Abstract
Heat shock protein 90 (Hsp90) has emerged as a promising therapeutic target for the treatment of cancer. Several Hsp90 inhibitors have entered clinical trials. However, some toxicological detriments have arisen, such as cardiotoxicity resulting from hERG inhibition following the administration of Hsp90 inhibitors. We sought to investigate this toxicity as hERG has been previously reported as a client protein that depends upon Hsp90 for its maturation and functional trafficking. In this study we show that hERG depends upon a single Hsp90 isoform. hERG preferentially co-immunoprecipitated with Hsp90α and genetic knockdown of Hsp90α, but not Hsp90β, resulted in a trafficking-defective hERG channel. This study demonstrates the importance of delineating the isoform dependence of Hsp90 client proteins and provides rationale for the design of isoform-selective Hsp90 inhibitors that avoid detrimental effects.
Keywords: Hsp90, isoform, hERG
Introduction
The 90-kDa heat shock protein (Hsp90) is an abundant molecular chaperone intimately involved in protein folding, activation, and stabilization. Hsp90 client proteins, those that rely on Hsp90 for their activity, include important signaling molecules, transcription factors, hormone receptors and kinases. Many of these client proteins are intimately involved in oncogenic progression, providing rationale for the development of Hsp90 inhibitors as a promising therapeutic strategy for the treatment of cancer.1 Several Hsp90 inhibitors have entered clinical trials; however, results from these trials have been somewhat disappointing. In many cases, clinical candidates have been plagued by a lack of efficacy, hepatotoxicity, cardiotoxicity or manifested detrimental off-target effects.2-3 While some of these detriments may be associated with the molecular scaffold, others may result from pan-Hsp90 inhibition, i.e. the inhibition of all Hsp90 isoforms.
In humans, there are four Hsp90 isoforms: Hsp90α and Hsp90β reside primarily in the cytoplasm and are stress-inducible and constitutively expressed, respectively.4 Glucose regulated protein 94 (Grp94) is localized to the endoplasmic reticulum, while tumor necrosis factor receptor associated protein1 (Trap1) is the mitochondrial-resident chaperone. Distinguishing the role played by each Hsp90 isoform has been a significant challenge, especially those manifested by the two cytosolic isoforms, which are structurally very similar (86% identical, 93% similar).4-5 The responsibility of each cytosolic isoform appears to overlap with some client proteins. However, recent evidence has emerged that suggests co-chaperones and client proteins interact with each isoform in a unique manner.5 All currently described Hsp90 inhibitors manifest pan-inhibition and since cardiotoxicity represents a major hurdle for Hsp90 inhibitor development, we investigated whether the hERG channel was an isoform-dependent Hsp90 client protein.
The α-subunit of the voltage gated potassium channel, the human ether-a-gogo-related gene product, hERG, constitutes a major component of the ion channel responsible for repolarization of cardiac action potential.6-7 Ficker et al. demonstrated previously that hERG depends upon Hsp90 for its functional maturation and that pharmacological inhibition of Hsp90 had deleterious effects on the hERG-related membrane potential.8-9 Defects in the hERG channel or off-target pharmacological inhibition of the hERG channel can cause long-QT syndrome, resulting in ventricular arrhythmias and even death.7,10 There are three primary classes of compounds that disrupt hERG function; 1) compounds that bind directly to and inhibit the hERG channel (hERG blockers), 2) compounds that inhibit the functional trafficking of hERG, or 3) compounds that exhibit both hERG blocking and hERG trafficking inhibitory abilities.11-12 Hsp90 inhibitors have been shown to disrupt hERG function and cause a reduction in hERG-related membrane currents in in-vitro studies. It has previously been demonstrated that the Hsp90 inhibitor, geldanamycin, inhibits hERG trafficking, presumably through an Hsp90-dependent mechanism.8 Given the intense pharmaceutical interest in the development of Hsp90 inhibitors as therapies for cancer and other diseases, the importance of identifying the isoform dependency of this client protein is clear, as pan-inhibition may result in undesired effects.
Experimental Section
Antibodies
The following antibodies were used for Western Blotting and/or co-immunoprecipitation: rabbit anti-Hsp90α (Neomarkers), goat anti-Hsp90β (SantaCruz), goat anti-hERG (SantaCruz), rabbit anti-Grp94 (SantaCruz) and rabbit anti-Actin (SantaCruz).
Cell Lines
The HEK-hERG13 (HEK 293 stably transfected with human hERG WT) cell line was a kind gift from Eckhard Ficker (MetroHealth Medical Center/Case Western Reserve University). The HEK-hERG cell line was maintained in DMEM supplemented with 10% FBS, streptomycin, penicillin, and geneticin at 37°C, 5% CO2. Inducible knockdown of Hsp90α or Hsp90β in the HEK-hERG cell line was accomplished using a tetracycline inducible shRNA construct containing a hairpin sequence specific for either the Hsp90α or Hsp90β isoform. These constructs were made by subcloning the isoform specific hairpin sequences from pGIPZ vectors (Open Biosystems, Lafayette, CO) into pTRIPZ vectors using MluI and XhoI restriction sites. The mature shRNA sequences were 5′–AGGAAGAATTTGGTCAAAA–3′ and 5′–ACTAAGAAGATCAAAGAGA–3′ for Hsp90α and Hsp90β respectively. Resulting clones where sequenced verified using pTRIPZ sequencing primers 5′–GGAAAGAATCAAGGAGG3′. The resulting pTRIPZ plasmids containing Hsp90 isoform specific shRNA were packaged into third generation lentivirus particles. Cells were cultured as above but with the addition of 2.5 μg/mL puromycin to select for stable, transduced cells. Induction of shRNA expression with tetracycline dose was monitored by the increase in TurboRFP fluorescence which is driven by the tetracycline response element (TRE). shRNA expression was induced with the addition of 1-24 μg/mL doxycyline.
siRNA and Transfection
Cells were plated in 24-well plates at 9.0×105 cells/mL (0.5 mL/well) in antibiotic free DMEM supplemented with 10% FBS. After 24 h., media was replaced with 0.2 mL OptiMEM (Invitrogen) and transfected with 43 uL of transfection mix containing 50 pmol siRNA and 2 uL oligofectamine (Invitrogen). Proteins were harvested 48 h. post transfection. Hsp90α siRNA was purchased from Ambion while Hsp90β siRNA was from SantaCruz Biotechnology.
Western Blot Analysis
The various HEK cells were harvested in cold PBS and lysed in mammalian protein extraction reagent (MPER, Pierce) lysis buffer containing protease inhibitors (Roche) on ice for 1 h. Lysates were clarified at 14,000g for 10 min at 4° C. Protein concentrations were determined using the Pierce BCA protein assay kit per the manufacturer’s instructions. Equal amounts of protein (2.5-10 μg) were electrophoresed under reducing conditions (6.5% acrylamide gel), transferred to a polyvinylidene fluoride membrane (PVDF), and immunoblotted with the corresponding specific antibodies. Membranes were incubated with an appropriate horseradish peroxidase-labeled secondary antibody, developed with a chemiluminescent substrate, and visualized.
Immunoprecipitation
Using a method analogous to Ficker et al.8 and Nanduri et al.9, cells were plated in 10 cm cell culture dishes and allowed to grow to 80% confluency. Cells were then washed once with phosphate-buffered saline (PBS) and then crosslinked by incubation with 1.5 mM dithiobis(succinimidyl propionate, DSP, Pierce) in PBS for 15 min at room temperature. DSP was quenched by the addition of pH 7.5 Tris (final concentration 10 mM). Cells in PBS/DSP/Tris were scraped into conical tubes, pelleted, and washed with PBS. Cells were harvested in lysis buffer containing 0.1% NP40, 50 mM Tris (pH 7.5), 150 mM NaCl, 20 mM MoO4, and protease inhibitors (Roche). Lysates were clarified and protein concentration was determined using BCA assay. For co-immunoprecipitation, 300 ug total protein was diluted to 500 uL total volume in lysis buffer and incubated with 2μg of either anti-hERG, anti-Hsp90α or anti-Hsp90β antibody overnight at 4°C with rocking. Immunocomplexes were captured with 35μL of DynaBeads Protein G (Invitrogen) for 2 h. with rocking at 4°C. Protein G Bead-complexes were washed three times with lysis buffer and eluted with sample buffer. Samples were then boiled and subjected to SDS-PAGE and Western Blot analysis.
Tryptic Digest and Mass Spectrometry
Lysates were co-immunoprecipitated as above except with 1 mg total protein, 6 ug anti-hERG antibody, and 100 μL DynaBeads Protein G in 1.5 mL total volume. After wash steps, samples were boiled and subjected to SDS-PAGE. Acrylamide gels were stained, bands excised, destained and washed using the Silver Stain Kit for Mass Spectrometry (Pierce). Excised bands were further washed (0.2 M NH4HCO3, 50% acetonitrile) and then dried. Gel pieces were suspended in 0.2 M NH4HCO3 and 10 mM DTT (final concentration) was added and heated at 60°C for 15 mins. 20 mM iodoacetamide (final concentration) was then added and gel bands incubated for 30 mins at 25°C in the dark. Gel bands were then washed (0.2 M NH4HCO3, 50% acetonitrile), shrunk in 100% acetonitrile, and dried. Gel bands were re-swelled in 0.2 M NH4HCO3 containing 0.5 μg trypsin and 0.2 M NH4HCO3 with 10% acetonitrile was added to keep gel pieces submerged. Digest was terminated after 16 h. of incubation at 37°C by the addition of 0.1% trifluoroacetic acid (final concentration). Supernatants were then subjected to LC-MS/MS at the University of Kansas Analytical Proteomics Lab. Results were analyzed using Scaffold software which integrates results from Mascot, Sequest and X!Tandem search engines.
Densitometry and Statistical Analysis
Western Blot films were digitally captured using a standard flatbed scanner (HP). The digital blots were then converted to 16-bit black and white images using ImageJ software. Densitometric measurements were then performed in ImageJ using the “Gels” tool. Peak areas were used as a measure of protein levels. Statistical analysis was performed using GraphPad Prism5, statistical significance was determined using a paired, two-tailed t-test.
Results and Discussion
hERG Interacts Solely with Hsp90α
The K+ channel encoded by the human ether-a-gogo-related gene (hERG) plays a major role in the repolarization of cardiac myocytes following normal action potential.6 Although pharmacological inhibition of hERG itself is a therapeutic target for the treatment of some cardiac arrhythmias, more often undesired inhibition of hERG is an off-target effect that results in cardiotoxicity and leads to the ultimate failure of pre-clinical or clinical candidates.7 hERG has been previously identified as an Hsp90/Hsp70–dependent client protein, and studies have shown that inhibition of Hsp90 with geldanamycin resulted in proteasome-mediated degradation of hERG which prevented maturation of a fully functional hERG channel.8-9 In addition, the Hsp90 co-chaperones Hsp-organizing protein (Hop), Hdj-2, and BCL-associated athanogene 2 (Bag-2), as well as the 38-kDa FK506 binding protein (FKBP38) and calnexin were found to interact with hERG, presumably to aid in hERG maturation.14 Based on these observations, we sought to determine whether hERG was dependent upon a sole Hsp90 isoform, which would provide rationale to support the development of Hsp90 isoform-selective inhibitors that avoid such detriments.
Using the method of Ficker et al.8 co-immunoprecipitation studies were performed to evaluate the interaction of hERG with each of the Hsp90 isoforms. hERG expressing HEK (HEK-hERG) cell lysates were chemically crosslinked and immunoprecipitated utilizing antibodies that recognize Hsp90α, Hsp90β, or hERG (Figure 1). hERG channels exist as either a functional, fully glycosylated mature form (fg) or an immature core-glycosylated protein (cg), and both species can be detected by Western Blot analysis.15-17 As previously determined, Hsp90 only interacts with the core glycosylated hERG (Figure 1).8 In addition, as evidenced in Figure 1, hERG only interacts with a single Hsp90 isoform, namely Hsp90α. The hERG-Hsp90α interaction can be captured by co-immunoprecipitation with either an anti-hERG or anti-Hsp90α antibody (Figure 1 and data not shown).
Figure 1. hERG co-immunoprecipitates with Hsp90αand not Hsp90β.
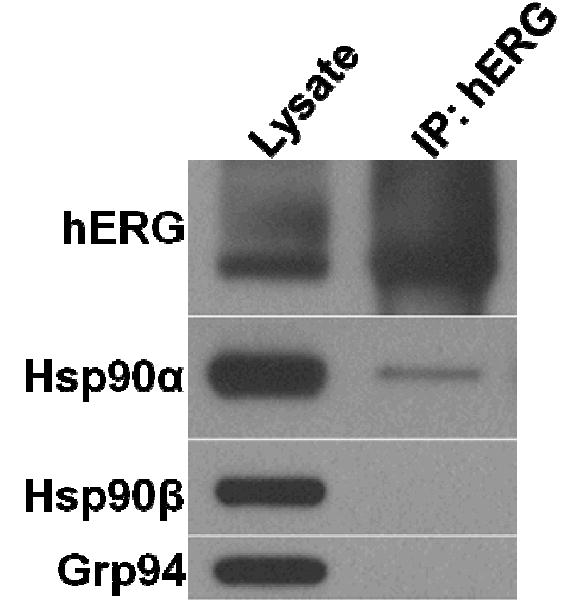
Cross-linked cell lysates were immunoprecipitated with an anti-hERG antibody, immunocomplexes were collected with Protein G beads. Proteins were eluted, uncrosslinked, separated by SDS-PAGE, and analyzed by Western Blotting (fg = fully glycosylated; cg = core glycosylated).
In order to identify other proteins involved in this interaction, we performed co-immunoprecipitations with anti-hERG antibody, separated the complexes on SDS-PAGE, excised protein bands and subjected the proteins to tryptic digest and mass spectrometry. Using this approach we identified Hsp90α in the hERG-associated complex. Since Hsp90α and Hsp90β are overall 86% identical, it is important to note that we observed overlapping peptides derived from both isoforms, however only unique peptides from Hsp90α were identified during these studies. In addition to the chaperones and co-chaperones previously identified to interact in the hERG complex,14 additional proteins were found (Table 1). The Hsp/c70 co-chaperone, Hsc70-interacting protein (Hip), was found in this complex and typically directs Hsc70 to the Hsp90 complex with the help of the Hsp organizing protein (Hop). Two additional peptidyl-prolyl cis-trans isomerases were also identified, FK506 binding protein 51 (FKBP51) and Cyclophilin A (CypA). FKBP51 has previously been identified in Hsp90 complexes, specifically those that contain the steroid hormone receptors.18 Hsp90 associated immunophilins typically bind to the C-terminus of Hsp90 and mediate interactions between Hsp90 and other co-chaperones. CypA and Hsp90 have been shown to be necessary for cell membrane translocation of toxins from both Bacillus anthracis and Clostridium difficile, however, the involvement of CypA in Hsp90-mediated client protein maturation has not been reported.19-20 Another tetratricopeptide repeat-containing protein, 14-3-3 protein epsilon (14-3-3) was also identified. Its role in client protein maturation is undocumented, however TPR-domain containing proteins typically mediate protein-protein interactions.
Table 1.
hERG interacting proteins (N=3)1.
| Protein | Accession Number (NCBI) | No. Unique Peptides | Sequence Coverage (%) |
|---|---|---|---|
|
| |||
| hERG | Q12809.1 | 9 | 11 |
| Hsp90α | P07900 | 6 | 22 |
| Hsc70* | P11142 | 13 | 29 |
| Hsp70 | P08107 | 21 | 40 |
| Calnexin* | P27824 | 3 | 7 |
| FKBP51 | Q02790 | 3 | 12 |
| Hip | P50502 | 2 | 7 |
| Hop* | P31948 | 6 | 16 |
| Cyclophilin A | P62937 | 7 | 47 |
| 14-3-3 | P62258 | 9 | 44 |
Confidence level of 95% or greater
Also identified in Ref. 14
Hsp90α and Hsp90β Knockdown have Differential Effects on hERG Trafficking
To investigate the relationship between each of the cytosolic Hsp90 isoforms and hERG maturation, we analyzed the effects of either Hsp90α or Hsp90β knockdown using RNA interference with siRNAs targeted to each isoform. Under control conditions (scrambled siRNA), both the fully glycosylated and core glycosylated hERG proteins were observed (Figure 2A). Previously, it was shown that Hsp90 inhibitors, such as geldanamycin and radicicol, cause both a dose-dependent reduction in the hERG-associated current amplitudes in patch clamp experiments and in the trafficking efficiency of hERG, or the ratio of fg hERG over total hERG (fg+cg) (Figure 2B).8 Knockdown of Hsp90α (60% reduction) caused approximately a 70% reduction in the trafficking efficiency of hERG and a 55% reduction in the total amount of hERG (Figure 2C). Conversely, the hERG trafficking efficiency and total amount of hERG remained unchanged after Hsp90β knockdown (60% reduction).
Figure 2. Hsp90α knockdown reduces hERG trafficking.
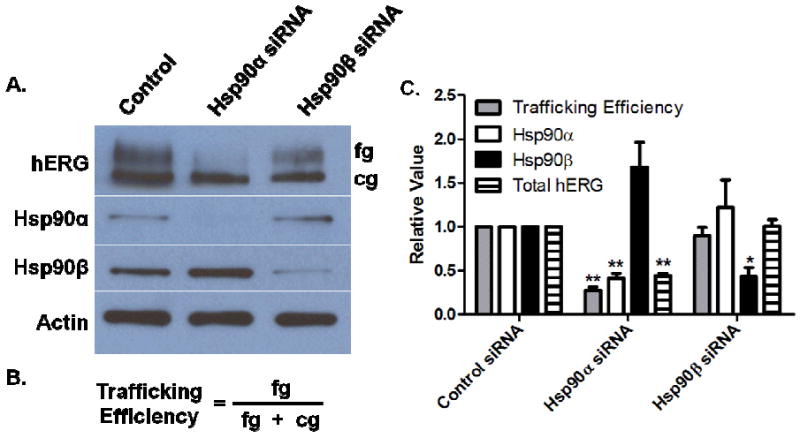
A) Control, Hsp90α, and Hsp90β targeted siRNA were transfected into hERG-HEK cells. The effect of siRNA was analyzed by Western Blot Analysis 48 h. post transfection. Actin represents a loading control. B) Equation for trafficking efficiency. C) Densitometry analysis of Western Blots from A (N=3); *, p < 0.02; **, p < 0.01 versus control siRNA values.
For further confirmation, we sought to demonstrate a direct relationship between the amount of functional Hsp90α and the trafficking efficiency of hERG. Using a tetracycline-induced promoter linked to the expression of either an Hsp90α or Hsp90β targeted shRNA, this relationship was investigated (Figure 3). Figure 3 demonstrates the direct relationship between the amount of Hsp90α and the trafficking efficiency of the hERG channel. Decreasing amount of Hsp90α in these cells causes a “dose-dependent” decrease in the amount of fully glycosylated, functional hERG after both 24 and 48 hours (Figure 3A/C and 3B/D/G). In addition, we observed a reduction in the total amount of hERG (fg+cg) in these cells following shRNA-mediated Hsp90α reduction (Figure 3 C/D). Conversely, reduction in the amount of Hsp90β had no effect on the glycosylation state, i.e. functional activity, or total hERG (Figure 3A/E and 3B/F/H). These data further demonstrate the isoform dependent nature of the hERG channel for Hsp90α.
Figure 3. Hsp90α levels directly affect hERG trafficking efficiency.
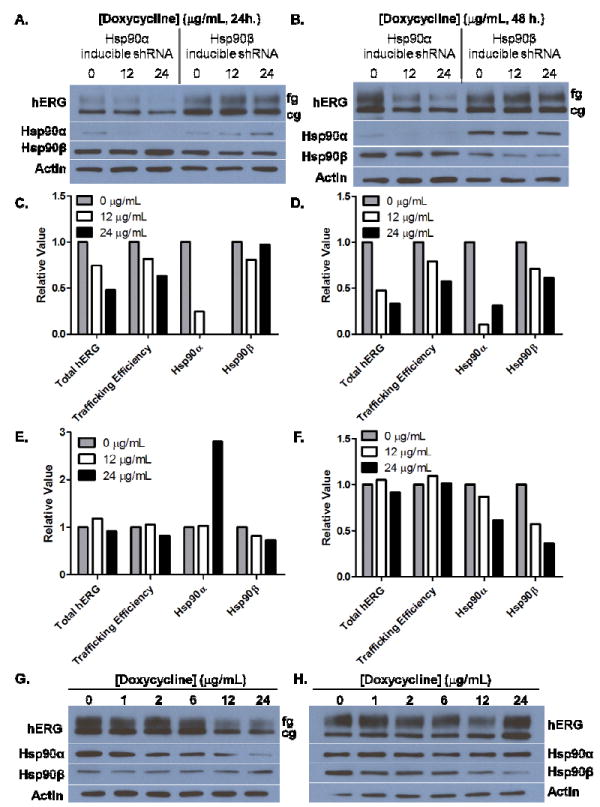
Western blot analysis of cell lystates from HEK-hERG cells that either express Hsp90α or Hsp90β targeted shRNA after doxycyline treatment for (A) 24 h. or (B) 48 h. treatment. C) and D) Densitometry analysis of Western blots from A and B (Hsp90α knockdown) respectively. E) and F) Densitometry analysis of Western blots from A and B (Hsp90β knockdown) respectively. Western blot analysis of cell lystates from HEK-hERG cells expressing G) Hsp90α targeted shRNA or H) Hsp90β targeted shRNA after 48 h. doxycycline treatment.
As pharmacological inhibition of hERG can lead to the ultimate failure of pre-clinical drug candidates, it is important to understand the different mechanisms by which hERG function can be affected.7 Drug-like molecules can bind directly to and inhibit hERG. However, it has also been demonstrated that Hsp90 inhibitors halt the functional maturation of hERG and can therefore inhibit hERG function by preventing trafficking to the membrane.11-12 Hsp90 inhibitors appear to prevent the glycosylation of hERG and halt translocation from the ER to the cell surface, which results in non-functional hERG accumulation in the cytosol.8 Since small molecule Hsp90 inhibitors are of great interest to the pharmaceutical industry, it is important to outline the liabilities associated with Hsp90 inhibition. These results clearly demonstrate that the cardiotoxic liability associated with pan-Hsp90 inhibition may be avoidable by the development of Hsp90 inhibitors that avoid Hsp90α.
In addition, these results suggest that deconvolution of the Hsp90-isoform proteome is needed in order to more thoroughly understand the consequences of Hsp90 inhibition. It has been difficult to biochemically distinguish the roles manifested by each Hsp90 isoform due to the high structural similarity, significant antibody cross reactivity, and the presence of Hsp90α/β heteromdimers. Furthermore, only a few Hsp90 isoform–dependent client proteins have been identified. Studies have demonstrated that the cellular inhibitor of apoptosis protein 1 (c-IAP1) depends upon Hsp90β,21 while some intermediates in the major histocompatibility complex (MHC) class I, but not MHC class II, pathway depend solely upon Hsp90α.22-23 Phenotypic screens have also been used to identify the unique roles played by each cytosolic Hsp90 isoforms. For example, Hsp90β appears to be critical for embryonic development, as an Hsp90β null mutation results in embryonic lethality.24 Meanwhile, Hsp90α is overexpressed in many cancer cells and has been linked to cancer cell invasiveness, which is directly related to matrix metalloproteinase 2 activity.25 Chadli et al. provided rationale to support Hsp90 isoform dependence of co-chaperones.26 In their studies, they found the general cell UNC45 (GCUNC45) binds preferentially to Hsp90β and was capable of stalling the chaperone machinery during the progression/activation of the progesterone receptor. In this example, the specific co-chaperone required for client protein activation/folding determines the isoform dependence. In a yeast model system in which the endogenous Hsp90 was replaced with either hHsp90α or hHsp90β as the sole Hsp90, differences the activation of client proteins and in the sensitivity to Hsp90 inhibitors were observed.27-28
In conclusion, we have shown herein that the hERG channel depends solely on Hsp90α, providing rationale for the design of isoform selective Hsp90 inhibitors. Furthermore, understanding the responsibilities manifested by each Hsp90 isoform will be crucial to the development of Hsp90 inhibitors and will allow for the development of Hsp90 inhibitors that produce fewer adverse effects.
Acknowledgments
The authors gratefully acknowledge the support of this project by NIH (CA109265), NIH Training Grant (T32 GM008545) on Dynamic Aspects in Chemical Biology (L.B.P.), and the ACS Division of Medicinal Chemistry Predoctoral Fellowship (L.B.P.).
Abbreviations
- Hsp90
Heat shock protein 90
- hERG
human ether-a-gogo-related gene
- Grp94
glucose regulated protein 94
- Trap1
tumor necrosis factor receptor associated protein1
- siRNA
small interfering RNA
- siRNA
short-hairpin RNA
- FKBP51
FK506 binding protein 51
- CypA
Cyclophilin A
- TPR
tetratricopeptide repeat
References
- 1.Bishop SC, Burlison JA, Blagg BSJ. Hsp90: a novel target for the disruption of multiple signaling cascades. Curr Cancer Drug Tar. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 2.Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, Trepel JB. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee W. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 4.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics. 2005;86:627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. BBA - Mol Cell Res. 2012;1823:607–613. doi: 10.1016/j.bbamcr.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Perrin MJ, Subbiah RN, Vandenberg JI, Hill AP. Human ether-a-go-go related gene (hERG) K+ channels: Function and dysfunction. Prog Biophys Mol Bio. 2008;98:137–148. doi: 10.1016/j.pbiomolbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. doi: 10.1016/s0165-6147(00)01662-x. [DOI] [PubMed] [Google Scholar]
- 8.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel hERG. Circ Res. 2003;92:e87–e100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 9.Nanduri J, Bergson P, Wang N, Ficker E, Prabhakar NR. Hypoxia inhibits maturation and trafficking of hERG K+ channel protein: Role of Hsp90 and ROS. Biochem Bioph Res Comm. 2009;388:212–216. doi: 10.1016/j.bbrc.2009.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2:439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 11.Brown AM. Cardiac Safety of Noncardiac Drugs. Humana Press; 2005. hERG Assay, QT Liability, and Sudden Cardiac Death; pp. 67–81. [Google Scholar]
- 12.Wible BA, Hawryluk P, Ficker E, Kuryshev YA, Kirsch G, Brown AM. HERG-Lite: A novel comprehensive high-throughput screen for drug-induced hERG risk. J Pharmacol Toxicol. 2005;52:136–145. doi: 10.1016/j.vascn.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker VE, Atanasiu R, Lam H, Shrier A. Co-chaperone FKBP38 Promotes HERG Trafficking. J Biol Chem. 2007;282:23509–23516. doi: 10.1074/jbc.M701006200. [DOI] [PubMed] [Google Scholar]
- 15.Ficker E, Dennis AT, Kuryshev YA, Wible BA, Brown AM. hERG channel trafficking. Novart Fdn Symp. 2005;266:57–74. [PubMed] [Google Scholar]
- 16.Petrecca K, Atanasiu R, Akhavan A, Shrier A. N-linked glycosylation sites determine HERG channel surface membrane expression. J Physiol. 1999;515:41–48. doi: 10.1111/j.1469-7793.1999.041ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong Q, Anderson CL, January CT, Zhou Z. Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am J Physiol Heart Circ Physiol. 2002;283:H77–H84. doi: 10.1152/ajpheart.00008.2002. [DOI] [PubMed] [Google Scholar]
- 18.Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF. Functional Specificity of Co-Chaperone Interactions with Hsp90 Client Proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. doi: 10.1080/10409230490892513. [DOI] [PubMed] [Google Scholar]
- 19.Dmochewitz L, Lillich M, Kaiser E, Jennings LD, Lang AE, Buchner J, Fischer G, Aktories K, Collier RJ, Barth H. Role of CypA and Hsp90 in membrane translocation mediated by anthrax protective antigen. Cell Microbiol. 2011;13:359–373. doi: 10.1111/j.1462-5822.2010.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser E, Kroll C, Ernst K, Schwan C, Popoff M, Fischer G, Buchner J, Aktories K, Barth H. Membrane Translocation of Binary Actin-ADP-Ribosylating Toxins from Clostridium difficile and Clostridium perfringens Is Facilitated by Cyclophilin A and Hsp90. Infect Immun. 2011;79:3913–3921. doi: 10.1128/IAI.05372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Didelot C, Lanneau D, Brunet M, Bouchot A, Cartier J, Jacquel A, Ducoroy P, Cathelin S, Decologne N, Chiosis G, Dubrez-Daloz L, Solary E, Garrido C. Interaction of heat-shock protein 90β isoform (HSP90β) with cellular inhibitor of apoptosis 1 (c-IAP1) is required for cell differentiation. Cell Death Differ. 2008;15:859–866. doi: 10.1038/cdd.2008.5. [DOI] [PubMed] [Google Scholar]
- 22.Houlihan JL, Metzler JJ, Blum JS. HSP90α and HSP90β Isoforms Selectively Modulate MHC Class II Antigen Presentation in B Cells. J Immunol. 2009;182:7451–7458. doi: 10.4049/jimmunol.0804296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunisawa J, Shastri N. Hsp90α Chaperones Large C-Terminally Extended Proteolytic Intermediates in the MHC Class I Antigen Processing Pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Voss AK, Thomas T, Gruss P. Mice lacking HSP90β fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Functional proteomic screens reveal an essential extracellular role for hsp90α in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 26.Chadli A, Felts SJ, Toft DO. GCUNC45 is the first Hsp90 co-chaperone to show α/β isoform specificity. J Biol Chem. 2008;283:9509–9512. doi: 10.1074/jbc.C800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millson SH, Prodromou C, Piper PW. A simple yeast-based system for analyzing inhibitor resistance in the human cancer drug targets Hsp90α/β. Biochem Pharmacol. 2010;79:1581–1588. doi: 10.1016/j.bcp.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Millson SH, Truman AW, Rácz A, Hu B, Panaretou B, Nuttall J, Mollapour M, Söti C, Piper PW. Expressed as the sole Hsp90 of yeast, the α and β isoforms of human Hsp90 differ with regard to their capacities for activation of certain client proteins, whereas only Hsp90β generates sensitivity to the Hsp90 inhibitor radicicol. FEBS J. 2007;274:4453–4463. doi: 10.1111/j.1742-4658.2007.05974.x. [DOI] [PubMed] [Google Scholar]


