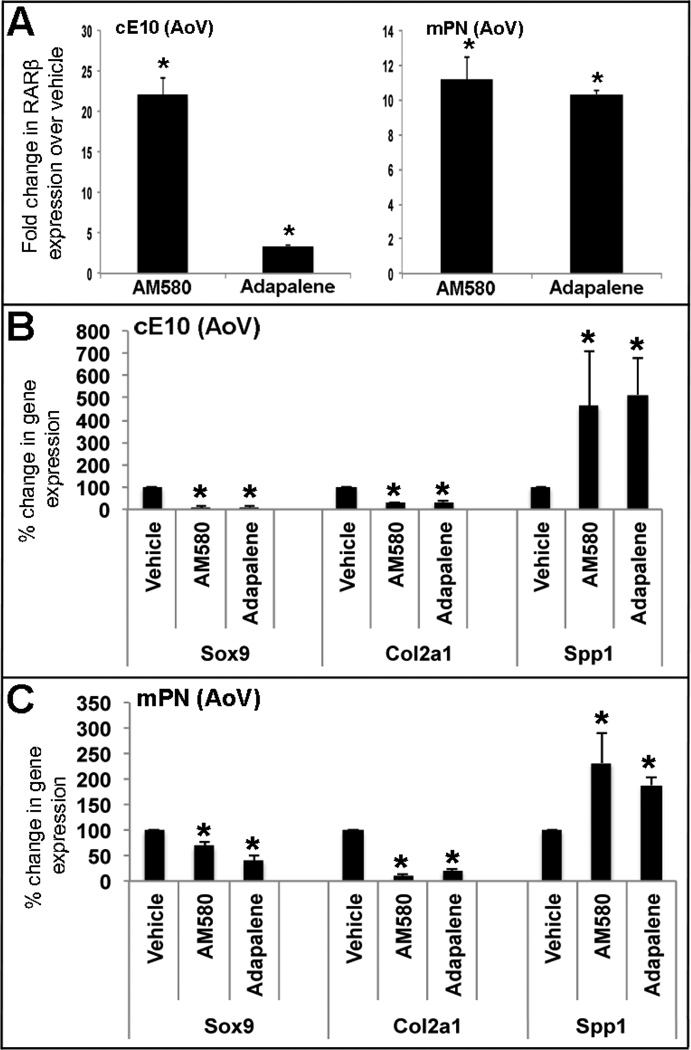
RARa and RARb,g agonist treatment of avian valve explants represses Sox9 and Col2a1 expression, and promotes Spp1
We, and others have shown that Sox9 promotes normal cartilaginous phenotypes in heart valves through direct activation of Col2a139, and prevents ectopic bone-like calcification potentially through direct repression of Spp1.48 To examine if this signaling pathway is downstream of RAR activity during heart valve calcification processes, gene expression analysis was performed in avian E10 and postnatal aortic valve explants treated with selective RAR agonists. To further delineate which RAR isoform might be responsible for this, specific RARa (AM580) and RARb,g (Adapalene) agonists were used. As expected, both AM580 and Adapelene increased RARb expression in treated avian E10 and murine postnatal aortic valve explants (Figure 5A), suggesting successful targeting. Similar to ATRA treatment (Figure 3), AM580 (RARa) and Adapalene (RARb,g) treatments repressed Sox9, suggesting that activity of all three RAR isoforms are sufficient to induce Sox9 repression. In addition to Sox9, expression of the cartilaginous marker, Col2a1 was also downregulated, while Spp1, associated with osteogenic processes was increased (Figure 5B, C). In contrast to Spp1, expression of the osteogenic transcription factor Runx2 was not significantly changed in this assay after 48 hour treatment (data not shown). These results suggest that in heart valves, activated RA signaling via RARa,b,g repress Sox9 leading to decreased Col2a1 (cartilage-like) and increased Spp1 (osteogenic).
Figure 5. Treatment with specific RAR isoform agonists promotes expression of osteogenic genes at the expense of cartilaginous markers.
(A) qPCR to show fold changes in RARb expression following treatment of cE10 or postnatal mouse aortic valve explants with RARa- (AM580) and RARb,g,-specific (Adapalene) agonists relative to vehicle controls. (B, C) qPCR to show changes in osteogenic (Spp1) and cartilaginous (Sox9, Col2a1) gene expression following treatments. * p<0.05 over vehicle controls.
Microarray analysis reveals significant changes in gene expression profiles associated with bone development and extracellular matrix in murine aortic heart valves following short-term ATRA treatment
In order to fully examine the differential gene expression changes in aortic valves in response to short-term ATRA treatment, a high throughput microarray approach was used. Compared to DMSO vehicle controls, a total of 432 probe sets were differentially expressed in ATRA treated aortic valve explants, with 118 being downregulated and 314 increased. The 432 probe sets were ranked based on false discovery rate (<0.1) and fold change (>2), and the top 100 are listed in Supplementary Table 1. As expected these top 100 hits include increased mRNA transcripts associated with RA metabolism and signaling (RARb (+4.76-fold), retinol binding protein 1 (rbp1) (+2.10), cytochrome P450 (Cyp26a1) (+2.37) and retinoic acid receptor responder (Rarres2) (+2.73). Of the 432 differentially expressed probe sets, 12 were successfully validated by qPCR using independent cDNA samples (Supplementary Table 2). Gene ontology analysis of the 432 probe sets altered by ATRA treatment revealed significant enrichment of genes associated with bone development and the extracellular matrix (Table 1). These data suggest that short-term ATRA treatment of aortic valve explants promotes calcification by activating gene expression profiles consistent to that observed in excised human calcified valves.3, 5
Table 1.
Gene ontology analysis of microarray data for ATRA treated aortic valve explants compared to DMSO vehicle controls shows enrichment of bone development and extracellular matrix gene programs.
| Gene Name | Gene Abbrev. |
Fold change ATRA vs. DMSO |
FDR |
|---|---|---|---|
|
Biological Process-Bone development GO:0060348 p value=0.0029 |
|||
| Bone morphogenetic protein 2 | Bmp2 | 2.67 | 0.004 |
| Acid phosphatase 5, tartrate resistant | Acp5 | 1.68 | 0.008 |
| Secreted phosphoprotein 1 | Spp1 | 1.87 | 0.019 |
| PDZ and LIM domain 7 | Pdlim7 | 1.41 | 0.22 |
| Insulin-like growth factor binding protein | Igfbp5 | 1.93 | 0.04 |
| Insulin-like growth factor 1 | Igf1 | 1.8 | 0.043 |
| MAD homolog 3 (Drosophila) | Smad3 | 1.69 | 0.044 |
| Forkhead box C2 | Foxc2 | 1.69 | 0.04 |
| Insulin-like growth factor binding protein 3 | Igfbp3 | 2.62 | 0.037 |
| Insulin-like growth factor 2 | Igf2 | 1.66 | 0.039 |
|
Cellular Component-Extracellular Matrix GO:0031012 p value=0.0005 |
|||
| Collagen triple helix repeat containing 1 | Cthrc1 | 1.92 | 0.08 |
| Collagen, type XIV, alpha 1 | Col14a1 | 1.85 | 0.058 |
| Fibulin1 | Fbln1 | 2.04 | 0.01 |
| Coiled-coil domain containing 80 | Ccdc80 | 1.6 | 0.058 |
| Matrix metallopeptidase 3 | Mmp3 | 5.12 | 0.021 |
| Hemicentin 1 | Hmcn1 | 4.49 | 0.001 |
| Microfibrillar-associated protein 2 | Mfap2 | 1.8 | 0.059 |
| Transforming growth factor, beta 3 | Tgfb3 | 1.67 | 0.04 |
| Osteoglycin | Ogn | 2.75 | 0.004 |
| Transglutaminase2, C polypeptide | Tgm2 | 4.99 | 0 |
| Lysyl oxidase | Lox | 1.93 | 0.03 |
| Spondin2, extracellular matrix protein | Spon2 | 3.41 | 0.02 |
Discussion
In this study we determined the effects of excess dietary vitamin A on heart valve structure and function and examined the signaling mechanisms underlying retinoic acid (RA)-induced changes in the gene expression profiles of valves. We show that long-term hypervitaminosis A in mice leads to aortic valve stenosis and leaflet calcification associated with increased osteogenic gene expression profiles in vivo. Using an established in vitro valve explant culture system, we also determined that RA-induced calcification signals through RAR and RXR, and leads to Sox9 repression and dysregulation of its target genes including decreased Col2a1 and increased Spp1. In support, gene ontology analysis of microarray findings, show that gene expression probe sets associated with bone development and extracellular matrix processes are enriched in ATRA-treated murine aortic valve explants. These results imply that RA signaling must be tightly regulated in adult mice to maintain heart valve connective tissue homeostatsis. Further, findings from this study suggest that excess exposure to retinoids could contribute to CAVD onset and progression in the human population.
Excess dietary intake of vitamin A promotes valve dysfunction in mice
We show that increased dietary vitamin A intake in adult mice promotes CAVD phenotypes in vivo. In humans, it is estimated that 75% of people ingest more than the recommended daily allowance of vitamin A on a regular basis.16 In pregnant women, the teratogenic potential of excess vitamin A and beta carotene on development of the heart in the embryo have been well established, however less is known of the long-term effects of hypervitaminosis A on cardiac structure and function in adults. Compared to mice fed 20IU/g of retinyl palmitate, wild type mice receiving 10 times excess develop aortic valve stenosis as evident by increased peak pressure gradient and velocity (Figure 1 E–H). This functional defect is attributed to formation of calcific nodules on the cusp surface (Figure 1A–B) and increased expression of osteogenic genes (Figure 1D), as commonly observed in human patients with CAVD.1 Therefore suggesting that high retinoid exposure is detrimental to heart valve structure and function in mice. In humans, clinical trial studies in smokers or lung cancer patients have shown that excess retinoid exposure is also associated with cardiovascular events and even mortality, although data is conflicting, reporting positive49, 50, negative51, or no52, 53 effects in promoting cardiovascular disease. In mice, 9-cis and beta carotene dietary supplements, or administration of the synthetic retinoid AM80 is beneficial in the regression of atherosclerotic lesion formation in genetically susceptible mouse models of atherosclerosis.54, 55 These studies suggest that excess retinoid exposure can have differential effects on the cardiovascular system that may be dependent on existing conditions and therefore, studies to determine if dietary hypervitaminosis A accelerates pathogenesis in susceptible mouse models of CAVD would be informative.
Retinoic acid treatment promotes osteogenic- and represses cartilage-like processes in adult heart valve connective tissue
Despite valve function deteriorating in mice exposed to excess dietary retinoid intake, no other overt cardiac defects were observed and myocardial contractility was comparable with controls. This suggests that in wild type adult mice, excess dietary vitamin A does not cause overt changes in all cardiovascular structures but specifically affects connective tissue homeostasis in the valves to promote CAVD. Calcification of other systems including the vasculature, have previously been associated with high levels of vitamin A and its derivatives.56, 57 In our previous study37, we showed that ATRA treatment promotes valve calcification via downregulation of Sox9, and mice with reduced Sox9 function develop early onset heart valve calcification in vivo.37 In these mice, calcific phenotypes are associated with increased expression of osteogenic markers and down regulation of cartilaginous genes within the valve structures.37 Similarly in this study, our microarray analysis reveals enrichment of gene expression profiles related to bone development (Table 1, Figure 5) and decreased expression of cartilaginous genes (Figure 5). In other systems, ATRA treatment has been shown to promote differentiation of stem cells towards the osteogenic lineage58, 59, and negatively regulate chondrogenic phenotypes in the skeletal system as a result of reduced Sox9 activity.60 All these data suggest that ATRA-induced calcification is converging on the Sox9 pathway, and therefore the opposing effects that ATRA treatment has on cartilage and bone-like processes-are likely due to altered regulation of Sox9 target genes leading to alleviated repression of the osteogenic glycoprotein Spp148 and lost transactivation of Col2a1.39 Our data showing that RAR and RXR are required for RA-mediated heart valve calcification (Figures 3, 4) suggests that Sox9 repression by ATRA is regulated via a classical RA signaling pathway.61 However, using transcription factor binding site prediction software, canonical RAREs were not identified within Sox9 and previous studies have not described direct interaction between RA receptors and Sox family members. Therefore we anticipate that RAR/RXRs could function indirectly to regulate Sox9, potentially by positively regulating known (co)repressors of the cartilage gene program, or inhibiting activators of osteogenesis62–65 in this system. Alternatively it is speculated that RA receptors cross talk with known nuclear receptor binding partners including VDRs, that when depleted, similarly cause aortic valve calcification in mice.66
Hypervitaminosis A as a model of CAVD
Defining the molecular and cellular phenotypes that contribute to the onset and progressive pathogenesis of CAVD has been challenging due to the limited number of suitable animal models available. In this study, we have identified a potentially new model of CAVD induced by increased dietary intake of vitamin A that recapitulates human disease at the functional level (Figure 1E–H). In addition to aortic valve stenosis, positive von Kossa reactivity (Figure 1A–B) is associated with increased expression of osteogenic genes Runx2 and Bglap (osteocalcin) (Figure 1D), consistent with previous human46, 67, 68, and animal3, 37, 69, 70 studies. As these increases are observed in 13 month old mice and not in valve explants exposed to short-term ATRA treatment (data not shown), it is considered that Runx2 and Bglap serve as molecular markers of late stage disease which is consistent with human data showing similar increases in adult, but not pediatric valve disease patients.46, 67 Despite increased Runx2, Spp1 (Osteopontin) expression was not altered in hypervitaminosis A mice, which is in contrast to other models of CAVD.3, 37, 46, 70 However, high levels of Spp1 were observed in our short-term explant assays consistent with decreased Sox9 (Figures 3, 5), a known negative regulator.48 Based on these observations and data from our previous studies,37, 71 we suggest that early Sox9 repression initiates pathological changes in aortic valves and promotes initial calcific nodule formation. While, increased levels observed in hypervitaminosis A mice and end-stage human degenerative valve disease,46, 72 suggests potential compensatory mechanisms in response to changes in matrix composition. In addition to differential changes in osteogenic and cartilage genes, previous studies have reported active Bmp and Wnt signaling pathways in valve and vascular calcification.69, 70, 73, 74 By microarray analysis, Bmp2 and Smad3 were increased in short-term ATRA-treated explants, however changes in pSmad1/5/8 were not observed in our in vivo model (data not shown). Further, expression of b-catenin or Wnt-associated pathways identified from Wikipathways analyses were not significantly altered. Therefore suggesting that canonical BMP and Wnt signaling is not active during late stages of pathogenesis in this mouse model. Collectively, these data suggest that hypervitaminosis A mice recapitulate functional and many molecular phenotypes of human and animal models of CAVD. In addition, this study supports work from others identifying early, and late markers of disease pathogenesis that may be important for improving diagnosis and therapeutic intervention. In addition, these studies highlight the complex, multifactorial nature of CAVD pathogenesis.
Summary and clinical perspectives
CAVD was once considered a passive ‘wear and tear’ disease most common in the aging population, however more recent work has described it as an actively regulated processes characterized by bone-like phenotypes.1 CAVD onset and progression has been associated with several genetic factors that underlie congenital valve malformations that increase susceptibility to calcification later in life. In addition, several clinical risk factors have been reported including older age, hypertension, serum lipoprotein levels and smoking1, 8, and while these can be attributed to genetic alterations, habitual lifestyles may also play a role. In this study, we have identified that long-term dietary intake of vitamin A results in aortic valve stenosis and leaflet calcification attributed to RAR/RXR dependent repression of Sox9. These studies suggest that patients exposed to retinol-based therapies or dietary hypervitaminosis A may be at an increased risk of developing CAVD. Vitamin A is found in a variety of food sources and the effects of excess supplementation by pregnant women on the developing embryo have been well described.16 However, the adverse effects of consuming large doses by healthy adults have been less well studied. Hypervitaminosis A induced by dietary intake may not be highly prevalent in developed countries, however, high retinol levels are more common in people ingesting dietary supplements as a beneficial antioxidant, or patients receiving retinol-based pharmaceuticals including Accutane and Tegison.16 At present, CAVD is largely diagnosed from functional analyses and biomarkers to indicate the clinical value of CAVD pathogenesis remain unknown. Based on this current study, retinol levels predicting CAVD susceptibility could improve risk stratification and help determine a therapeutic window to improve patient outcome.
Supplementary Material
Acknowledgements
We thank Blair Austin, Agata Levay and Ge Tao for technical assistance and Dr. T. Michael Underhill for sharing reagents. In addition, we thank Dr. David Willoughby (Ocean Ridge Biosciences, LLC) for performing the microarray study.
Sources of funding
This work was supported by NIH (R01-HL091878 (JL)) and The Research Institute at Nationwide Children’s Hospital.
Abbreviations
- ATRA
all-trans retinoic acid
- CAVD
calcific aortic valve disease
- ECM
extracellular matrix
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- RXR
retinoid x receptor
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors of this paper have nothing to disclose.
Conflict of Interest
The authors of this paper have no conflicts of interest.
References
- 1.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lincoln J, Yutzey KE. Molecular and developmental mechanisms of congenital heart valve disease. Birth defects research. Part A, Clinical and molecular teratology. 2011;91:526–534. doi: 10.1002/bdra.20799. [DOI] [PubMed] [Google Scholar]
- 3.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. Journal of molecular and cellular cardiology. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirrig EE, Snarr BS, Chintalapudi MR, O'Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (crtl1), an extracellular matrix component playing an important role in heart development. Developmental biology. 2007;310:291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Gersh B, Bonow RO. Calcific aortic stenosis: From bench to the bedside--emerging clinical and cellular concepts. Heart. 2003;89:801–805. doi: 10.1136/heart.89.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. The Journal of heart valve disease. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- 7.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in notch1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 8.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 9.Mohler ER, 3rd, Wang H, Medenilla E, Scott C. Effect of statin treatment on aortic valve and coronary artery calcification. The Journal of heart valve disease. 2007;16:378–386. [PubMed] [Google Scholar]
- 10.Nus M, MacGrogan D, Martinez-Poveda B, Benito Y, Casanova JC, Fernandez-Aviles F, Bermejo J, de la Pompa JL. Diet-induced aortic valve disease in mice haploinsufficient for the notch pathway effector rbpjk/csl. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- 11.Barrick CJ, Roberts RB, Rojas M, Rajamannan NM, Suitt CB, O'Brien KD, Smyth SS, Threadgill DW. Reduced egfr causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in c57bl/6j but not 129s1/svimj mice. American journal of physiology. Heart and circulatory physiology. 2009;297:H65–H75. doi: 10.1152/ajpheart.00866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. Journal of molecular and cellular cardiology. 2009;47:828–834. doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin s deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt N, Brandsch C, Kuhne H, Thiele A, Hirche F, Stangl GI. Vitamin d receptor deficiency and low vitamin d diet stimulate aortic calcification and osteogenic key factor expression in mice. PloS one. 2012;7:e35316. doi: 10.1371/journal.pone.0035316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in c57bl/6j mice. Journal of the American College of Cardiology. 2006;47:850–855. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 16.Duerbeck NB, Dowling DD. Vitamin a: Too much of a good thing? Obstetrical & gynecological survey. 2012;67:122–128. doi: 10.1097/OGX.0b013e318244c52d. [DOI] [PubMed] [Google Scholar]
- 17.Theodosiou M, Laudet V, Schubert M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cellular and molecular life sciences : CMLS. 2010;67:1423–1445. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (rars) Nuclear receptor signaling. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the ppar-rxr complex. FEBS letters. 2008;582:32–38. doi: 10.1016/j.febslet.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 20.Vermot J, Niederreither K, Garnier JM, Chambon P, Dolle P. Decreased embryonic retinoic acid synthesis results in a digeorge syndrome phenotype in newborn mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1763–1768. doi: 10.1073/pnas.0437920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryckebusch L, Bertrand N, Mesbah K, Bajolle F, Niederreither K, Kelly RG, Zaffran S. Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of digeorge syndrome. Circulation research. 2010;106:686–694. doi: 10.1161/CIRCRESAHA.109.205732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakabe M, Kokubo H, Nakajima Y, Saga Y. Ectopic retinoic acid signaling affects outflow tract cushion development through suppression of the myocardial tbx2-tgfbeta2 pathway. Development. 2012;139:385–395. doi: 10.1242/dev.067058. [DOI] [PubMed] [Google Scholar]
- 23.Gruber PJ, Kubalak SW, Pexieder T, Sucov HM, Evans RM, Chien KR. Rxr alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. The Journal of clinical investigation. 1996;98:1332–1343. doi: 10.1172/JCI118920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. Rxr alpha mutant mice establish a genetic basis for vitamin a signaling in heart morphogenesis. Genes & development. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 25.Pavan M, Ruiz VF, Silva FA, Sobreira TJ, Cravo RM, Vasconcelos M, Marques LP, Mesquita SM, Krieger JE, Lopes AA, Oliveira PS, Pereira AC, Xavier-Neto J. Aldh1a2 (raldh2) genetic variation in human congenital heart disease. BMC medical genetics. 2009;10:113. doi: 10.1186/1471-2350-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang X, Choudhary B, Merki E, Chien KR, Maxson RE, Sucov HM. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mechanisms of development. 2002;117:115–122. doi: 10.1016/s0925-4773(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 28.Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation; research in biological diversity. 2012;84:25–40. doi: 10.1016/j.diff.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brade T, Kumar S, Cunningham TJ, Chatzi C, Zhao X, Cavallero S, Li P, Sucov HM, Ruiz-Lozano P, Duester G. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial igf2. Development. 2011;138:139–148. doi: 10.1242/dev.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran CM, Sucov HM. The rxralpha gene functions in a non-cell-autonomous manner during mouse cardiac morphogenesis. Development. 1998;125:1951–1956. doi: 10.1242/dev.125.10.1951. [DOI] [PubMed] [Google Scholar]
- 31.Lin SC, Dolle P, Ryckebusch L, Noseda M, Zaffran S, Schneider MD, Niederreither K. Endogenous retinoic acid regulates cardiac progenitor differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9234–9239. doi: 10.1073/pnas.0910430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorrell MR, Waxman JS. Restraint of fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Developmental biology. 2011;358:44–55. doi: 10.1016/j.ydbio.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of wt1 and raldh2 in the embryonic avian heart: A model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (epdcs) Developmental biology. 2002;247:307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- 34.von Gise A, Zhou B, Honor LB, Ma Q, Petryk A, Pu WT. Wt1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Developmental biology. 2011;356:421–431. doi: 10.1016/j.ydbio.2011.05.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braitsch CM, Combs MD, Quaggin SE, Yutzey KE. Pod1/tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Developmental biology. 2012;368:345–357. doi: 10.1016/j.ydbio.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- 37.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circulation research. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lincoln J, Lange AW, Yutzey KE. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Developmental biology. 2006;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P. Sox9 binds DNA, activates transcription, and coexpresses with type ii collagen during chondrogenesis in the mouse. Developmental biology. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 40.Levay AK, Peacock JD, Lu Y, Koch M, Hinton RB, Jr, Kadler KE, Lincoln J. Scleraxis is required for cell lineage differentiation and extracellular matrix remodeling during murine heart valve formation in vivo. Circulation research. 2008;103:948–956. doi: 10.1161/CIRCRESAHA.108.177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin a deficiency decreases and high dietary vitamin a increases disease severity in the mouse model of asthma. J Immunol. 2008;180:1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- 42.Sagazio A, Piantedosi R, Alba M, Blaner WS, Salvatori R. Vitamin a deficiency does not influence longitudinal growth in mice. Nutrition. 2007;23:483–488. doi: 10.1016/j.nut.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Kitaoka K, Hattori A, Chikahisa S, Miyamoto K, Nakaya Y, Sei H. Vitamin a deficiency induces a decrease in eeg delta power during sleep in mice. Brain research. 2007;1150:121–130. doi: 10.1016/j.brainres.2007.02.077. [DOI] [PubMed] [Google Scholar]
- 44.Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, De Luca LM, Ozato K. Vitamin a deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349–3356. [PubMed] [Google Scholar]
- 45.Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the camp response element-binding protein in normal human bronchial epithelial cells. Molecular biology of the cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. Journal of the American College of Cardiology. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umemiya H, Fukasawa H, Ebisawa M, Eyrolles L, Kawachi E, Eisenmann G, Gronemeyer H, Hashimoto Y, Shudo K, Kagechika H. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the rxr-rar heterodimers. Journal of medicinal chemistry. 1997;40:4222–4234. doi: 10.1021/jm9704309. [DOI] [PubMed] [Google Scholar]
- 48.Peacock JD, Huk DJ, Ediriweera HN, Lincoln J. Sox9 transcriptionally represses spp1 to prevent matrix mineralization in maturing heart valves and chondrocytes. PloS one. 2011;6:e26769. doi: 10.1371/journal.pone.0026769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. The New England journal of medicine. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 50.Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–1720. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 51.Street DA, Comstock GW, Salkeld RM, Schuep W, Klag MJ. Serum antioxidants and myocardial infarction. Are low levels of carotenoids and alpha-tocopherol risk factors for myocardial infarction? Circulation. 1994;90:1154–1161. doi: 10.1161/01.cir.90.3.1154. [DOI] [PubMed] [Google Scholar]
- 52.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. The New England journal of medicine. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 53.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: The women's health study. Journal of the National Cancer Institute. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 54.Takeda N, Manabe I, Shindo T, Iwata H, Iimuro S, Kagechika H, Shudo K, Nagai R. Synthetic retinoid am80 reduces scavenger receptor expression and atherosclerosis in mice by inhibiting il-6. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1177–1183. doi: 10.1161/01.ATV.0000214296.94849.1c. [DOI] [PubMed] [Google Scholar]
- 55.Harari A, Harats D, Marko D, Cohen H, Barshack I, Kamari Y, Gonen A, Gerber Y, Ben-Amotz A, Shaish A. A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in ldl receptor knockout mice. The Journal of nutrition. 2008;138:1923–1930. doi: 10.1093/jn/138.10.1923. [DOI] [PubMed] [Google Scholar]
- 56.Strebel RF, Girerd RJ, Wagner BM. Cardiovascular calcification in rats with hypervitaminosis a. Archives of pathology. 1969;87:290–297. [PubMed] [Google Scholar]
- 57.Jacobson A, Johansson S, Branting M, Melhus H. Vitamin a differentially regulates rankl and opg expression in human osteoblasts. Biochemical and biophysical research communications. 2004;322:162–167. doi: 10.1016/j.bbrc.2004.07.092. [DOI] [PubMed] [Google Scholar]
- 58.Roberts SJ, Chen Y, Moesen M, Schrooten J, Luyten FP. Enhancement of osteogenic gene expression for the differentiation of human periosteal derived cells. Stem cell research. 2011;7:137–144. doi: 10.1016/j.scr.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, Bi Y, Luo X, Luo J, Teven C, Shi Q, Kim SH, Gao JL, Huang E, Yang K, Rames R, Liu X, Li M, Hu N, Liu H, Su Y, Chen L, He BC, Zuo GW, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC. Bmp-9 induced osteogenic differentiation of mesenchymal stem cells: Molecular mechanism and therapeutic potential. Current gene therapy. 2011;11:229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 60.Weston AD, Chandraratna RA, Torchia J, Underhill TM. Requirement for rar-mediated gene repression in skeletal progenitor differentiation. The Journal of cell biology. 2002;158:39–51. doi: 10.1083/jcb.200112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee EJ, Nallamshetty S, Plutzky J. Retinoid metabolism and its effects on the vasculature. Biochimica et biophysica acta. 2012;1821:230–240. doi: 10.1016/j.bbalip.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Kawakami Y, Tsuda M, Takahashi S, Taniguchi N, Esteban CR, Zemmyo M, Furumatsu T, Lotz M, Izpisua Belmonte JC, Asahara H. Transcriptional coactivator pgc-1alpha regulates chondrogenesis via association with sox9. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2414–2419. doi: 10.1073/pnas.0407510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semba I, Nonaka K, Takahashi I, Takahashi K, Dashner R, Shum L, Nuckolls GH, Slavkin HC. Positionally-dependent chondrogenesis induced by bmp4 is co-regulated by sox9 and msx2. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;217:401–414. doi: 10.1002/(SICI)1097-0177(200004)217:4<401::AID-DVDY7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 64.Furumatsu T, Tsuda M, Yoshida K, Taniguchi N, Ito T, Hashimoto M, Ito T, Asahara H. Sox9 and p300 cooperatively regulate chromatin-mediated transcription. The Journal of biological chemistry. 2005;280:35203–35208. doi: 10.1074/jbc.M502409200. [DOI] [PubMed] [Google Scholar]
- 65.Rau MJ, Fischer S, Neumann CJ. Zebrafish trap230/med12 is required as a coactivator for sox9-dependent neural crest, cartilage and ear development. Developmental biology. 2006;296:83–93. doi: 10.1016/j.ydbio.2006.04.437. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tassone F, Hertz-Picciotto I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the charge (childhood autism risks from genetics and environment) case-control study. The American journal of clinical nutrition. 2012;96:80–89. doi: 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. Journal of molecular and cellular cardiology. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC, Jr, Fullerton DA. Bone morphogenic protein 2 induces runx2 and osteopontin expression in human aortic valve interstitial cells: Role of smad1 and extracellular signal-regulated kinase 1/2. The Journal of thoracic and cardiovascular surgery. 2009;138:1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 69.Rajamannan NM. The role of lrp5/6 in cardiac valve disease: Experimental hypercholesterolemia in the apoe−/− /lrp5−/− mice. Journal of cellular biochemistry. 2011;112:2987–2991. doi: 10.1002/jcb.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the lrp5 receptor pathway. Circulation. 2005;112:I229–I234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Developmental biology. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wirrig EE, Yutzey KE. Transcriptional regulation of heart valve development and disease. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2011;20:162–167. doi: 10.1016/j.carpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-mediated activation of beta-catenin signaling has a critical role in warfarin-induced vascular calcification. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:123–130. doi: 10.1161/ATVBAHA.111.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Developmental biology. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.