Abstract
BACKGROUND
Soluble ST2 (sST2) is a cardiac biomarker whose concentration rises in response to myocardial strain. Increased sST2 concentrations may predict adverse outcomes in patients with heart failure and myocardial infarction. Because sST2 was largely undetectable with first-generation assays in ambulatory individuals, there are few data regarding its distribution and correlates in community-based populations.
METHODS
We measured sST2 using a highly sensitive ELISA in 3450 Framingham Heart Study participants who attended a routine examination. We used multivariable linear regression models to identify covariates associated with sST2 in the general sample. We obtained a reference sample (n = 1136) by excluding individuals with prevalent coronary disease, heart failure, atrial fibrillation, diabetes, hypertension, obesity, valvular disease, left ventricular systolic dysfunction, and pulmonary and renal dysfunction. We used empiric and quantile regression techniques to estimate the 2.5th, 50th, 97.5th, and 99th quantiles.
RESULTS
In the general sample (mean age 59 years, 55% women), systolic blood pressure (P = 0.006), antihypertensive medication use (P = 0.03), and diabetes (P < 0.001) were associated with sST2 concentrations. In the reference sample (mean age 55, 59% women), male sex (P < 0.0001) and older age (P = 0.004) were predictive of higher sST2 concentrations. Quantile and empirical methods were used to define the reference intervals. Using the empirical approach, upper 99% percentile values in different age groups ranged from 46.6 to 64.4 μg/L in men and 36.7 to 53.0 μg/L in women.
CONCLUSIONS
In a well-characterized, community-based cohort, values for sST2 differ between men and women, increase with age, and are associated with diabetes and hypertension.
ST2 is a member of the interleukin-1 (IL-1)9 receptor family that appears to play several roles in health and disease. Originally studied in allergic and immunologic diseases, the cardiovascular role of ST2 was initially identified by examining gene transcripts that are up-regulated with myocardial strain, where the gene for ST2 was noted to be intensely transcribed (1, 2). ST2 consists of 2 isoforms, a transmembrane ligand (ST2L) and a soluble component (sST2). The biological effects of IL-33 are transduced by ST2L, mitigating cellular responses to mechanical stress. Loss of intact IL-33/ST2L signaling results in unchecked remodeling of ventricular myocardium characterized by excessive myocyte hypertrophy, fibrosis, and worsening of left ventricular (LV) function, along with a higher risk of death from ventricular failure (3). The favorable responses to IL-33/ST2L function are thought to be mediated by inhibition of apoptosis and cell death (4). In contrast to ST2L, sST2 may act as a “decoy” receptor for IL-33, and when present in large enough amounts, sST2 likely interferes significantly with the actions of IL-33, potentially leading to loss of the beneficial effects of this hormone (5). Clinically, increased sST2 concentrations predict adverse outcomes in acute myocardial infarction (6 – 8), acutely decompensated heart failure (9 –12), and chronic heart failure (13, 14). Increased sST2 concentrations are also present in multiple non-cardiac entities (asthma (15), pulmonary disease (16), sepsis (17, 18), and trauma(18)), consistent with its role of mitigating type 2 helper (Th2) cell responses.
Despite the powerful biological and prognostic data regarding sST2 across a wide range of disease states, there are relatively few data regarding its distribution and correlates in the community. Such information may become important as the use of this biomarker increases, and may provide insight regarding factors that influence the ST2/IL-33 system. Although earlier versions of the assay for sST2 measurement were insufficiently precise to measure very low concentrations of the biomarker in healthy individuals, such measurement was now possible with the development of a highly sensitive ELISA (19). With this in mind, we measured sST2 in a large population-based study of well-characterized, ambulatory individuals.
Methods
STUDY SAMPLE
The study design of the Framingham Offspring Study has been described (20(. Participants who attended Examination 6 and had sST2 concentrations checked from banked serum were eligible for the current investigation (1996–1998; n = 3450).
We excluded the following participants in hierarchical fashion: prevalent LV dysfunction (n = 302), prevalent heart failure (n = 39), missing sST2 concentration (n = 4), missing high-sensitivity troponin (n = 35), and no blood sample available (offsite examination; n = 43). The general sample contained 3109 individuals.
A healthy reference sample was also selected, from which participants with the following characteristics were excluded: missing biomarker information (n = 4), high-sensitivity troponin concentration extreme outlier (n = 1), age <35 or ≥75 years (because of too few individuals in these age groups to determine a reliable reference limit; n = 23), renal dysfunction [defined by estimated glomerular filtration rate (GFR) < 60 mL/min/1.73m2 (21); n = 85], pulmonary dysfunction [forced expiratory volume in 1 s (FEV1) < lower limit of normal for the population as calculated by Hankinson et al. (22); n = 114], LV dysfunction (echocardiographically determined by fractional shortening <0.30, ventricular function coded as borderline or mild to severe dysfunction; n = 86), valve disease (systolic murmur graded 3/6 or worse or any diastolic murmur; n = 19), obesity [body mass index (BMI) ≥30 kg/m2; n = 367], hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or use of an antihypertensive medication; n = 1019), diabetes [fasting blood glucose >125 mg/dL (>6.94 mmol/L); n = 312], atrial fibrillation (n = 61), heart failure (n = 13), or coronary heart disease (myocardial infarction, angina pectoris, or coronary insufficiency; n = 292). After applying these exclusions, the resulting reference sample included 1136 individuals. Of note, we excluded individuals with renal disease (on the basis of GFR) to maintain the similarity between our reference population and reference populations used in other biomarker studies. There was no significant correlation between log(ST2) and log(GFR) in our analysis (data not shown). This is consistent with the work from Dieplinger et al. (19), who did not find any difference in sST2 concentrations between healthy controls and individuals with renal disease.
Given previous links to asthma, a secondary analysis was performed on the entire Examination 6 population to investigate the relationship between sST2 concentrations, asthma, and measures of pulmonary function by pulmonary function testing. For this analysis, participants were excluded for prevalent heart failure (n = 56), coronary heart disease (n = 159), or chronic kidney disease stage IV (n = 27). Additionally, participants with missing covariates including BMI, height, weight, pack-years smoked, smoking status, and diabetes (n = 421) and missing pulmonary function testing (n = 518) were excluded. After applying these exclusions, the sample for the secondary analysis included 2374 individuals.
All participants in the Framingham Heart Study provided written informed consent, and the study protocol was approved by the Institutional Review Board of the Boston University Medical Center.
CLINICAL ASSESSMENT
All participants underwent a clinical examination as described elsewhere (20). A general medical history (including alcohol and smoking history) was performed. Standard 12-lead electrocardiogram (ECG), blood pressure, and anthropomorphic measures were recorded. Pulmonary function testing was performed, and measurement and derivation of the predicted values have been described elsewhere (23). An echocardiogram was also performed, and standard 2-dimensional and M-mode measurements were recorded (24). All measurements were performed in accordance with American Society of Echocardiography recommendations and by use of a leading-edge to leading-edge technique. Fractional shortening was calculated by the following equation: LV end-diastolic diameter − LV end-systolic diameter)/(LV end-diastolic diameter) (25(. Metabolic syndrome was defined by the National Cholesterol Education Program Adult Treatment Panel III and has been used in previous Framingham Studies (26, 27). C-reactive protein (CRP) was measured from a morning fasting blood sample as described previously (28).
MEASUREMENT OF sST2
Participants provided a morning, fasting blood sample that was stored at −80 °F until thawed for measurement of sST2. We measured sST2 in citrated plasma by use of a clinically available, highly sensitive ELISA (Presage® ST2 assay, Critical Diagnostics) whose performance characteristics have been recently described (the reference change value from this study was reported to be 29.8%) (19). The lower limit of detection of the assay is 2 μg/L. The assay has a within-run CV of 2.4% and a total CV of 4.0% at a mean concentration of 11 μg/L, within-run CV of 2.0% and total CV of 3.9% at a mean concentration of 87 μg/L, and within-run CV of 2.2% and total CV of 3.9% at a mean concentration of 140 μg/L.
STATISTICAL METHODS
We used descriptive statistics to obtain the characteristics of the reference and general populations. Means (SDs) are presented for continuous variables [with the exception of the hormone replacement therapy (HRT) variable, for which median and first and third quartiles are presented] and percentages for categorical variables in the general and reference samples.
Given nonnormality of distribution, sST2 concentrations were log-transformed for modeling. We used multivariable linear regression to determine the clinical correlates of log(sST2) in the reference and general samples. Continuous covariates were standardized (1-SD increment) to facilitate comparison among covariates. Candidate covariates included age, sex, BMI, systolic blood pressure, use of antihypertensive medication, smoking status, total and HDL cholesterol, LV hypertrophy (by ECG), diabetes, and atrial fibrillation. We used multivariable linear regression to determine the correlates of individual pulmonary function measures and asthma. Candidate covariates included log(ST2), age, sex, height, smoking status, pack-years smoked, BMI, and diabetes.
We derived reference values for the 2.5th, 50th, 97.5th, and 99th quantiles of sST2 using 2 techniques described previously in the Framingham Heart Study: empirical and quantile regression (29). To estimate the empirical reference limits, the reference sample was divided by sex and placed into 10-year age bins. Because the empirical reference limits can vary substantially when the subgroup size is small, we also performed linear quantile regression (PROC QUANTREG) to estimate the 2.5th-, 50th-, 97.5th-, and 99th-percentile sex-specific regressions with age as the sole predictor. Analyses were performed with SAS version 9.1.3 (SAS Institute). All P values are 2-sided, with values <0.05 considered statistically significant.
Results
BASELINE CHARACTERISTICS
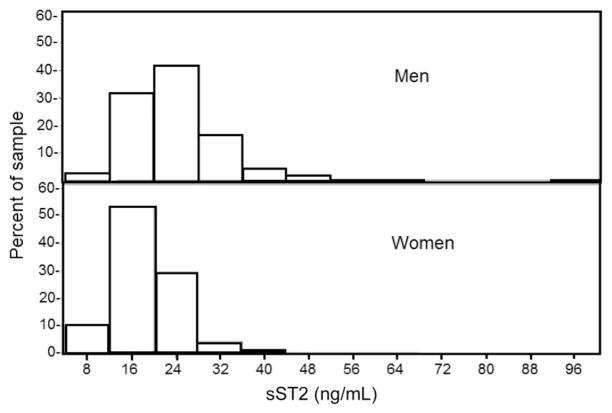
Characteristics of the general and reference samples are presented in Table 1. In the general sample, the mean age was 59 years, and 55% were women. Plasma concentrations of sST2 were detectable in 100% of individuals with plasma tested. The distribution of sST2 concentrations in men and women in the reference sample is shown in Fig. 1.
Table 1.
Characteristics of the reference and general samples.a
| General sample
|
Reference sample
|
|||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| n | 1388 | 1721 | 462 | 674 |
|
| ||||
| Age, years | 59 (10) | 59 (10) | 55 (9) | 56 (8) |
|
| ||||
| BMI, kg/m2 | 28.5 (4.4) | 27.4 (5.7) | 26.1 (2.4) | 24.4 (2.8) |
|
| ||||
| Blood pressure, mmHg | ||||
|
| ||||
| Systolic | 130 (17) | 127 (20) | 119 (11) | 116 (12) |
|
| ||||
| Diastolic | 78 (9) | 74 (9) | 74 (7) | 71 (8) |
|
| ||||
| Total cholesterol, | ||||
|
| ||||
| mg/dL | 200 (40) | 212 (38) | 202 (39) | 209 (39) |
|
| ||||
| mmol/L | 5.17 (1.03) | 5.48 (0.98) | 5.22 (1.01) | 5.41 (1.01) |
|
| ||||
| HDL cholesterol | ||||
|
| ||||
| mg/dL | 43.8 (12.3) | 58.1 (16.3) | 46.5 (12.6) | 61.9 (15.8) |
|
| ||||
| mmol/L | 1.13 (0.32) | 1.50 (0.42) | 1.20 (0.33) | 1.60 (0.41) |
|
| ||||
| Smoker, % | 15 | 16 | 14 | 14 |
|
| ||||
| Antihypertensive therapy, % | 29 | 25 | ||
|
| ||||
| Diabetes, % | 12 | 9 | ||
|
| ||||
| Atrial fibrillation, % | 3 | 1 | ||
Data are mean (SD) unless otherwise noted.
Fig. 1.
ST2 concentrations in men and women in the reference sample.
CLINICAL CORRELATES
Correlates of sST2 in the general and reference samples are shown in Table 2. In the general sample, age, sex, systolic blood pressure, use of antihypertensive medication, and presence of diabetes were associated with higher plasma sST2 concentrations (P < 0.05 for each covariate).
Table 2.
Clinical correlates of sST2.
| Covariates included in the modela | Regression coefficientb | SE | P |
|---|---|---|---|
| General sample | |||
| All | |||
| Female sex | −0.206 | 0.013 | <0.001 |
| Age, per 10 years | 0.027 | 0.007 | <0.001 |
| BMI, per 5.2 kg/m2 | 0.011 | 0.007 | 0.09 |
| Systolic blood pressure, per 19 mmHg | 0.019 | 0.007 | 0.006 |
| Use of antihypertensives | 0.032 | 0.017 | 0.03 |
| Diabetes | 0.104 | 0.020 | <0.001 |
| Men | |||
| Age, per 10 years | 0.010 | 0.010 | 0.35 |
| BMI, per 5.7 kg/m2 | −0.015 | 0.011 | 0.20 |
| Systolic blood pressure, per 21 mmHg | 0.031 | 0.011 | 0.005 |
| Use of antihypertensives | 0.024 | 0.022 | 0.27 |
| Diabetes | 0.121 | 0.030 | <0.001 |
| Women | |||
| Age, per 10 year | 0.042 | 0.009 | <0.001 |
| BMI, per 4.4 kg/m2 | 0.024 | 0.008 | 0.002 |
| Systolic blood pressure, per 17.0 mmHg | 0.009 | 0.009 | 0.31 |
| Use of antihypertensives | 0.043 | 0.020 | 0.03 |
| Diabetes | 0.091 | 0.029 | 0.002 |
| Reference sample | |||
| All | |||
| Female sex | −0.233 | 0.038 | <0.001 |
| Age, per 8.9 years | 0.029 | 0.010 | 0.006 |
| BMI, per 2.8 kg/m2 | −0.020 | 0.011 | 0.07 |
| Men | |||
| Age, per 8.9 years | 0.029 | 0.016 | 0.07 |
| BMI, per 2.4 kg/m2 | −0.023 | 0.019 | 0.22 |
| Women | |||
| Age, per 9 years | 0.026 | 0.014 | 0.06 |
| BMI, per 2.8 kg/m2 | −0.019 | 0.013 | 0.15 |
For the reference sample, covariates in the model included age, sex, BMI, systolic blood pressure, smoking status, and total and HDL cholesterol. For the general sample, covariates in the model included age, sex, BMI, systolic blood pressure, use of an antihypertensive medication, smoking status, total and HDL cholesterol, LV hypertrophy (by ECG), diabetes, and atrial fibrillation.
Regression coefficients represent expected increment in log(sST2) for presence vs absence (or 1 SD increment) of the categorical (or continuous) covariates shown.
To further evaluate the association of sST2 with sex, we examined sST2 concentrations in women by HRT status. HRT includes estrogen therapy in women with or without the use of progestin. The median concentration of sST2 in men was 23.50 μg/L (first and third quartiles, 19.18 and 28.96); for women not on HRT (n = 1267), it was 19.51 μg/L (15.76 and 23.83); and for women on HRT (n = 452), it was 17.07 μg/L (13.91 and 20.68) (P for difference in mean between all 3 groups = <0.0001). Additionally, when adding HRT status to the multivariable model, we found that HRT was strongly associated with log(sST2) (regression coefficient −0.353, SE 0.052, P < 0.001).
To explore the association with diabetes, we created additional models. Concentrations of CRP [standardized log(CRP), added to the main sex-pooled model] were significantly associated with log(sST2) (regression coefficient 0.024, SE 0.007, P < 0.0006), but standardized log(triglyceride) concentrations were not (regression coefficient −0.005, SE 0.008, P = 0.54). Presence of metabolic syndrome was not associated with log(sST2) (regression coefficient 0.004, SE 0.016, P = 0.82). In a separate model, we replaced BMI with waist circumference (WC). In this model, WC was also significantly associated with log(sST2) concentrations (regression coefficient 0.003, SE 0.001, P = 0.03); however, when added to the model in addition to BMI, WC was not independently significant.
In an analysis of the correlates of pulmonary function tests and asthma, although log(sST2) was significantly associated with percent predicted FEV1 (regression coefficient −0.022, SE 0.009, P = 0.01) and percent predicted forced vital capacity (FVC) (regression coefficient −0.206, SE 0.008, P < 0.001), it was not associated with the percent predicted FEV1/FVC ratio (regression coefficient 0.002, SE 0.005, P = 0.71) or a clinical diagnosis of asthma (regression coefficient 0.176, SE 0.176, P = 0.321). A 2-SD increase change in log(sST2) is associated with a 1.5% decline in the per-cent predicted FEV1 and a 1.8% decline in the percent predicted FVC.
REFERENCE LIMITS
Given the important effect of sex on sST2 values, we generated sex-specific reference limits for the biomarker. The sex- and age-specific 2.5th-, 50th-, 97.5th-, and 99th-percentile reference limits (as calculated by empirical and quantile regression methods) for sST2 are presented in Table 3. The similarity between the empirical and quantile methods is particularly notable in the 2.5th- and 50th-percentile reference limits, whereas the empirical and quantile methods diverged in the 97.5th percentile in women and in the 99th percentile for men (Fig. 2). This variability may relate to subgroup size. sST2 concentrations were generally higher in men than women. Both sexes showed small increases in sST2 with advancing age, but a steep increase in sST2 concentrations in older women resulted in similar empirical percentiles between sexes above age 65 years.
Table 3.
Reference limits for sST2 (ng/mL) by sex and age.
| Age group, years | Men, percentile
|
Women, percentile
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2.5th | 50th | 97.5th | 99th | 2.5th | 50th | 97.5th | 99th | |
| Empirical reference limits | ||||||||
|
| ||||||||
| 35–44 | 10.6 | 22.9 | 47.6 | 49.3 | 10.4 | 17.1 | 33.2 | 45.9 |
|
| ||||||||
| 45–54 | 11.5 | 22.3 | 43.7 | 64.4 | 9.8 | 17.7 | 30.7 | 36.7 |
|
| ||||||||
| 55–64 | 12.4 | 22.7 | 43.3 | 46.4 | 9.9 | 17.5 | 34.3 | 39.3 |
|
| ||||||||
| 65–74 | 13.2 | 24.5 | 45.2 | 54.7 | 9.3 | 19.2 | 45.1 | 53.0 |
|
| ||||||||
| Quantile regression reference limits | ||||||||
|
| ||||||||
| 35–44 | 10.3 | 21.3 | 46.5 | 46.7 | 10.2 | 16.6 | 29.4 | 29.5 |
|
| ||||||||
| 45–54 | 11.2 | 22.0 | 45.8 | 48.7 | 10.0 | 17.2 | 31.2 | 34.0 |
|
| ||||||||
| 55–64 | 12.1 | 22.8 | 45.2 | 50.8 | 9.8 | 17.8 | 33.2 | 39.3 |
|
| ||||||||
| 65–74 | 13.1 | 23.6 | 44.6 | 53.0 | 9.6 | 18.5 | 35.3 | 45.3 |
Fig. 2.
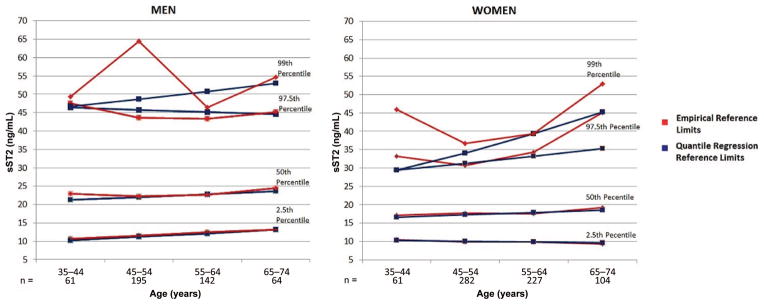
2.5th, 50th, and 97.5th percentiles of ST2 for men and women in the reference sample: quantile and empirical approaches.
Discussion
Several recent studies have highlighted the biological and clinical importance of sST2 in a broad range of individuals, including those with heart failure syndromes (9 –14), acute myocardial infarction (6 – 8), and other medical conditions (15, 16). However, the vast majority of studies have considered sST2 in the context of disease, whereas limited data have been published regarding concentrations of the biomarker in healthy individuals. As use of this biomarker increases, it is critical to understand the normal variability in sST2 concentrations and establish reference values. One previous issue limiting the ability to perform such an important study was the fact that prior methods for sST2 measurement were insensitive and imprecise in patients with very low concentrations of the biomarker. This issue has been surmounted with the development of a highly-sensitive ELISA method for measurement of sST2 (19).
Accordingly, in a well-characterized community-based cohort, we measured sST2 using the Presage ST2 assay and, remarkably, found that circulating concentrations of the biomarker are universally detectable in apparently-healthy individuals. The strongest correlates of sST2 in this cohort were age, sex, and presence of diabetes, whereas many variables such as renal function or BMI that influence other biomarker concentrations were not important correlates of sST2. Additionally, in contrast to prior studies, we did not find that ST2 was associated with a clinical diagnosis of asthma, though sST2 values did have modest correlation to pulmonary function testing, which is of unclear relevance. Last, we established age- and sex-based reference limits for sST2, which offers utility for both clinical and research applications.
CLINICAL CORRELATES OF sST2 IN A GENERAL SAMPLE
An important finding of our study was that age and sex represent important determinants of “normal” sST2 concentrations. Heterogeneity of the biomarker in women was characterized by lower values in women compared with men and an age-associated rise in sST2 among older women, although even at older age, men still had higher sST2 values than women. This finding suggests that there are potentially important effects of sex on sST2 concentrations, with women demonstrating lower values than age-matched men. This trend may represent the effects of sex hormones: when we stratified analyses by estrogen replacement status, women taking estrogen had the lowest concentrations of sST2. In contrast, Dieplinger et al. (30) did not find any clear association between measured sex hormone concentrations and sST2 concentrations in a study of healthy blood donors, albeit in a smaller study cohort. Adiposity is an important factor to consider in this context, given the production of sex hormones by adipose tissue. In the general sample, the effect of BMI appeared somewhat sex specific, with an association in women only; however, this relationship was not evident in the reference sample. It is worth noting that these relatively minor effects of BMI are considerably less significant than what has been reported in this cohort for natriuretic peptide concentrations (31).
Interestingly, we found sST2 to be associated with blood pressure (systolic pressure in men and use of antihypertensive medications in women). In patients with heart failure, Rehman et al. (12) reported a similar association between hypertension and sST2. Mechanistically, this may reflect cardiac production related to strain: Bartunek et al. (32) found that sST2 was strongly associated with diastolic load in healthy subjects, as well as in patients with aortic stenosis or cardiomyopathy. In the study by Bartunek et al., ST2 production was found in myocardium as well as venous and arterial endothelial cells. Thus, sST2 may be a marker of the vascular effects associated with increased afterload and myocardial stress. Further studies examining sST2 concentrations and direct measurements of vascular and endothelial function are needed to better understand this relationship.
A striking finding was the association of sST2 with diabetes mellitus in both men and women. To investigate this relationship further, we created models that included covariates that are associated with diabetes. WC and CRP but not triglyceride concentration were correlated with sST2. CRP and WC are associated with other cardiometabolic risk markers, such as hypertension, cholesterol, and insulin resistance [as measured by homeostatic model assessment–insulin resistance (HOMA-IR)] (33). The association with diabetes and other cardiometabolic risk markers suggests that sST2 may also be a marker of cardiometabolic risk. The pathways by which this occurs are not known, although animal studies have shown that sST2 signaling may be important in modulating the autoimmune effects on the pancreas associated with diabetes (34). More investigation is needed before firm conclusions can be made; however, it is reasonable to infer that the relationship between diabetes and sST2 might represent a signal of potential cardiometabolic risk.
We observed an association of sST2 with FEV1 and FVC, but not with the FEV1/FVC ratio or a clinical diagnosis of asthma. Therefore, our data suggest that sST2 is associated with restrictive pulmonary physiology but not obstructive physiology. We do not have additional data to determine if participants with restrictive physiology by pulmonary function tests had subclinical evidence of pulmonary fibrosis, but other investigators have found that ST2 concentrations are increased during acute exacerbations of symptomatic pulmonary fibrosis (35). Prior studies have reported an association between sST2 concentrations and atopic asthma (15). Differing definitions of asthma and lack of radioallergosorbent testing in the current study may have contributed to these discrepant results. The mechanism by which elevation of sST2 occurs in both pulmonary fibrosis and asthma has been suggested to be Th2 cell proliferation via tumor necrosis factor-α, IL-1, IL-4, IL-5, IL-13, and IL-1β (15, 35).
REFERENCE LIMITS
We determined sST2 reference limits using both empirical and quantile regression approaches, the latter used to minimize the influence of outlier values. There was excellent agreement in the 2 approaches for the 2.5th- and 50th-percentile values, but more variability for the 97.5th percentile was noted in women, consistent with an effect of outliers in the empirical approach. Our reference values are similar to those of Lu et al. (36) but higher than those reported by Dieplinger et al. (19). Our more extensive criteria for excluding individuals with cardiovascular risk factors or subclinical LV dysfunction may have accounted for some of the differences. Indeed, a major strength of our study is the well-characterized population that made up our reference sample and the large size of our cohort compared with other studies. In addition to clinical history, diagnostic testing with routine electrocardiograms and echocardiograms allowed us to minimize inclusion of individuals with subclinical cardiovascular dysfunction in the reference sample.
sST2 values previously linked with a higher risk for cardiovascular outcomes in patients with established heart disease (e.g., ≥35 μg/L) (13) generally represented values at or above the 95th-percentile concentration in our general population. This observation implies that, much like other biomarkers of risk in the general population (such as highly sensitive troponin), there are “apparently well” subjects with values of sST2 associated with considerable risk. Recently published work from this cohort confirms that sST2 (alone or in combination with other cardiovascular biomarkers) predicts incident cardiovascular disease (37). More data are needed to better understand this observation. In-depth echocardiographic analyses are underway in this cohort and will be examined as a separate effort.
LIMITATIONS
Our study is limited in that we examined a population cohort that was primarily white, and there were few elderly individuals in the cohort. Thus, our results cannot necessarily be generalized to individuals outside this demographic or age range. That said, the quality of the data from our cohort is well established for studies such as ours, and a major strength of the analysis is the size and well-characterized nature of the cohort, as noted above.
Conclusions
We have demonstrated that sST2 concentrations are associated with sex, age (in women), systolic blood pressure (more notably in men), use of antihypertensive medication, and diabetes in a sample of individuals without heart failure. We have also established reference intervals for sST2 using a highly sensitive assay. Our results imply that when using the Presage ST2 assay, it would be expected that concentrations of the biomarker are detectable in most (and likely all) apparently healthy individuals; for measurement in such individuals, upper reference limits might be adjusted for age and sex. Because sST2 has been established as a potentially useful biomarker for risk prediction in patients with established heart disease, our data are important, as they may now allow for investigations of the potential role of sST2 testing in a considerably broader, community-based population.
Acknowledgments
Measurement of sST2 was performed by Critical Diagnostics, Inc. This work was completed during E.E. Coglianese’s tenure as a Research Fellow of the Heart Failure Society of America.
Footnotes
Nonstandard abbreviations: IL, interleukin; ST2L, ST2 ligand; sST2, soluble ST2; LV, left ventricular; Th2, type 2 helper; GFR, glomerular filtration rate; FEV1, forced expiratory volume in 1 s; BMI, body mass index; ECG, electrocardiogram; CRP, C-reactive protein; HRT, hormone replacement therapy; WC, waist circumference; FVC, forced vital capacity; HOMA-IR, homeostatic model assessment–insulin resistance.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: T.J. Wang, Roche, Quest, Diasorin.
Research Funding: This study was supported by N01-HC-25195, a contract to the Framingham Heart Study. S. Cheng, grant K99HL107642 and the Ellison Foundation; T.J. Wang, R01-HL-086875; J.J. Januzzi, endowment from the Roman W. DeSanctis Clinical Scholar Fund.
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.Weinberg EO. ST2 protein in heart disease: from discovery to mechanisms and prognostic value. Biomark Med. 2009;3:495–511. doi: 10.2217/bmm.09.56. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538 – 49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–91. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 5.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 7.Eggers KM, Armstrong PW, Califf RM, Simoons ML, Venge P, Wallentin L, James SK. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J. 2010;159:788–94. doi: 10.1016/j.ahj.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, McInnes IB, Dargie HJ, McMurray JJ. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55:243–50. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Boisot S, Beede J, Isakson S, Chiu A, Clopton P, Januzzi J, Maisel AS, Fitzgerald RL. Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Card Fail. 2008;14:732–8. doi: 10.1016/j.cardfail.2008.06.415. [DOI] [PubMed] [Google Scholar]
- 10.Bayes-Genis A, Pascual-Figal D, Januzzi JL, Maisel A, Casas T, Valdes Chavarri M, Ordonez-Llanos J. Soluble ST2 monitoring provides additional risk stratification for outpatients with decompensated heart failure. Rev Esp Cardiol. 2010;63:1171–8. doi: 10.1016/s1885-5857(10)70231-0. [DOI] [PubMed] [Google Scholar]
- 11.Manzano-Fernandez S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259 – 67. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458 – 65. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 13.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–7. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–9. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–81. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Rumayor A, Camargo CA, Green SM, Baggish AL, O’Donoghue M, Januzzi JL. Soluble ST2 plasma concentrations predict 1-year mortality in acutely dyspneic emergency department patients with pulmonary disease. Am J Clin Pathol. 2008;130:578 – 84. doi: 10.1309/WMG2BFRC97MKKQKP. [DOI] [PubMed] [Google Scholar]
- 17.Hoogerwerf JJ, Tanck MW, van Zoelen MA, Wittebole X, Laterre PF, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36:630–7. doi: 10.1007/s00134-010-1773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30:1468–73. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 19.Dieplinger B, Januzzi JL, Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma: the Presage ST2 assay. Clin Chim Acta. 2009;409:33–40. doi: 10.1016/j.cca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179 – 87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009;5:e1000429. doi: 10.1371/journal.pgen.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 25.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, et al. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718–26. doi: 10.2337/db07-0078. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 29.Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study) Am J Cardiol. 2011;108:1341–5. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieplinger B, Egger M, Poelz W, Gabriel C, Haltmayer M, Mueller T. Soluble ST2 is not independently associated with androgen and estrogen status in healthy males and females. Clin Chem Lab Med. 2011;49:1515–8. doi: 10.1515/CCLM.2011.239. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594 – 600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 32.Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008;52:2166–74. doi: 10.1016/j.jacc.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen I. Influence of age on the relation between waist circumference and cardiometabolic risk markers. Nutr Metab Cardiovasc Dis. 2009;19:163–9. doi: 10.1016/j.numecd.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Zdravkovic N, Shahin A, Arsenijevic N, Lukic ML, Mensah-Brown EP. Regulatory T cells and ST2 signaling control diabetes induction with multiple low doses of streptozotocin. Mol Immunol. 2009;47:28–36. doi: 10.1016/j.molimm.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–14. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Snider JV, Grenache DG. Establishment of reference intervals for soluble ST2 from a United States population. Clin Chim Acta. 2010;411:1825–6. doi: 10.1016/j.cca.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596 – 604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]