Abstract
Fifty-three patients with resected pancreatic adenocarcinoma were studied to determine if adjuvant chemo-radiation causes severe lymphopenia and if this is associated with adverse outcomes. Total lymphocyte counts (TLC) were normal in 91% before adjuvant chemo-radiation. Two months later, TLC fell by 63% (p <.0001) with 45% of patients having TLC <500 cells/mm3. Median survival in patients with low TLC was 14 versus 20 months (p = .048). Multivariate analysis revealed a significant association between treatment related lymphopenia and survival (HR 2.2, p = .014). Adjuvant chemo-radiation induced lymphopenia is frequent, severe, and an independent predictor for survival in patients with resected pancreatic adenocarcinoma.
Keywords: Lymphopenia, Pancreatic cancer, Immunosuppression, Chemotherapy, Radiation
INTRODUCTION
Over a century ago, Paul Erlich noted the potential role of the immune system in cancer suppression (1). Subsequent studies demonstrated that tumor-infiltrating lymphocytes found within cancers is a positive prognostic sign and that immune surveillance can eliminate preclinical cancers (2–4). These observations spawned extensive research to stimulate the immune system in an effort to improve the outcome of patients with cancer. To date, immunotherapy approaches have been associated with modest success and a full understanding of the relationship between the immune system and regulation of malignancies remains an area of active research (5).
Recent evidence has highlighted the association between treatment-related lymphopenia and shortened survival in patients with newly diagnosed glioblastomas (6). Similar to patients with high-grade gliomas, patients with resectable pancreatic adenocarcinoma usually receive postoperative radiation and chemotherapy and have a poor prognosis (5, 7–9). Although prior reports suggest that lymphopenia and an elevated neutrophil to lymphocyte ratio at diagnosis are predictive of survival, the development and consequences of lymphopenia that occurs as a result of postoperative radiation and chemotherapy has not been studied (10, 11). As a result, we performed this retrospective study in patients with newly diagnosed, resected pancreatic adenocarcinoma who received postoperative radiation and chemotherapy. The purpose of this study was to document the frequency of severe treatment-related lymphopenia in this setting and to determine if this was associated with changes in overall survival.
PATIENTS AND METHODS
Using the Pancreatic Tumor Registry database at Johns Hopkins Hospital, we retrospectively identified all patients with newly diagnosed and resected pancreatic adenocarcinoma who: (1) had their primary surgery at our institution between 1997 and 2008, (2) were ≥18 years of age, (3) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, (4) received postoperative adjuvant radiation with concomitant chemotherapy at our institution, and (5) had their baseline and follow-up laboratory values done at John Hopkins so they were accessible in the Electronic Patient Record (EPR). Patients were excluded if they had evidence of distant metastatic disease or had received any prior therapy for their pancreatic adenocarcinoma. This study was reviewed and approved by the Institutional Review Board of the Johns Hopkins University.
Information relating to known prognostic factors in pancreatic cancer was obtained from the records of each patient (12, 13). Baseline values collected postoperatively but prior to initiation of radiation and chemotherapy included: CA 19-9, BUN, albumin, AST, alkaline phosphatase, complete blood count (CBC), and ECOG performance status (12, 13). Surgical procedures were categorized as a pylorus preserving (classic) pancreaticoduodenectomy, total pancreatectomy, or distal pancreatectomy. Information was recorded on tumor location, size, grade, nodal status, and resection margins. Margins were considered positive if the carcinoma was within 1 mm of the margin or present at the final pancreatic neck, uncinate process, bile duct, or duodenal or retroperitoneal soft tissue. In addition, the dose of radiation and whether the chemotherapy regimen was 5FU or gemcitabine based were collected. CBCs’ were routinely performed monthly after radiation and chemotherapy were initiated and these values were also recorded. Baseline total lymphocyte counts (TLC) before beginning radiation and chemotherapy were classified as normal (≥1,000 cells/mm3) or abnormal. Version 3.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) was used to classify the severity of treatment-related lymphopenia. The TLC 2 months after the initiation of radiation and chemotherapy were dichotomized to grade 0–II versus grade III–IV for the relevant analyses. For patients with missing lymphocyte counts at 2 months, the TLC at 1 month was used. Overall survival was measured from the date of initial surgery to the date of death due to any cause. Survival was censored if the subject was alive at the time of last follow-up.
Patient baseline characteristics were summarized using descriptive statistics. Chi-square test statistics were used for proportional comparison. Student’s t-test and paired t-test were used for continuous data between and within group comparison, respectively. Survival probability was estimated using the Kaplan–Meier method (14). The confidence interval of median time survival was constructed by the method of Brookmeyer–Crowley (15). Univariate analysis was used to assess an association between the known prognostic factors of patients at baseline and overall survival. Important patient characteristics associated with survival were identified in the univariate analysis using a value of p value ≤.3. These characteristics and baseline lymphocyte counts were selected as covariates to construct the multivariate proportional hazards regression model. The proportional hazards regression model was used to estimate the hazard ratio (HR) for death attributable to prognostic factors. All p values are reported as two-sided and all analyses were conducted using the SAS software (version 9.1, SAS Institute).
RESULTS
A total of 77 patients met the inclusion criteria outlined above. However, 24 of these patients received chemotherapy before radiation was initiated. These patients were excluded from the analysis. As a result, this report focuses on 53 patients who received concurrent radiation and chemotherapy as their initial nonsurgical therapy for resected pancreatic cancer. Baseline information on these patients is outlined in Table 1. The median age of the patients was 64 years and 43% of the patients were over the age of 65. Fifty-five percent were male, 85% were Caucasian, and 62% had an ECOG performance status of zero. The tumor was located in the pancreatic head in 81% of patients, the median size of the tumors was 3.0 cm, and 83% of the patients underwent a pancreaticoduodenectomy. Poorly differentiated adenocarcinoma was noted in 43% of the patients, the surgical margins were positive in 47%, and 92% had positive lymph nodes. The baseline laboratory data revealed that 91% of patients had a normal lymphocyte count. The median postoperative CA19-9 was 41. The mean administered dose of radiation was 50.4 Gy in 1.8 Gy fractions. The initial 45 Gy of radiation encompassed the tumor bed, regional lymph nodes, and anastomoses and was followed by a 5.4 Gy boost to the tumor bed plus a 1.5–2 cm margin. Concomitant with this radiation, 59% of patients were treated with a 5FU based chemotherapy regimen while the remaining 41% received a gemcitabine-based regimen.
Table 1.
Baseline Characteristics of Patients with Lymphocyte Counts Above and Below 500 at 2 Months
| All Patients (N = 53) | Patients with Lymphocyte Counts 500 at 2 Months (N = 29) | Patients with Lymphocyte Counts < 500 at 2 Months (N = 24) | p value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age: median (range) | 64 (47–84) | 63 (47–81) | 62 (47–84) | .86 |
| Age 65 years: # (%) | 23 (43) | 13 (45) | 10 (42) | .82 |
| Male: # (%) | 29 (55) | 19 (66) | 10 (42) | .08 |
| Caucasian: # (%) | 45 (85) | 26 (90) | 19 (79) | .29 |
| ECOG performance score = 0: # (%) | 33 (62) | 19 (66) | 14 (58) | .59 |
| Baseline laboratory data | ||||
| Total lymphocyte count: median (range) | 1945 (580–4200) | 2030 (580–3280) | 1599 (870–4200) | .57 |
| Lymphocyte ≥ 1,000: # (%) | 48 (91) | 26 (90) | 22 (92) | .80 |
| AST: median (range) | 28 (12–70) | 28 (12–53) | 28 (12–70) | .42 |
| BUN: median (range) | 12 (5–34) | 14 (5–34) | 12 (5–27) | .31 |
| CA19-9: median (range) | 41 (0.1–400) | 29 (0.1–440) | 60 (4.9–203) | .39 |
| Albumin: median (range) | 4 (2.8–5.1) | 4.1 (2.8–4.7) | 3.9 (3–5.1) | .56 |
| Alk phos: median (range) | 113 (54–493) | 103 (54–493) | 129 (59–492) | .18 |
| Tumor staging data | ||||
| Tumor location: No. head (%) | 43 (81) | 22 (76) | 21 (88) | .54 |
| Tumor size: median centimeters (range) | 3 (1.4–9.5) | 3.5 (1.4–9.5) | 3 (1.5–6) | .69 |
| Poorly differentiated pathology: #(%) | 23 (43) | 11 (40) | 12 (50) | .38 |
| Positive margins: # (%) | 25 (47) | 13 (45) | 12 (50) | .71 |
| Positive nodes: # (%) | 48 (92) | 27 (93) | 21 (91) | .81 |
| Whipple procedure: # (%) | 44 (83) | 23 (79) | 21 (88) | 0.43 |
| Postoperative treatment data | ||||
| Radiation dose: median cGy (range) | 5040 (2700–5760) | 5040 (2750–5580) | 5000 (2700–5760) | .25 |
| 5-fU based chemotherapy: # % | 31 (59) | 16 (55) | 15 (63) | .59 |
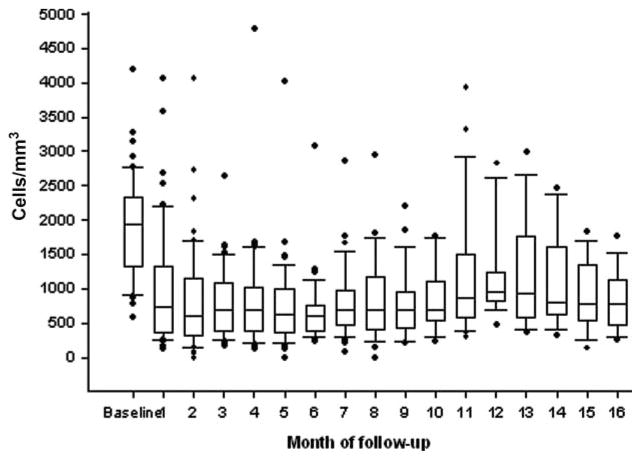
Three patients had missing lymphocyte counts at 2 months and in these patients the TLC at 1 month were used. Two months after initiating radiation and chemotherapy 24 patients (45%) had a TLC below 500 cells/mm3 while 29 (55%) had had counts above 500 cells/mm3. The median reduction in TLC in all patients was 63% (p < .0001). As shown in Figure 1, TLC remained low for many months thereafter. As noted in Table 1, there was no significant difference in the baseline demographic, surgical, pathology, laboratory, or adjuvant treatment data in patients whose TLC remained above 500 cells/mm3 or fell significantly.
Figure 1.
Total lymphocyte counts (TLC) over time.
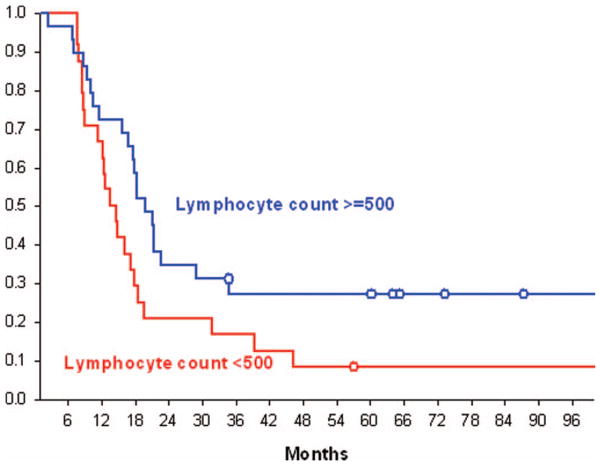
The overall median survival time for the 53 patients was 17.7 months (95%CI = 12.6–19.7). The median survival for patients whose lymphocyte count was <500 cells/mm3 at 2 months was 14 months (95%CI = 11.3–17.8) compared to 20 months (95%CI= 16.8–28.9) for patients who had higher lymphocyte counts (p = .0485, log-rank test). Kaplan–Meier survival curves for these two populations are presented in Figure 2. The cause of death in this patient population was uniformly due to progressive cancer. No major opportunistic infections were noted.
Figure 2.
Kaplan–Meier survival curves for patients with total lymphocyte counts (TLC) above and below 500 cells/mm3.
Table 2 presents univariate and multivariate associations between patient characteristics and survival in these 53 patients. No significant univariate associations with survival were seen within patient demographics. The only laboratory category associated with inferior survival was a lower lymphocyte count at 2 months (HR 1.82). Treatment with different chemotherapy regimens was also not significantly associated with different lymphocyte counts or survival outcomes. The multivariate analysis demonstrated significant associations between survival and the TLC at 2 months (HR 2.2; 95%CI = 1.17–4.12, p = .014) after taking into account baseline nodal status (HR = 3.79; 95%CI = 0.86–16.67, p = .08) and baseline lymph count normality (HR = 3.06; 95%CI = 1–9.41, p = .05).
Table 2.
Associations Between Patient Characteristics and Survival
| Characteristic | Hazard Ratio (95% CI) | p value |
|---|---|---|
| Univariable association | ||
| Age: < 65 vs. > 65 | 1.18 (0.65–2.18) | .58 |
| ECOG: 1 vs. 0 | 1.67 (0.91–3.07) | .1 |
| Tumor location: head vs. other | 1.4 (0.65–3.04) | .39 |
| Tumor resection: whipple vs. distal and total | 1.24 (0.52–2.93) | .63 |
| Gender: female vs. male | 1.58 (0.87–2.9) | .14 |
| Pathology: poorly differentiated vs. other | 1.62 (0.88–2.93) | .13 |
| Margin: negative vs. positive | 1.12 (0.62–2.05) | .7 |
| Staging: node positive vs. negative | 2.28 (0.55–9.45) | .26 |
| Tumor size/per cm increase | 1.2 (0.99–1.45) | .06 |
| Lymph count at 2 months: < 500 vs. 500** | 1.82 (0.995–3.3) | .05 |
| Baseline lymphocyte count* < 1,000 vs. ≥ 1,000 | 1.58 (0.56–4.41) | .39 |
| Baseline AST | 1.002 (0.99–1.03) | .86 |
| Baseline BUN | 1.007 (0.95–1.07) | .84 |
| Baseline CA19-9 | 1.002 (0.999–1.01) | .17 |
| Baseline albumin | 0.86 (0.44–1.68) | .67 |
| Baseline alkaline phosphatase | 1.001 (0.999–1.0) | .35 |
| Chemotherapy: 5-fU vs. gemcitabine based | 0.84 (0.46–1.55) | .58 |
| Multivariable association | ||
| Lymph count at 2 months: < 500 vs. ≥ 500 | 2.20 (1.17–4.12) | .01 |
| Baseline lymph count: < 1,000 vs. ≥ 1,000 | 3.06 (1–9.14) | .05 |
| Staging: node positive vs. negative | 3.79 (0.86–16.67) | .08 |
Pretreatment lymphocyte count is dichotomized at 1,000 (abnormal vs. normal).
Lymphocyte count at 2 months is dichotomized at 500 (per the CTC NIH grade 3–4 treatment induced lymphopenia).
DISCUSSION
This retrospective analysis demonstrates that TLC were remarkably normal in patients with resected pancreatic adenocarcinoma prior to the initiation of postoperative radiation and chemotherapy. However, two months later their median lymphocyte counts were reduced by 63% and 45% of patients had developed TLC of less than 500 cells/mm3. Furthermore, those patients who developed grade III–IV lymphopenia (<500 cells/mm3) 2 months after starting postoperative combined radiation and chemotherapy had shorter survival times than those with higher TLC (14 vs. 20 months, p = .0485).
Several studies had previously shown that preoperative lymphocyte counts are associated with poorer prognosis in patients with pancreatic adenocarcinoma (10, 16). The median survival time for patients with lymphocyte counts above 1500 cells/mm3 was 14.3 months as compared to 8.8 months for those with lower lymphocyte counts. However, these studies do not address the survival impact of lymphopenia that occurs as a consequence of postoperative chemotherapy and radiation. In the study reported in this manuscript, the preoperative lymphocyte count was not of prognostic value as virtually all patients had normal baseline lymphocyte counts. The greatest degree of lymphopenia was observed two months after beginning chemotherapy and radiation therapy and was clearly related to the administration of these antineoplastic therapies.
The design of the study reported in this manuscript was based on recent findings in patients with newly diagnosed high-grade gliomas who received standard radiation and chemotherapy (6). Forty percent of these patients developed severe reductions in total lymphocyte and CD4 counts 2 months following initiation of therapy. Multivariate analysis demonstrated that this lymphopenia was independently associated with a significantly shorter overall survival (median 13.1 vs. 19.7 months, p = .002). Only 2% of deaths were related to infection while the remainders were secondary to tumor progression. This observation might be expected in patients with brain tumors who receive high doses of glucocorticoids, radiation, and temozolomide as each of these treatment modalities are known to be toxic to lymphocytes (17, 18). The current study was conducted to determine if similar treatment-related lymphopenia occurs in other solid tumors and if there is a relationship between posttreatment lymphocyte counts and survival.
We chose to study this in patients with resected pancreatic cancer as a result of similarities to the brain tumor population. In both settings, surgery infrequently results in cure and postoperative concurrent radiation and chemotherapy are commonly administered (19–23). Our results demonstrate that the frequency and severity of treatment-related lymphopenia in patients with pancreatic cancer and newly diagnosed high-grade gliomas are comparable. Forty-five percent of patients with resected pancreatic cancer who recovered sufficiently to receive aggressive adjuvant postoperative therapy develop grade III–IV lymphopenia 2 months after initiating radiation and chemotherapy. Furthermore, as in the high-grade glioma study, multivariate analysis reveals that this severe lymphopenia is independently associated with early death from progressive cancer.
These findings suggest that treatment-induced lymphopenia in patients with brain tumors was not specifically related to glucocorticoids or temozolomide as these were not used in the pancreatic cancer population. Radiation without chemotherapy has been reported to cause substantial and long-lasting lymphopenia in patients with malignancies or accidental exposures (24–33). This suggests that radiation may play a significant role in the development of treatment-related lymphopenia in patients with high-grade gliomas and pancreatic cancer. This is plausible given the extreme sensitivity of lymphoctyes to radiation and the lymphopenia that has been well documented following radiation administered to the brain or other tissues devoid of lymphoid and marrow, or even when radiation is directed at blood circulating through a renal dialysis machine (23, 31, 33).
Given that treatment-related lymphopenia is common in patients with newly diagnosed pancreatic cancer and high-grade gliomas and is associated with poor survival outcome, additional studies are needed to further define the mechanisms and implications of these findings. These studies should be designed to determine whether treatment-related lymphopenia is causal or if it is merely a prognostic variable. The results could steer future treatment approaches toward reducing the toxicities of therapy to lymphocytes and defining the proper timing and use of immunologic interventions such as vaccines or other immune response modifiers.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. This study was conducted without financial support from pharmaceutical companies. The authors had no assistance in writing this manuscript and there are no potential conflicts of interests by the authors.
References
- 1.Ehrlich P. Über den jetzigen stand der karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273–290. [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Prestwich RJ, Errington F, Hatfield P, Merrick AE, Ilett EJ, Selby PJ, Melcher AA. The immune system–is it relevant to cancer development, progression and treatment? Clin Oncol (R Coll Radiol) 2008;20:101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy. 2011;3:517–537. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S for the NABTT CNS Consortium. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;15:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, Robinson R, Laheru DA, Jaffee E, Hruban RH, Campbell KA, Wolfgang CL, Asrari F, Donehower R, Hidalgo M, Diaz LA, Jr, Yeo C, Cameron JL, Schulick RD, Abrams R. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg ML, Benedetti JK, Macdonald JS, Seay TE, Neubauer MA, George CS, Tanaka MS, Jr, Giguere JK, Pruitt BT, Abbruzzese JL. Phase II trial of 5-fluorouracil plus eniluracil in patients with advanced pancreatic cancer: a Southwest Oncology Group study. Ann Oncol. 2002;13:1576–1582. doi: 10.1093/annonc/mdf274. [DOI] [PubMed] [Google Scholar]
- 9.Ammori JB, Colletti LM, Zalupski MM, Eckhauser FE, Greenson JK, Dimick J, Lawrence TS, McGinn CJ. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7:766–772. doi: 10.1016/s1091-255x(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 10.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, Pedrazzoli S, Plebani M. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 11.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 12.Stocken DD, Hassan AB, Altman DG, Billingham LJ, Bramhall SR, Johnson PJ, Freemantle N. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer. 2008;99:883–893. doi: 10.1038/sj.bjc.6604568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda M, Endo I, Nakashima M, Minami Y, Takeda K, Matsuo K, Nagano Y, Tanaka K, Ichikawa Y, Togo S, Kunisaki C, Shimada H. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009;33:104–110. doi: 10.1007/s00268-008-9807-2. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meir P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 15.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 16.Clark EJ, Connor S, Taylor MA, Madhavan KK, Garden OJ, Parks RW. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:456–460. doi: 10.1080/13651820701774891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Kleinberg L, Grossman SA, Piantadosi S, Zeltzman M, Wharam M. The effects of sequential versus concurrent chemotherapy and radiotherapy on survival and toxicity in patients with newly diagnosed high-grade astrocytoma. Int J Radiat Oncol Biol Phys. 1999;44:535–543. doi: 10.1016/s0360-3016(99)00060-7. [DOI] [PubMed] [Google Scholar]
- 19.Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 20.Yeo C, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, Pitt HA, Lillemoe KD, Cameron JL. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival: a prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. discussion 633–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. discussion 782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Buchler MW European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 23.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KK. Radiation-induced lymphocyte-immune deficiency: a factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch Surg. 1970;101:114–121. doi: 10.1001/archsurg.1970.01340260018003. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe RT, Fuks ZY, Strober S, Kaplan HS. The long term effects of radiation of T and B lymphocytes in the peripheral blood after regional irradiation. Cancer. 1977;40:2071–2078. doi: 10.1002/1097-0142(197711)40:5<2071::aid-cncr2820400513>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Idestrom K, Petrini B, Blomgren H, Wasserman J, Wallgren A, Baral E. Changes of the peripheral lymphocyte population following radiation therapy to extended and limited fields. Int J Radiat Oncol Biol Phys. 1979;5:1761–1766. doi: 10.1016/0360-3016(79)90558-3. [DOI] [PubMed] [Google Scholar]
- 27.Louagie H, Van Eijkeren M, Philippe J, Thierens H, de Ridder L. Changes in peripheral blood lymphocyte subsets in patients undergoing radiotherapy. Int J Radiat Biol. 1999;75:767–771. doi: 10.1080/095530099140113. [DOI] [PubMed] [Google Scholar]
- 28.Lissoni P, Meregalli S, Bonetto E, Mancuso M, Brivio F, Colciago M, Gardani G. Radiotherapy-induced lymphocytopenia: changes in total lymphocyte count and in lymphocyte subpopulations under pelvic irradiation in gynecologic neoplasms. J Biol Regul Homeost Agents. 2005;19:153–158. [PubMed] [Google Scholar]
- 29.Standish LJ, Torkelson C, Hamill FA, Yim D, Hill-Force A, Fitzpatrick A, Olsen M, Schildt S, Sweet E, Wenner CA, Martzen MR. Immune defects in breast cancer patients after radiotherapy. J Soc Integr Oncol. 2008;6:110–121. [PMC free article] [PubMed] [Google Scholar]
- 30.Lissoni P, Rovelli F, Brivio F, Fumagalli L, Brera G. A study of immunoendocrine strategies with pineal indoles and interleukin-2 to prevent radiotherapy-induced lymphocytopenia in cancer patients. In Vivo. 2008;22:397–400. [PubMed] [Google Scholar]
- 31.United Nations Scientific Committee on the Effects of Atomic Radiation - UNSCEAR. United Nations 2nd volume Appendix D. Vienna, Austria: United Nations Office; 2006. Effects of ionizing radiation on the immune system; pp. 81–195. [Google Scholar]
- 32.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:64. doi: 10.1186/1471-2407-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]