Abstract
Cure rates for American cutaneous leishmaniasis (ACL) range between 60% and 90%. Early evidence suggests lower cure rates for early ACL before the development of the ulceration. We evaluated risk factors for treatment failure in patients with early and classic ulcerative ACL. Patients (n = 136) were 13–60 years of age and had lesions with a duration of 15–90-days. Patients were treated with antimony (20 mg/kg/day for 20 days). The primary outcome was lesion cure by 90 days without recurrence. Patients with early ACL (n = 16) had papules, nodules, plaques, or superficial ulcerations with less than 30 days of illness. Patients with classic ulcerative ACL (n = 120) had ulcerated classic lesions, longer duration, larger lesions, and higher levels of interferon-γ and tumor necrosis factor-α (P ≤ 0.01 for all comparisons). Ulcerated lesions were associated with a lower treatment failure rate compared with early ACL (25.8% versus 75.0%; P < 0.001). Early treatment of ACL does not prevent lesion ulceration and is associated with higher rates of treatment failure.
INTRODUCTION
Leishmaniasis is a vector-borne protozoal disease endemic to 88 tropical and sub-tropical countries.1 There are a presumed 12 million cases of leishmaniasis and 2 million new cases each year, of which 1.5 million are cutaneous leishmaniasis.1 American cutaneous leishmaniasis (ACL) is a major health problem in Brazil and has an incidence of 8.1 cases per 1,000 persons in the southern part of the state of Bahia where Leishmania braziliensis accounts for more than 95% of all cases of ACL.2 American cutaneeous leishmaniasis typically manifests as a single ulcerated lesion with elevated borders on exposed inferior limbs. Lesions may also be vegetative, verrucous, sporotricoid, or lupoid.3,4 Host and parasite factors may influence the clinical outcome and response to therapy for leishmaniasis.5–7 In the Old World, ulcers caused by L. major heal even without antimony therapy.8 Ulcer healing in ACL usually occurs 50–90 days after initiation of therapy.9,10
Innate and type 1 immune responses play a central role in macrophage killing intracellular Leishmania spp. and consequently in response to therapy.11,12 When cellular immune response to Leishmania antigen is decreased, patients infected with L. amazonensis developed diffuse cutaneous leishmaniasis characterized by multiple nodular lesions with macrophages filled with parasites and poor therapeutic response to all known leishmanicidal drugs.3,13–15 Interestingly, the development of a type 1 immune response is not indicative of protection. Although a modulated Th1 response that controls L. braziliensis infection is observed in persons who do not develop disease (sub-clinical L. braziliensis infection),16,17 exaggerated production of interferon-γ (IFN-γ) and tumor-necrosis factor-α (TNF-α) is observed in patients with ACL and patients with mucosal leishmaniasis.11,18 Evidence in humans that tissue damage in leishmaniasis is immune-mediated includes the presence of a local inflammatory infiltrate and high levels of IFN-γ and TNF-α, despite few or no parasites19,20 and accelerated re-epithelization of mucosal and cutaneous lesions with immunomodulators such as granulocyte–monocyte colony-stimulating factor or pentoxifylline in association with antimony.9,21–24
Most persons with ACL have classic ulcers 30–60 days after initial appearance of the lesion. However, through a surveillance program developed in our disease-endemic area, we have been able to identify persons in the early phase of the disease, when they only have lymphadenopathy or non-ulcerated lesions.25,26 We reported previously a series of cases with low cure rates despite early treatment of ACL.27 In the present study, we compared response to therapy in patients with early cutaneous lesion (early ACL) and patients with classic ulcerated lesions (classic ulcerative ACL). We also evaluated clinical and immunologic features associated with treatment failure.
METHODS
Study site and patient selection
This study was a compilation of two prospective cohort studies of patients who came to a leishmaniasis referral center in a disease-endemic area in the southern part of the state of Bahia, Brazil. This clinic treats an average of 800 cases per year and serves a population of 500,000 people living within a 30-km radius.28 Patients 13–60 years of age with two distinct forms of ACL were enrolled into cohort studies during two different times (Figure 1). Patients with classic ulcerative ACL were recruited into an observational study assessing the effects of helminth co-infection on response to antimony treatment during 2004–2005.28 Patients with early ACL, including papules, nodules, or small superficial ulcerations, were recruited during 2005–2006. Patients with classic ulcerative ACL had well-delimited deep ulcers with raised borders. Early forms had a duration of less than 30 days. Classic ulcerative forms had a duration of 15–90 days. Other inclusion and exclusion criteria were the same in the two cohorts. Patients had no previous history of Leishmania spp. infection or antimonial treatment. Criteria for ACL diagnosis were an early or classic ulcerative lesion associated with parasite isolation or a positive Montenegro antigen skin test result (> 5 mm induration at 48–72 hours) and a histopathologic feature of leishmaniasis. The Leishmania antigen used was obtained from a strain of L. braziliensis (IOC L2463, MHOM/BR/2001) as previously reported;29 25 μg of antigen in 0.1 mL of solution was injected into the volar forearm. Patients with evidence of mucosal disease or dissemination (≥ 10 lesions on ≥ 2 body regions involved), women who were pregnant or breast-feeding, and patients with diabetes, infected with human immunodeficiency virus, or with venous insufficiency were excluded.
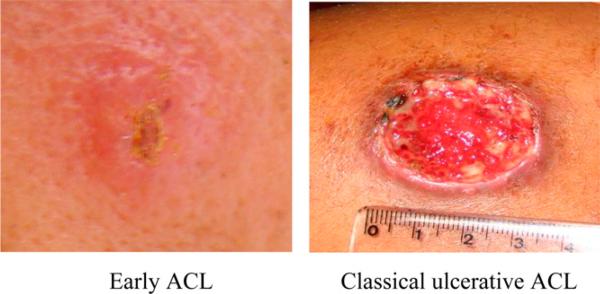
Figure 1.
Non-classic lesion in patients with early American cutaneous leishmaniasis (ACL) and ulcerative lesion in patients with classic ulcerative ACL. This figure appears in color at www.ajtmh.org.
Patient follow-up and laboratory analyses
After diagnosis, all patients provided 30 mL of blood and 3 stool samples, and began treatment with 20 mg/kg/day of intravenous antimony (pentavalent antimony, meglutamine antimony; Sanofi Aventis, Bridgewater, NJ) for 20 days. Patients returned for follow-up at 15–30 day intervals until treatment cure and every 3 months up until one year to evaluate reactivation of the disease or appearance of new lesion. There was no lost in the follow-up until day 90, and all patients were seen after one year of therapy. All lesions were characterized and photographed, and area of lesions and lymph nodes were measured at each visit. The criteria for cure included complete re-epithelization of the lesion on day 90 as confirmed by two experienced clinicians after one course of antimony, and no reactivation or no detection of a new lesion after one-year of follow-up. Patients with helminth co-infection were treated with the appropriate anti-helminth oral regimen 60 days after the initiation of antimony treatment. Parasitologic assay of feces consisted of sedimentation, Baermann method, and Kato-Katz method for all three samples.
Immunologic testing was performed on a convenience sample of 40 patients with classic ulcerative ACL and all 16 patients with early ACL. Levels of Th-1 cytokine IFN-γ and TNF-α in the supernatants of peripheral blood mononuclear cells (PBMCs) were measured by using an enzyme-linked immunosorbent assay after stimulation with L. braziliensis antigens.18 Briefly, PBMCs were obtained by density-gradient centrifugation using a lymphocyte separation medium (Organon Teknika, Durham, NC). Cells were washed in saline and adjusted to a concentration of 3 × 106 cells/mL in RPMI 1640 medium (Gibco-BRL, Gaithersburg, MD) supplemented with 10% AB+ serum that contained 100 U of penicillin/g and 10 μg/mL of streptomycin. Supernatants were stored at –20°C. Results of cytokine assays were expressed in picograms per milliliter on the basis of a standard curve generated by use of recombinant cytokines.
Statistical analysis and ethics
Stata Version 7.0 software package (StataCorp LP, College Station, TX) was used for all analyses. Normally-distributed continuous variables were compared using the unpaired Student t tests and non-parametric continuous variables were compared with the Wilcoxon rank sum test. Fisher's exact test was used to analyze categorical data. The cumulative probability of healing stratified by lesion type was estimated using the Kaplan-Meier method, and the log-rank test was used to compare the curves. Univariate and multivariate Cox proportional-hazard models were used to analyze the association between multiple variables and time to healing. Written informed consent was obtained from all adult patients and from parents or guardians of minors. This study was reviewed and approved by the ethics committee of the Hospital Universitário Professor Edgard Santos, Salvador, Brazil, and by the institutional review board of Weill Cornell Medical College, New York, New York (Institutional Review Board Protocol # 0412007660).
RESULTS
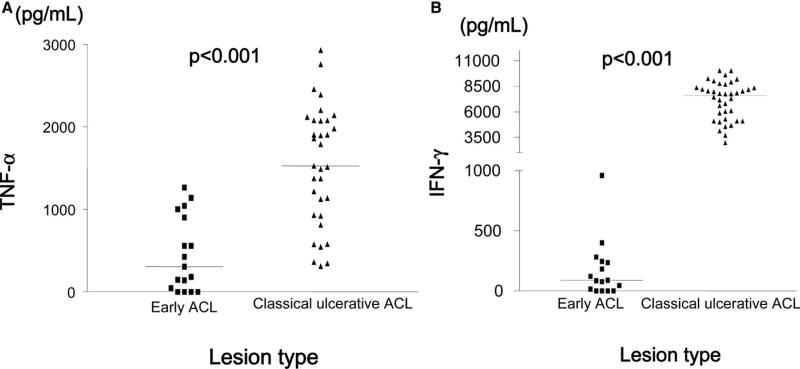
A total of 136 patients were enrolled in the study during 2004–2006. There were 16 patients with early ACL lesions, including papules, nodule, plaques, and superficial ulcerations. Classic ulcerative lesions were present in 120 patients. Most patients in both groups had a single lesion (72.8%) in the lower limbs. However, patients with early ACL had more lesions on the face and on the superior limbs. Specifically, 43.8% of the patients with early ACL had lesions on the lower extremity, 37.9% on the head or neck, 12.5% on the arm, and 6.3% on the hands. There was no association between location of primary lesion and treatment failure (P = 0.78). Helminth co-infection was common (88.2%) and most patients had more than one helminth (73%). There was no difference in participants with early ACL forms and classic ulcerative forms for sex, age, number of lesions, or helminth burden (Table 1). There was a tendency for higher prevalence of Schistosoma mansoni and Strongyloides stercoralis in patients with classic ulcerative lesion but no statistically significant difference was found. Compared with patients with early ACL, patients with classic ulcerative ACL had significantly larger primary lesions (median = 265 mm2 [interquartile range (IQR) = 110–475] versus 30 mm2, IQR = 20–54, P < 0.001) and longer duration (mean ± 37.7 ± 19.9 days versus 21.1 ± 5.9 days; P < 0.001). Ulcerated lesions were also associated with larger intradermal immune response to skin testing (median = 210 mm2, IQR = 132–323 versus 144 mm2, IQR = 99–192, P = 0.01), and higher median levels of IFN-γ (median = 7,579 pg/mL, IQR = 5,098–8,355 versus 86 pg/mL, IQR = 0–236, P < 0.01) and TNF-α (median = 1,526 pg/mL. IQR = 928–2,078 versus 307 pg/mL, IQR = 46–901, P < 0.001) in supernatants of lymphocyte cultures (Figure 2). Healthy persons usually have undetectable levels of IFN-γ levels and undetectable levels of TNF-α or levels less than 50 pg/mL.
Table 1.
Demographic and clinical characteristics of study participants with American cutaneous leishmaniasis (ACL)
| Characteristics | Early ACL (n = 16) | Classic ulcerative ACL (n = 120) | P * |
|---|---|---|---|
| Demographics | |||
| No. (%) female | 3 (18.8) | 43 (35.8) | 0.26 |
| Age, years, mean (SD) | 27.7 (12.0) | 29.3 (12.3) | 0.69 |
| Clinical data | |||
| Lesion duration, days , mean (SD) | 21.1 (5.9) | 37.7 (19.9) | < 0.001 |
| Leishmania skin test area, mm2, median (interquartile range) | 144 (99–192) | 210 (132–323) | 0.001 |
| No. (%) with 1 lesion | 11 (68.8) | 88 (73.3) | 0.71 |
| Lesion area, median mm2 (interquartile range) | 30 (20–54) | 265 (110–475) | < 0.001 |
| Helminth co-infection, no. (%) | 14 (87.5) | 106 (88.2) | 1.00 |
| Ancylostosoma duodenale, no. (%) | 13 (81.3) | 97 (80.9) | 1.00 |
| Ascaris lumbricoides, no. (%) | 10 (62.5) | 58 (48.3) | 0.43 |
| Schistosoma mansoni, no. (%) | 1 (6.3) | 20 (16.7) | 0.47 |
| Strongyloides stercoralis, no. (%) | 0 | 12 (10.0) | 0.36 |
| Cytokine levels | |||
| Interferon-γ,† pg/mL, median (interquartile range) | 86 (0–236) | 7,579 (5,098–8,355) | < 0.001 |
| Tumor necrosis factor-α,† pg/mL, median (interquartile range) | 307 (46–901) | 1,526 (928–2,078) | < 0.001 |
| Clinical outcome | |||
| No. (%) requiring second course of antimony treatment | 12 (75.0) | 31 (25.8) | < 0.001 |
Normally distributed and non-normally distributed continuous variables were compared using unpaired t tests and Wilcoxon rank sum tests, respectively. Categorical variables were compared using Fisher's exact test.
Cytokine data were based on a convenience sample of 40 persons with ulcerated lesions and all 16 persons with early non-ulcerated lesions.
Figure 2.
Pre-treatment levels of A, tumor necrosis factor-α and B, interferon-γ in supernatants of peripheral blood mononuclear cells stimulated with Leishmania braziliensis antigens, in patients with early non-classic American cutaneous leishmaniasis (ACL) and classic ulcerative ACL. Horizontal lines denote medians. Wilcoxon rank sum test was used for analysis.
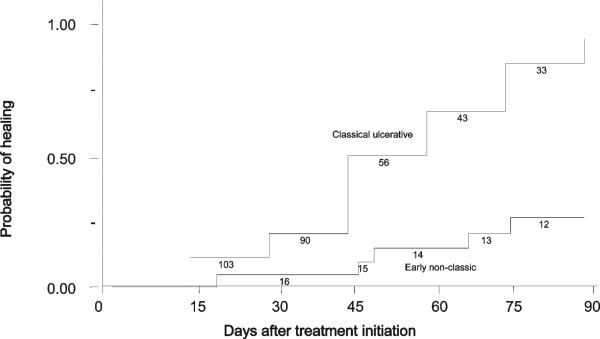
Classic ulcerative ACL was associated with lower treatment failure rates at the 90-day study endpoint compared with early ACL (25.8% versus 75.0%, respectively; P < 0.001). All early ACL patients who failed to antimony therapy developed ulcerative lesions. The Kaplan-Meier analysis presented in Figure 3). shows that patients with early ACL took longer to heal than those with classic ulcerative ACL. We used crude and adjusted Cox proportional hazards models to quantify the effect of demographic and clinical variables on lesion healing time (Table 2). In these models, a hazard ratio (HR) > 1 means that the variable was associated with shorter healing time. In the univariate model, lesion ulceration was strongly associated with lesion healing (HR = 4.50, 95% confidence interval [CI] = 1.65–12.30). Intradermal immune response (HR = 1.24, 95% CI = 1.08–1.42) and IFN-γ were weakly associated with lesion healing (HR = 1.25, 95% CI = 1.10–1.42. Helminth co-infection was associated with delayed lesion healing (HR = 0.42, 95% CI = 0.24–0.74). The multivariate Cox proportional hazard model was adjusted for ulceration, LST area, and helminth co-infection. After adjustment, the effect of lesion ulceration was strengthened (HR = 5.33, 95% CI = 1.67–16.99). Intradermal immune response was again associated with lesion healing (HR = 1.16, 95% CI = 1.14–1.33), and helminth co-infection remained associated with prolonged healing (HR = 0.43, 95% CI = 0.24–0.77).
Figure 3.
Lesion healing in patients with classic ulcerative American cutaneous leishmaniasis (ACL) (n = 120) and early ACL (n = 16), P < 0.01 by log rank test. Number of patients still at risk is indicated underneath the curve.
Table 2.
Association of risk factors with time to lesion healing in patients with American cutaneous leishmaniasis treated with antimony*
| Risk factor | Crude hazard ratio (95% CI) | P | Multivariate hazard ratio† (95% CI) | P |
|---|---|---|---|---|
| Demographics | ||||
| Female | 0.84 (0.55–1.28) | 0.43 | – | – |
| Age per 10 years | 1.04 (0.86–1.20) | 0.87 | – | – |
| Clinical data | ||||
| Classic ulceration | 4.50 (1.65–12.30) | < 0.001 | 5.33 (1.67–16.99) | 0.005 |
| LST area per 100 mm2 | 1.24 (1.08–1.42) | 0.003 | 1.16 (1.14–1.33) | 0.03 |
| One lesion only | 1.11 (0.82–1.51) | 0.51 | – | – |
| Lesion area per 100 mm2 | 1.00 (0.95–1.05) | 0.99 | – | – |
| Helminth co-infection | 0.42 (0.24–0.74) | 0.006 | 0.43 (0.24–0.77) | 0.004 |
| Cytokines | ||||
| IFN-γ per 1,000 pg/mL‡ | 1.25 (1.10–1.42) | < 0.001 | ||
| TNF-α per 1,000 pg/mL‡ | 1.86 (1.16–2.97) | 0.01 |
CI = confidence interval; LST = Leishmania skin test; IFN-γ = interferon-γ; TNF-α = tumor necrosis factor-α.
Multivariate Cox proportional hazard models adjusted for ulceration, LST area, and helminth co-infection.
n = 46 persons with cytokine data.
DISCUSSION
The natural history of ACL is well-documented. After initial bite by the sandfly, a small papule or nodule appears within a few weeks and develops into the classic ulcer over 2–3 weeks.30–32 However, patients only look for medical care after development of the classic ulcerative lesion. Recent attention has been given to detecting and treating ACL in early forms to limit the risk of disfiguring ulcers or the development of mucosal or disseminated forms.25,26 It is known that large ulcers, multiple cutaneous lesions and failure with antimony therapy are risk factors for development of mucosal disease.33,34 Early evidence in one case series suggested high rates of treatment failure despite early treatment of ACL,27 and in a recent study in Peru, failure in therapeutic response was associated with lesions treated with a duration of less than five weeks.35 Previously, therapeutic failure or delayed healing had been associated with age, increased duration of disease, presence of multiple lesions, large lesions, parasite species, and helminth infections.28,36–38 In this study, we confirm that treatment of early ACL does not prevent lesion ulceration and is associated with higher rates of treatment failure than classic ulcerative lesions of longer duration.
Our study is the first to compare patients with early ACL with those who have classic ulcerative ACL. The paradigm for treating most infectious diseases is that early diagnosis and treatment generally results in a higher cure rate. This finding is of utmost importance in ACL caused by L. braziliensis where long-term ulceration is a risk factor associated with development of mucosal disease.33,34 We showed that use of antimony in patients with early ACL was associated with a high failure rate. This finding could not be explained by the presence of more risk factors for treatment failure in patients with early ACL. In fact, these patients had a shorter duration of illness and smaller lesion sizes compared with patients with classic ACL. Moreover, the number of lesions and frequency of helminths were similar in both groups.
Studies of patients with early ACL are difficult to perform. Most patients present only after recognition of the classic ulceration. The nature of this disease and its disease-endemic area make early diagnosis difficult. Duration of the lesion is also subject to substantial recall bias. We believe that lesion characteristics in this study were a better marker for duration of disease than the duration reported by participants. Regional lymphadenopathy, degree of ulceration, or local immune response may be better markers of lesion duration.26,27,39
It is known that immune response plays a pivotal role in the pathogenesis of ACL. Interferon-γ produced by T cells activates macrophages leading to killing of Leishmania spp. However, high levels of IFN-γ and TNF-α are found in supernates of PBMCs and at the lesion site in patients with classic ACL and mucosal leishmaniasis, and cure of infection is associated with decreasing in cytokine level.12,18,40 Additionally, there is a correlation between type 1 immune response and lesion size39 and a correlation between numbers of cells expressing TNF-α and the intensity of the inflammation.20 The documentation here that patients with early ACL had lower levels of TNF-α and IFN-γ and had a worse prognosis indicate that a poor type 1 immune response as observed in the early phase of CL is also harmful because it may contribute to parasite persistence and non-healing of the lesion. Subsequently, a strong cellular immune response develops in these patients that is associated with ulcer development. This model is supported by the observation that interleukin-10 is produced in high amounts in patients with early ACL41 and plays an important role in parasite persistence after infection by down modulate IFN-γ production.42
The documentation that in early phase of ACL antimony alone is not effective indicates that alternative drugs should be used for treatment of leishmaniasis patients previous to ulcer development. Unfortunately, we have few options because miltefosine and aminosidine are not commercially available in Brazil. Amphotericin B is the second-line drug and is quite effective in patients with ACL and in patients with mucosal leishmaniasis who fail to antimony therapy, but the side effects and the need for hospitalization limit its use in patients that have an initial lesion, when patients do not recognize that he or she has leishmaniasis. Pentamidine has been successful used in the treatment of patients with cutaneous leishmaniasis caused by L. guyanensis in northern Brazil,43 and we have shown that pentamidine is effective in treating patients infected with L. braziliensis.44 Therefore, a good option would be an association between pentamidine and antimonial compounds for treating these patients.
Acknowledgments
We thank Elbe Silva for secretarial assistance in the preparation of the manuscript.
Financial support: This study was supported by the National Institutes of Health (NIH) grant T32 AI-07613, NIH/Fogarty International Center grant D43 TW007127, a Fogarty/Ellison fellowship to Alon Unger, and Fundação de Amparo à Pesquisa do Estado da Bahia.
Contributor Information
Alon Unger, Departments of Medicine and Pediatrics, University of California, 10833 Le Conte Avenue, 12-335 MDCC, Mailcode 175217, Los Angeles, CA 90095, AUnger@mednet.ucla.edu..
Seth O'Neal, Department of Public Health and Preventive Medicine, Oregon Health and Sciences University, 3181 SW Sam Jackson Park Road, CCB 669, Portland OR 97239, oneals@ohsu.edu..
Paulo R. L. Machado, Serviço de Imunologia, 5° Andar, Hospital Universitário Professor Edgard Santos, Rua João das Botas, s/n 40110160 Canela, Salvador, Bahia, Brazil, prlmachado@uol.com.br.
Luiz H. Guimarães, Serviço de Imunologia, 5° Andar, Hospital Universitário Professor Edgard Santos, Rua João das Botas, s/n 40110160 Canela, Salvador, Bahia, Brazil.
Daniel J. Morgan, Department of Epidemiology and Preventive Medicine, University of Maryland School of Medicine, 100 North Greene Street, Lower Level, Baltimore, MD 21201, danieljosiahmorgan@yahoo.com.
Albert Schriefer, Serviço de Imunologia, 5° Andar, Hospital Universitário Professor Edgard Santos, Rua João das Botas, s/n 40110160 Canela, Salvador, Bahia, Brazil, aschriefer@globo.com..
Olívia Bacellar, Serviço de Imunologia, 5° Andar, Hospital Universitário Professor Edgard Santos, Rua João das Botas, s/n 40110160 Canela, Salvador, Bahia, Brazil, olivinha@ufba.br..
Marshall J. Glesby, Department of Medicine, Weill Cornell Medical College 525 East 68th Street, Box 566, New York, NY 10021, mag2005@med.cornell.edu.
Edgar M. Carvalho, Serviço de Imunologia, 5° Andar, Hospital Universitário Professor Edgard Santos, Rua João das Botas, s/n 40110160 Canela, Salvador, Bahia, Brazil..
REFERENCES
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Jones TC, Johnson WD, Jr, Barretto AC, Lago E, Badaro R, Cerf B, Reed SG, Netto EM, Tada MS, Franca TF, Wiese K, Golightly L, Fikrig E, Costa JM, Cuba CC, Marsden PD. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987;156:73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- 3.Barral-Netto M, Machado P, Bittencourt A, Barral A. Recent advances in the pathophysiology and treatment of human cutaneous leishmaniasis. Curr Opin Dermatol. 1997;4:51–58. [Google Scholar]
- 4.Llanos Cuentas EA, Cuba CC, Barreto AC, Marsden PD. Clinical characteristics of human Leishmania braziliensis braziliensis infections. Trans R Soc Trop Med Hyg. 1984;78:845–846. doi: 10.1016/0035-9203(84)90043-9. [DOI] [PubMed] [Google Scholar]
- 5.Bittencourt AL, Costa JM, Carvalho EM, Barral A. Leishmaniasis recidiva cutis in American cutaneous leishmaniasis. Int J Dermatol. 1993;32:802–805. doi: 10.1111/j.1365-4362.1993.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–325. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 7.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 8.Weina P, Neafie R, Wortmann G, Polheumus M, Aronson N. Old world leishmaniasis: an emerging infection among deployed US military and civilian workers. Clin Infect Dis. 2004;39:1674–1680. doi: 10.1086/425747. [DOI] [PubMed] [Google Scholar]
- 9.Almeida R, d'Oliveira A, Jr, Machado P, Bacellar O, Ko A, de Jesus A, Mobashery N, Santos JB, Carvalho EM. Randomized, double-blind study of stibogluconate plus human granulocyte macrophage colony-stimulating factor versus stibogluconate alone in the treatment of cutaneous Leishmaniasis. J Infect Dis. 1999;180:1735–1737. doi: 10.1086/315082. [DOI] [PubMed] [Google Scholar]
- 10.Romero GA, Guerra MV, Paes MG, Macedo VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985;135:4144–4148. [PubMed] [Google Scholar]
- 12.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 13.Bittencourt A, Barral A, de Jesus A, de Almeida R, Grimaldi Junior G. In situ identification of Leishmania amazonensis associated with diffuse cutaneous leishmaniasis in Bahia, Brazil. Mem Inst Oswaldo Cruz. 1989;84:585–586. doi: 10.1590/s0074-02761989000400022. [DOI] [PubMed] [Google Scholar]
- 14.Barral A, Costa JM, Bittencourt AL, Barral-Netto M, Carvalho EM. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Int J Dermatol. 1995;34:474–479. doi: 10.1111/j.1365-4362.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 15.Bomfim G, Nascimento C, Costa J, Carvalho EM, Barral-Netto M, Barral A. Variation of cytokine patterns related to therapeutic response in diffuse cutaneous leishmaniasis. Exp Parasitol. 1996;84:188–194. doi: 10.1006/expr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 16.Follador I, Araujo C, Bacellar O, Araujo CB, Carvalho LP, Almeida RP, Carvalho EM. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin Infect Dis. 2002;34:E54–E58. doi: 10.1086/340261. [DOI] [PubMed] [Google Scholar]
- 17.Castes M, Trujillo D, Rojas M, Fernandez CT, Araya L, Cabrera M, Blackwell J, Convit J. Serum levels of tumor necrosis factor in patients with American cutaneous leishmaniasis. Biol Res. 1993;26:233–238. [PubMed] [Google Scholar]
- 18.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado P, Kanitakis J, Almeida R, Chalou A, Araujo C, Carvalho E. Evidence of in situ cytotoxicity in American cutaneous leishmaniasis. Eur J Dermatol. 2002;12:449–451. [PubMed] [Google Scholar]
- 20.Faria D, Gollob K, Barbosa JJ, Schriefer A, Machado PR, Lessa H, Carvalho LP, Romano-Silva MA, de Jesus AR, Carvalho EM, Dutra WO. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and ctyotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado PR, Lessa H, Lessa M, Guimarães LH, Bang H, Ho JL, Carvalho EM. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 22.Lessa HA, Machado P, Lima F, Cruz AA, Bacellar O, Guerreiro J, Carvalho EM. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65:87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 23.Sadeghian G, Nilforoushzadeh M. Effect of combination therapy with systemic glucantime and pentoxifylline in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2006;45:819–821. doi: 10.1111/j.1365-4632.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- 24.Báfica A, Oliveira F, Freitas L, Nascimento E, Barral A. American cutaneous leishmaniasis unresponsive to antimonial drugs: successful treatment using combination of N-methylglucamine antimoniate plus pentoxifylline. Int J Dermatol. 2003;42:203–207. doi: 10.1046/j.1365-4362.2003.01868.x. [DOI] [PubMed] [Google Scholar]
- 25.Barral A, Barral-Netto M, Almeida R, de Jesus AR, Grimaldi G, Jr, Netto EM, Santos I, Bacellar O, Carvalho EM. Lymphadenopathy associated with Leishmania braziliensis cutaneous infection. Am J Trop Med Hyg. 1992;47:587–592. doi: 10.4269/ajtmh.1992.47.587. [DOI] [PubMed] [Google Scholar]
- 26.Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am J Trop Med Hyg. 1995;53:256–259. doi: 10.4269/ajtmh.1995.53.256. [DOI] [PubMed] [Google Scholar]
- 27.Machado P, Araujo C, Da Silva AT, Almeida RP, d'Oliveira A, Jr, Bittencourt A, Carvalho EM. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clin Infect Dis. 2002;34:E69–E73. doi: 10.1086/340526. [DOI] [PubMed] [Google Scholar]
- 28.O'Neal SE, Guimaraes LH, Machado PR, Alcântara L, Morgan DJ, Passos S, Glesby MJ, Carvalho EM. Influence of helminth infections on the clinical course of and immune response to Leishmania braziliensis cutaneous leishmaniasis. J Infect Dis. 2007;195:142–148. doi: 10.1086/509808. [DOI] [PubMed] [Google Scholar]
- 29.Reed SG, Badaro R, Masur H, Carvalho EM, Lorenco R, Lisboa A, Teixeira R, Johnson WD, Jr, Jones TC. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 30.Herwaldt B, Arana B, Navin T. The natural history of cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992;165:518–527. doi: 10.1093/infdis/165.3.518. [DOI] [PubMed] [Google Scholar]
- 31.Weigle K, Saravia N. Natural history, clinical evolution, and the host-parasite interaction in New World cutaneous leishmaniasis. Clin Dermatol. 1996;14:433–450. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez C. Natural history of cutaneous and mucocutaneous leishmaniasis. Biomedica (Bogota) 2006;26:10–12. [PubMed] [Google Scholar]
- 33.Llanos-Cuentas EA, Marsden PD, Cuba CC, Barreto AC, Campos M. Possible risk factors in development of mucosal lesions in leishmaniasis. Lancet. 1984;2:295. doi: 10.1016/s0140-6736(84)90346-5. [DOI] [PubMed] [Google Scholar]
- 34.Machado-Coelho G, Caiatta W, Genaro O, Magalhaes P, Mayrink W. Risk factors for mucosal manifestations of ACL. Trans R Soc Trop Med Hyg. 2005;99:59–61. doi: 10.1016/j.trstmh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, Ramirez L, Lazo M, De Doncker S, Boelaert M, Robays J, Dujardin JC, Arevalo J, Chappuis F. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46:223–231. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 36.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. Influence of Leishmania (Viannia11) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues M, Hueb M, Santos T, Fontes CJ. Factors associated with treatment failure of cutaneous leishmaniasis with meglumine antimoniate. Rev Soc Bras Med Trop. 2006;39:139–145. doi: 10.1590/s0037-86822006000200001. [DOI] [PubMed] [Google Scholar]
- 38.Palacios R, Osorio L, Grajalew L, Ochoa M. Treatment failure in children in a randomized clinical trial with 10 and 20 days of meglumine antimonate for cutaneous leishmaniasis due to Leishmania viannia species. Am J Trop Med Hyg. 2001;64:187–193. doi: 10.4269/ajtmh.2001.64.187. [DOI] [PubMed] [Google Scholar]
- 39.Antonelli L, Dutra W, Almeida R, Bacellar O, Carvalho E, Gollob K. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Da-Cruz A, Bittar R, Mattos M, Oliveira-Neto MP, Nogueira R, Pinho-Ribeiro V, Azeredo-Coutinho RB, Coutinho SG. T-cell mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol. 2002;9:251–256. doi: 10.1128/CDLI.9.2.251-256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salhi A, Rodrigues V, Jr, Santoro F, Dessein H, Romano A, Castellano LR, Sertorio M, Rafati S, Chevillard C, Prata A, Alcaïs A, Argiro L, Dessein A. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 42.Rocha PN, Almeida RP, Bacellar O, Ribeiro de Jesus A, Correia Filho D, Cruz Filho A, Barral A, Coffman RL, Carvalho EM. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J Infect Dis. 1999;180:1731–1734. doi: 10.1086/315071. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira Guerra JA, Talhari S, Paes MG, Garrido M, Talhari JM. Clinical and diagnostic aspects of American tegumentary leishmaniasis in soldiers simultaneously exposed to the infection in the Amazon region. Rev Soc Bras Med Trop. 2003;36:587–590. [PubMed] [Google Scholar]
- 44.Correia D, Macedo VO, Carvalho EM, Barral A, Magalhães AV, Abreu MVA, Orge Orge MG, Marsden P. Estudo comparativo entre antimoniato de meglumina, isotianato de pentamidina e sulfato de aminosidine, no tratamento de lesões cutâneas primárias causadas por Leishmania (viannia) braziliensis. Rev Soc Bras Med Trop. 1996;29:447–453. doi: 10.1590/s0037-86821996000500007. [DOI] [PubMed] [Google Scholar]