Abstract
Loliginid and sepiolid squid light organs are known to host a variety of bacterial species from the family Vibrionaceae, yet little is known about the species diversity and characteristics among different host squids. Here we present a broad-ranging molecular and physiological analysis of the bacteria colonizing light organs in loliginid and sepiolid squids from various field locations of the Indo-West Pacific (Australia and Thailand). Our PCR-RFLP analysis, physiological characterization, carbon utilization profiling, and electron microscopy data indicate that loliginid squid in the Indo-West Pacific carry a consortium of bacterial species from the families Vibrionaceae and Photobacteriaceae. This research also confirms our previous report of the presence of Vibrio harveyi as a member of the bacterial population colonizing light organs in loliginid squid. pyrH sequence data were used to confirm isolate identity, and indicates that Vibrio and Photobacterium comprise most of the light organ colonizers of squids from Australia, confirming previous reports for Australian loliginid and sepiolid squids. In addition, combined phylogenetic analysis of PCR-RFLP and 16S rDNA data from Australian and Thai isolates associated both Photobacterium and Vibrio clades with both loliginid and sepiolid strains, providing support that geographical origin does not correlate with their relatedness. These results indicate that both loliginid and sepiolid squids demonstrate symbiont specificity (Vibrionaceae), but their distribution is more likely due to environmental factors that are present during the infection process. This study adds significantly to the growing evidence for complex and dynamic associations in nature and highlights the importance of exploring symbiotic relationships in which non-virulent strains of pathogenic Vibrio species could establish associations with marine invertebrates.
Introduction
The family Vibrionaceae (gamma-proteobacteria) is a highly diverse group containing both symbiotic and free-living species [1]. Vibrionaceae is comprised of seven main genera, including Vibrio, Listonella, Photobacterium, Enterovibrio, Aliivibrio, Grimontia, and Salinivibrio [2], although recent debates question the overall systematic classification [3]. Vibrios are highly abundant in aquatic environments, where they actively participate in the re-cycling of nutrients and detritus [4]. In addition, a number of luminescent symbionts play a key role in antipredatory behaviors documented in a number of marine organisms [5–7].
Members of the family Vibrionaceae have been frequently detected and isolated from freshwater, estuarine, and marine habitats [8, 9]. Several species such as Vibrio fischeri [10, 11], Vibrio logei [7], Vibrio harveyi [12, 13], and Photobacterium leiognathi [14] play important ecological roles because of their life history strategies, including both mutualistic associations with marine organisms and free-living planktonic lifestyles. Moreover, the genus Vibrio encompasses several pathogens of humans (e.g., Vibrio cholerae [15, 16], Vibrio parahaemolyticus [17–22], and Vibrio vulnificus [23, 24]) as well as other eukaryotic organisms. Some of these pathogens are known to attach to surfaces of live marine animals without causing disease to their invertebrate host. Examples of such associations include V. cholerae and its copepod host, which constitutes an important factor in the epidemiology of cholera disease [16], as well as V. harveyi, which causes disease in marine animals, producing mass mortalities in shrimp farms around the world (luminous vibriosis [25]) and also infecting pearl oysters, fish, seahorses, and lobsters [26]. However, V. harveyi is also found mutualistically with the hydrozoan Aglaophenia octodonta [13] and fish light organs [27]. Similarly to V. cholerae, these non-pathogenic associations are likely to play a role in the epidemiology of vibriosis by V. harveyi.
Understanding bacterial diversity in natural environments is of pivotal importance because this information provides a phylogenetic framework to clarify the degree of variation among species in a particular environment. Similarly, characterizing microbial populations is also essential to helping define the structure and diversity of a particular community of microorganisms [28, 29]. Because of the large fraction of non-culturable microbes in nature, establishing these parameters via conventional culture-dependent, physiology-based methods has serious limitations [30]. However, complementing culture-based with molecular methods is an excellent approach to elucidate the nature of bacterial communities, without acquiring the large costs affiliated with wide-scale, genomic-based approaches.
In the specific case of squid symbionts, light organ homogenate, spread-plate cultures yield a great number of luminescent colonies very similar to each other in shape, color, size, and texture [12], which makes them difficult to identify without a combination of microbiological and genetic approaches. Recent studies by our group that explore the diversity of bacterial isolates colonizing light organs of loliginid squids in Thailand have provided evidence that colonization is achieved by multiple species of Vibrio [12, 31], including V. harveyi, constituting the first report of a marine pathogen in a molluscan mutualism. However, further studies were not implemented to determine the physiological characteristics of these isolates. Here, we report the physiological characterization of Thailand isolates and the results of comparative studies of 16S ribosomal RNA genes using polymerase chain reaction (PCR) in combination with restriction fragment length polymorphism (RFLP). To better characterize variation among loliginid symbionts, we used PCR-RFLP of the 16S rRNA locus to type and identify marine Vibrios associated with light organs of squids in the family Loliginidae (Mollusca: Cephalopoda) from Australian and Thai locations. This method has proven to be time and cost efficient, and is increasingly used as a standard technique to address questions regarding the ecology, distribution, and biodiversity of natural isolates of bacteria [32–38]. Additionally, a battery of microbiological assays were completed in parallel to type and identify isolates through culture based tests including Gram stain, light production (luminescence), growth on thiosulfate/citrate/bile salts (TCBS) agar, and growth on seawater tryptone (SWT) agar at various temperatures in addition to phenotypes of each isolate through electron microscopy.
Materials and Methods
Bacterial Strains, Growth Conditions, and DNA Extraction
Bacterial strains used in this study are listed in Table 1. To isolate bacteria from squid light organs, ten specimens from each location were captured by trawl netting for dissection and their light organs removed and homogenized in sterile seawater [39]. Collection sites in the Indo-West Pacific (a zoogeographical region including the Indian and Pacific oceans) are indicated in Table 1. Serial tenfold dilutions (1/10,000) of the homogenate were plated on seawater tryptone agar (SWT; 70 % seawater v/v, 0.5 % tryptone w/v, 0.3 % yeast extract w/v, 0.3 % glycerol v/v and 15 % technical grade agar) and grown at 28 °C for 16 h. Individual colonies of luminous bacteria were isolated and used to inoculate 5 mL of SWT broth and incubated for 18 h at 250 revolutions per minute (rpm). An aliquot (900 µL) of the resulting culture was combined with the same volume of 40 % glycerol to be stored at −80 °C for further studies.
Table 1.
Environmental and laboratory isolates used in this study
| Strain Name | Squid host or source | Location |
|---|---|---|
| Group A | Uroteuthis chinensis | Cairns, QLD, Australia |
| Group B | Uroteuthis etheriogei | Townsville, QLD, Australia |
| Group C | Photololigo noctiluca | Sydney, NSW, Australia |
| Vibrio fischeri CG101 | Cleidopus gloriamaris | Townsville, QLD, Australia |
| Vibrio fischeri ET101 | Euprymna tasmanica | Crib Point, VIC, Australia |
| Vibrio fischeri ETJB | Euprymna tasmanica | Jervis Bay, NSW, Australia |
| Vibrio fischeri ES915 | Euprymna scolopes | Paiko, O’ahu, Hawaii, USA |
| Vibrio fischeri MJ101 | Monocentris japonica | Tokyo, Japan |
| Vibrio fischeri SL518 | Sepiola ligulata | Banyuls-sur-mer, France |
| Vibrio fischeri SR5 | Sepiola robusta | Banyuls-sur-mer, France |
| Vibrio fischeri WH1 | Free-living | Woods Hole, MA |
| Vibrio fischeri VLS2 | Euprymna scolopes | Kaneohe Bay, O’ahu, Hawaii, USA |
| Vibrio fischeri ES191 | Euprymna scolopes | Paiko, O’ahu, Hawaii, USA |
| Photobacterium phosphoreum | Laboratory strain | ATCC 11004 |
| Photobacterium leiognathi | Laboratory strain | ATCC 25521 |
| Photobacterium leiognathi RM1 | Rondeletiola minor | Banyuls-sur-mer, France |
| Photobacterium leiognathi LN101 | Uroteuthis noctiluca | Sydney, NSW, Australia |
| Vibrio fischeri PP3 | Free-living | Kaneohe Bay, O’ahu, Hawaii, USA |
| Vibrio fischeri PP42 | Free-living | Kaneohe Bay, O’ahu, Hawaii, USA |
| Vibrio anguillarum | Laboratory strain | ATCC 19264 |
| EHP group | Euprymna hyllebergi | Phuket, Thailand |
| UCP group | Uroteuthis chinensis | Phuket, Thailand |
| UCR group | Uroteuthis chinensis | Rayong, Thailand |
| UDP group | Uroteuthis duvauceli | Phuket, Thailand |
QLD Queensland, NSW New South Wales, VIC Victoria
Total 16S rRNA Gene Amplification and Sequencing from Bacterial Isolates
Isolates were recovered from glycerol stocks by growing them overnight on SWT agar at 28 °C. An individual colony was recovered from each plate and inoculated in 5 mL of SWT broth and incubated overnight on a shaking incubator (250 rpm) at 28 °C. Genomic DNA was isolated from these liquid cultures using the DNAeasy Isolation Kit (Qiagen®, Valencia, CA). Concentration and purity of genomic DNA was estimated with a Thermo Scientific NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA). DNA integrity was validated by 1 % agarose gel electrophoresis in 1× TAE buffer (40 mM Tris–acetate, 1 mM EDTA, pH 8.0).
16S rRNA amplification and sequencing was completed using universal primers 16SF (5′-GCAAGCCTGATGCAGCCATG-3′) and 16SR (5′-ATCGTTTACGGCGTGGACTA-3′) at a 0.2 mM concentration per reaction. PCR and sequencing reactions were completed in a DNA peltier thermal cycler (MJ Research, Inc., Watertown, MA). Amplification reactions were executed using 0.05 U/µL of Amplitaq Gold (Applied Biosystems, Foster City, CA) and consisted of an initial hot start at 94 °C for 2 min followed by 29 cycles of: 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. After cycling, the process was terminated at 72 °C for 7 min. Each PCR reaction mix also contained 2.5 mM of MgCl2, 0.5 mM dNTPs (25 µM each, Promega, Madison, WI) and 0.05 U/µL of Taq DNA Polymerase (Promega, Madison, WI), and 10× reaction buffer (10 mM Tris–HCl, pH 9.0, 50 mM KCl, and 0.1 % Triton X-100). PCR reactions yielded a gene product of about 1,500 bp when analyzed through gel electrophoresis.
16S rDNA amplicons were purified from primers and unincorporated nucleotides using the GeneClean® II DNA purification kit (Bio 101, Carlsbad, CA) and used for subsequent applications. Sequencing reactions were executed by the dideoxy chain termination method using the Big Dye™ Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Sequences were obtained through the ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA) and edited using Sequencher v4.6 (Gene Codes Corporation, Ann Arbor, MI). DNA sequences were then compared with the National Center for Biotechnology Information (NCBI) database using BLAST 2.2.11 (Basic Local Alignment Search Tool, NCBI, NLM, NIH, Bethesda, MD) for initial identification of bacterial isolates.
PCR Amplification and Sequencing of Partial 16S rRNA Gene and Uridilate Kinase Gene (Pyrh) for Species Identification
Amplification of partial 16S rRNA gene was completed using primers 16S2F (5′-GCAAGCCTGATGCAGCCATG-3′) and 16S3R (5′-ATCGTTTACGGCGTGGACTA-3′) in a DNA thermal cycler (MJ Research, Inc., Watertown, MA). PCR conditions were the following for both genes: hot start at 94 °C for 2 min, followed by 25 cycles of: 94 °C for 2 min, 45 °C for 1.5 min and 72 °C for 2 min. A final termination step at 72 °C for 8 min completed the process. PCR component concentrations were the same as previously stated.
16S rRNA and pyrH amplicons were purified and sequenced as mentioned above. DNA sequences were then compared with the NCBI database using BLAST 2.2.11 for initial confirmation of sequence identity. Upon confirmation, partial 16S rRNA gene sequences were incorporated in the combined phylogenetic analysis described below.
Restriction Fragment Length Polymorphism (RFLP) Analysis
RFLP analysis was completed as described by Urakawa et al. [33, 35] using three restriction endonucleases: RsaI (5′GTAC3′), HhaI (5′GCGC3′) and DdeI (5′CTNAG3′; Promega Corporation, Madison, WI). Fragments were separated through gel electrophoresis at 2 V/cm in a 1.5 % agarose gel in 0.5× TAE buffer (20 mM Tris acetate, and 0.5 mM EDTA). The BenchTop 1 Kb Ladder was used as a molecular DNA marker (Promega Corporation, Madison, WI). Image analysis and estimation of fragment size were completed with the Kodak Molecular Imaging software v5.0 (Carestream Health Inc., Rochester, NY). Informative electrophoresis bands derived from restriction endonucleases digestion were scored for presence or absence and entered into a migration distance matrix to determine specific banding patterns for each enzyme. PCR-RFLP restriction pattern, presence–absence matrix, and 16S rRNA gene sequence data were analyzed using the direct optimization method described by Wheeler [40, 41] and implemented in the computer program POY [42, 43]. Previously sequenced 16S rRNA gene sequences were retrieved from GenBank.
Phenotypic Characterization of Bacterial Isolates
Bacterial squid isolates were phenotypically identified following the schemes of Alsina and Blanch [44] and Farmer et al. [2]. Physiological and morphological tests were completed and are listed in Table 2.
Table 2.
Results of physiological and morphological assays of Vibrio harveyi squid isolates
| Characteristic assay |
Thailand bacterial isolates |
Vibrio harveyia |
Results from Stabili et al. [13] |
Results from Farmer III et al. [2] | ||||
|---|---|---|---|---|---|---|---|---|
|
Vibrio harveyi |
Vibrio fischeri |
Vibrio alginolyticus |
Vibrio campbelli |
Vibrio damsela |
||||
| Gram reaction | − | − | − | − | − | − | − | − |
| Cell morphology | r | r | r | r | r | r | r | r |
| Luminescence | + | + | + | d | + | − | − | − |
| 0/129 sensitivity | ||||||||
| 10 µg | + | + | + | d | + | d | nd | + |
| 150 µg | + | + | + | + | + | d | nd | + |
| Growth in 0 % NaCl | − | − | − | − | − | − | − | − |
| Growth in 3 % NaCl | + | + | + | + | + | + | + | + |
| Growth in 8 % NaCl | + | + | + | + | − | + | + | − |
| Growth at 4 °C | − | − | − | − | − | − | − | nd |
| Growth at 30 °C | + | + | + | + | + | + | + | + |
| Growth at 35 °C | + | + | + | + | d | + | + | + |
| Oxidation/fermentation | F | F | F | F | nd | nd | nd | nd |
| Decarboxylase (NaCl)b | ||||||||
| Arginine | + | − | − | − | − | − | − | + |
| Lysine | − | − | + | + | + | + | + | + |
| Ornithine | + | + | + | + | − | − | − | − |
| Acid from Inositol | − | − | nd | nd | nd | nd | nd | nd |
| Acid from Arabinose | − | − | nd | nd | nd | nd | nd | nd |
| Acid from Sucrose | − | − | nd | nd | nd | nd | nd | nd |
| Oxidase | + | + | + | + | + | + | + | + |
| Catalase | + | + | + | + | +c | nd | nd | nd |
| Voges–Proskauer | − | − | − | − | − | + | − | + |
| Indole | + | + | + | + | − | + | + | − |
| Gelatinase | + | + | + | + | − | + | + | − |
| Lipase | − | − | + | + | + | + | + | − |
| Citrate | − | − | − | + | d | + | d | − |
| Carbon sources | ||||||||
| l-Arabinose | − | − | − | d | − | − | − | − |
| Mannose | + | + | + | + | + | d | d | + |
| Cellobiose | + | + | + | + | + | − | d | − |
| Glucose | + | + | + | nd | nd | nd | nd | nd |
| Galactose | d | + | − | d | + | d | − | nd |
| Trehalose | + | + | + | + | d | + | + | − |
| Melibiose | − | − | − | − | − | − | − | − |
| Lactose | − | − | − | − | − | − | − | − |
| Mannitol | + | + | + | + | + | + | d | − |
| Sorbitol | − | − | − | − | − | − | − | − |
| Inositol | − | − | − | − | − | − | − | − |
| Sucrose | − | + | − | d | − | + | − | − |
| Identification | Vibrio harveyi | Vibrio harveyi | Vibrio harveyi | |||||
d diverse, nd no data, + positive reaction, − negative reaction, F fermentative, r rod shape
Vibrio harveyi ATCC 14126
(NaCl) indicates that NaCl was added to the standard media to enhance growth
Visick and Ruby [63]
Observation of Bacterial Cellular Morphology
Isolates from individual bacterial colonies were used to inoculate 5 mL of SWT media and grown overnight at 28 °C in a shaking incubator (250 rpm). Bacterial samples were prepared using a technique modified from Allen and Baumann [45] for examination of cell appendages by transmission electron microscopy. Briefly, 5 µL of culture was added onto a 200-mesh Formvar coated nickel grid (Electron Microscopy Sciences, Hartfield, PA) and allowed to sit for 10 s. Excess culture media was blotted dry with filter paper. This was followed by the addition and removal of 5 µL of distilled water, which provided the initial wash. Staining was completed with a 1 % aqueous solution of uranyl acetate for 10 s. Excess stain was removed and the grid was allowed to air dry. Conversely, cells grown in solid media were harvested by addition of 5 µL of sterile seawater directly on solid agar media, and immediately homogenized by slow pipetting. Five microliters of the homogenate was collected and processed as mentioned previously. Micrographs were obtained using a Hitachi H-7650 (Hitachi High Technologies America, Pleasanton, CA) transmission electron microscope (TEM) at an accelerating voltage of 80 kV.
Accession Numbers
The 16S rRNA and pyrH gene sequences determined in this study were deposited in GenBank and are listed in Tables 3 and 4.
Table 3.
16S rRNA sequence information from Thailand Vibrio harveyi isolates from loliginid squid light organs
| Species name | Squid host | Isolate name | Location | Accession number |
|---|---|---|---|---|
| Vibrio harveyi | Uroteuthis chinensis | UCP6 | Phuket, Thailand | AY332404 |
| Vibrio harveyi | Uroteuthis chinensis | UCP8 | Phuket, Thailand | FJ227109 |
| Vibrio harveyi | Uroteuthis chinensis | UCP9 | Phuket, Thailand | FJ227110 |
| Vibrio harveyi | Uroteuthis chinensis | UCP10 | Phuket, Thailand | FJ227111 |
| Vibrio harveyi | Euprymna hyllebergi | EHP6 | Phuket, Thailand | FJ227112 |
| Vibrio harveyi | Euprymna hyllebergi | EHP7 | Phuket, Thailand | FJ227113 |
| Vibrio harveyi | Euprymna hyllebergi | EHP8 | Phuket, Thailand | FJ227114 |
| Vibrio harveyi | Euprymna hyllebergi | EHP9 | Phuket, Thailand | FJ227115 |
| Vibrio harveyi | Euprymna hyllebergi | EHP10 | Phuket, Thailand | FJ227116 |
| Vibrio harveyi | Euprymna hyllebergi | EHP11 | Phuket, Thailand | FJ227117 |
| Vibrio harveyi | Euprymna hyllebergi | EHP12 | Phuket, Thailand | FJ227118 |
| Vibrio harveyi | Euprymna hyllebergi | EHP13 | Phuket, Thailand | FJ227119 |
UCP6 16S rRNA sequence from Guerrero-Ferreira and Nishiguchi [12]
Table 4.
Species identification of Australian isolates based on protein BLAST of pyrH gene sequences. Species names correspond to the highest score of significant alignment using BLAST
| Isolate name | Squid host | Symbiont species identification | Accession number |
|---|---|---|---|
| A1-1 | Uroteuthis chinensis | Vibrio harveyi | HQ226045 |
| A1-5 | Uroteuthis chinensis | Photobacterium angustum/P. leioghnati [64] | HQ226046 |
| A1-6 | Uroteuthis chinensis | P. angustum/P. leioghnati | HQ226047 |
| A2-1 | Uroteuthis chinensis | V. harveyi | HQ226048 |
| B1-1 | Uroteuthis etheriogei | V. cyclitrophicus | HQ226049 |
| C1-1 | Photololigo noctiluca | P. angustum/P. leioghnati | HQ226050 |
| C2-7 | Photololigo noctiluca | V. cyclitrophicus/V. fischeri | HQ226051 |
| C3-5 | Photololigo noctiluca | P. angustum/P. leioghnati | HQ226052 |
| C4-23 | Photololigo noctiluca | V. harveyi | HQ226053 |
| C5-10 | Photololigo noctiluca | V. cyclitrophicus | HQ226054 |
Results and Discussion
Phenotypic Characterization of Thailand Squid Bacterial Isolates
A number of isolates from Thailand loliginid squids had been previously identified as members of the genus Vibrio on the basis of their 16S rRNA gene sequence [12] (Table 3). However, these isolates exhibited sequence similarities of 98 % or higher to the 16S rRNA gene sequence of Vibrio alginolyticus [46], V. harveyi [46], and Vibrio charchariae (synonym of V. harveyi) [46]. This high percent of sequence similarity did not allow for the specific identification of these isolates solely using their 16S rRNA gene sequence.
We carried out additional tests for physiological, morphological, and biochemical characterization of Thai isolates (Table 1) which provided a more precise species identification. Results from these assays are shown in Table 2. The isolates surveyed belonged to a single species on the basis of their biochemical, physiological, and morphological analysis and were definitively identified as V. harveyi. Previous research by Dunlap et al. [27] provided similar evidence of the presence of V. harveyi in the light organ of the marine fish Nuchequula nuchalis (Perciformes: Leiognathidae) identified by luxA sequences. However, our research constitutes the first report confirming the presence of V. harveyi as a member of the bacterial population colonizing light organs in loliginid squid.
All Thailand isolates were found to be Gram-negative, luminescent rods, sensitive to the vibriostatic agent 0/129 (at both 10 and 150 µg). These isolates were unable to grow in liquid media without sodium chloride, and exhibited no growth at 4 °C in SWT. As shown in Table 2, the results are consistent with the characteristics of a laboratory strain incorporated in this analysis, as well as other V. harveyi laboratory [2] and natural [13] isolates. Similar results were also attained with the oxidase, catalase, Voges–Proskauer, indole, and gelatinase tests. Uniformity between isolates was also achieved in the output of carbon utilization profiles. When compared with other isolates of V. harveyi, Thailand strains were equally capable of utilizing l-arabinose, mannose, cellobiose, glucose, trehalose, melibiose, lactose, mannitol, sorbitol, and inositol as unique carbon sources (Table 2).
Interestingly, some differences were evident regarding the ability of individual isolates to produce the enzymes lysine and arginine decarboxylase when compared to a laboratory strain of V. harveyi, which is pathogenic in marine environments. Similar results have been reported for lysine decarboxylase in V. harveyi, where contradictory results were obtained in tests from the Centers for Disease Control and Prevention Vibrio reference lab using standardized enteric media supplemented with marine cations [2]. This may be due to the particular environmental niche each isolate has adapted to, despite being from the same species.
The presence of arginine decarboxylase (ADC) in strains isolated from loliginid squid light organs (Table 2) may be indirectly related with the formation of bacterial biofilms within squid tissues of the light organ, an important factor for successful colonization. ADC is responsible for catalytic reactions occurring in alternative pathways for the synthesis of putrescine, a precursor of many polyamines [47]. In bacteria, they play a significant role in the formation of biofilms. Patel et al. [48] demonstrated that polyamines are responsible for the formation of biofilms by Yersinia pestis. Similarly, polyamines are also responsible for the modulation of bacterial biofilms within Vibrionaceae species. For example, Karatan et al. [49] reported that formation of biofilms by V. cholerae is activated by an increase in the environmental concentration of norspermidine, a polyamine. Most importantly, the gene for ADC was previously found to be expressed solely by symbiotic V. fischeri ETJB1A in the light organ of the sepiolid squid Euprymna tasmanica [50, 51]. This indicates that ADC expression is highly specific during growth and persistence of V. fischeri in the light organ, suggesting that this gene has an important role in establishing and maintaining the symbiosis. For instance, some Vibrio species also use ADC to regulate pH, which may be linked to the shift between aerobic and fermentative states while colonizing the sepiolid light organ [50]. The source of the V. fischeri strain in Farmer et al. [2] is not indicated in their study. However, the negative ADC result reported may indicate that it was a seawater isolate and not a symbiotic one. Furthermore, Guerrero-Ferreira and Nishiguchi [31] reported the expression of ADC gene by symbiotic V. harveyi, hypothesizing that environmental production of ADC to degrade ArgA (a molecule associated to V. cholerae pathogenesis [52]) may play a pivotal role in the transition of V. harveyi from a pathogenic to mutualistic state [53].
Phenotypic variation is not an isolated occurrence in V. harveyi strains. In a study by Vidgen et al. [26], differences were evident in phenotypic profiles of five V. harveyi strains (four seawater isolates and a pathogenic strain from a diseased prawn Penaeus monodon). Those differences were associated with the presence of a specific mobile genetic element, named V. harveyi myovirus-like bacteriophage (VHML), which caused the bacterium to elicit variable responses to several phenotypic tests [26]. Another example of this phenomenon is found in the bacterium Aeromonas veronii, a digestive tract symbiont of the medicinal leech Hirudo medicinalis [54, 55]. When tested for the presence of the enzyme arginine dehydrolase, isolates of A. veronii obtained from clinical sources (i.e., respiratory secretions, infected wounds, and stools) were negative [56]. Conversely, strains isolated in their symbiotic state (i.e., in the digestive tract of their leech host) were positive for arginine dehydrolase. Therefore, existing data suggests that within-species variation in arginine metabolism is common in members of the family Vibrionaceae. More interestingly, occurrence of this variation in other bacterial species may be niche related.
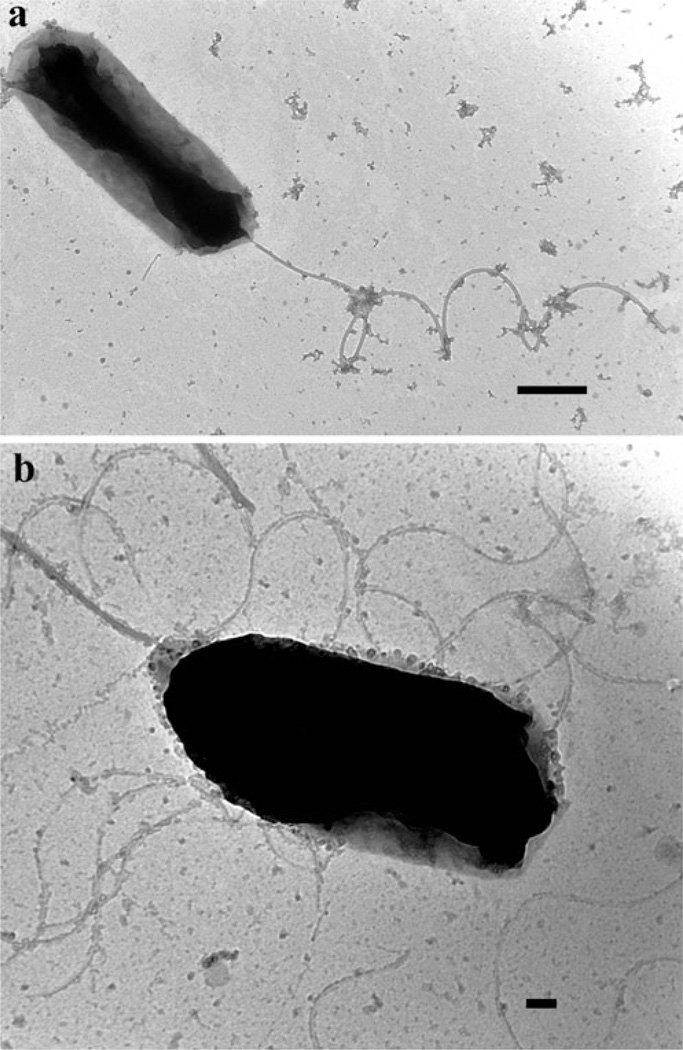
Morphology and flagellation patterns of each Vibrio symbiont were completed by negative staining TEM (representative micrographs in Fig. 1a, b). After extensive screening, it is evident that isolates appeared rod shaped and displayed a single polar flagellum when grown in liquid media (Fig. 1a). This type of flagellum is commonly observed in species of the genus Vibrio grown under these conditions, with the exception of V. fischeri, which exhibits lophotrichous flagella (two to eight polar flagella; [57]). Although flagellation pattern was not considered diagnostic for species identification, our microscopic survey confirms that the isolates are not V. fischeri. When grown on solid medium, peritrichous (lateral) flagella were observed in addition to the polar flagellum (Fig. 1b). Production of both polar and peritrichous flagella has previously been reported to occur in several species of Vibrio (e.g., V. harveyi, V. parahaemolyticus, and V. alginolyticus [57]).
Figure 1.
Transmission electron micrographs of Vibrio harveyi squid isolates grown in (a) seawater tryptone liquid media or (b) seawater tryptone agar. Scale bar=500 (a) and 100 nm (b)
Amplification and Sequencing of Uridilate Kinase Gene (pyrH) for Characterization of Bacterial Consortia in Loliginid Squids
With the purpose of confirming the multi-specific nature of the population of bacteria colonizing loliginid light organs, ten isolates of luminescent strains representing several geographical areas off the coast of Australia were selected for sequencing the uridilate kinase gene (pyrH; Table 4). The use of this genetic marker for species identification within the Vibrionaceae family extends from bacterial pathogenesis studies to ecological analysis of both marine and freshwater environments [58–60]. Our results confirm that loliginid light organs are colonized by a luminescent bacterial consortium. This condition is at least common for the selected squid hosts examined in this study, including representatives of the genera Uroteuthis and Photololigo. Of the selected isolates, three were identified as V. harveyi, further indicating that this species exists in a mutualistic association with loliginid squids in Australia (Table 4).
Polymerase Chain Reaction/Restriction Fragment Length Polymorphism (PCR/RFLP) Confirms Multi-Species Symbiosis in Squids from Thailand and Australia
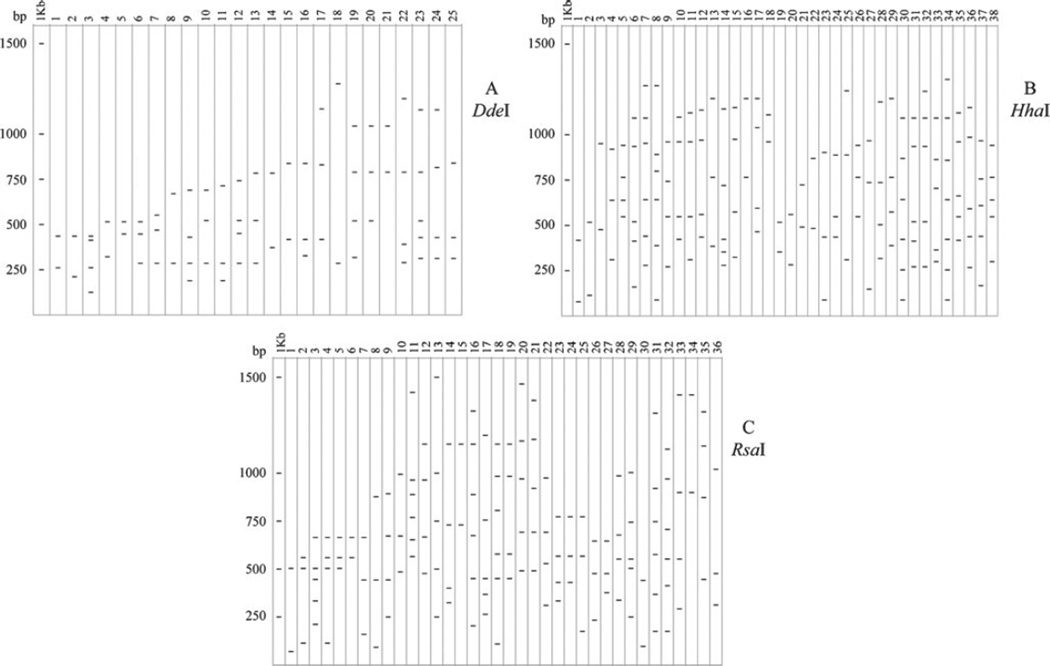
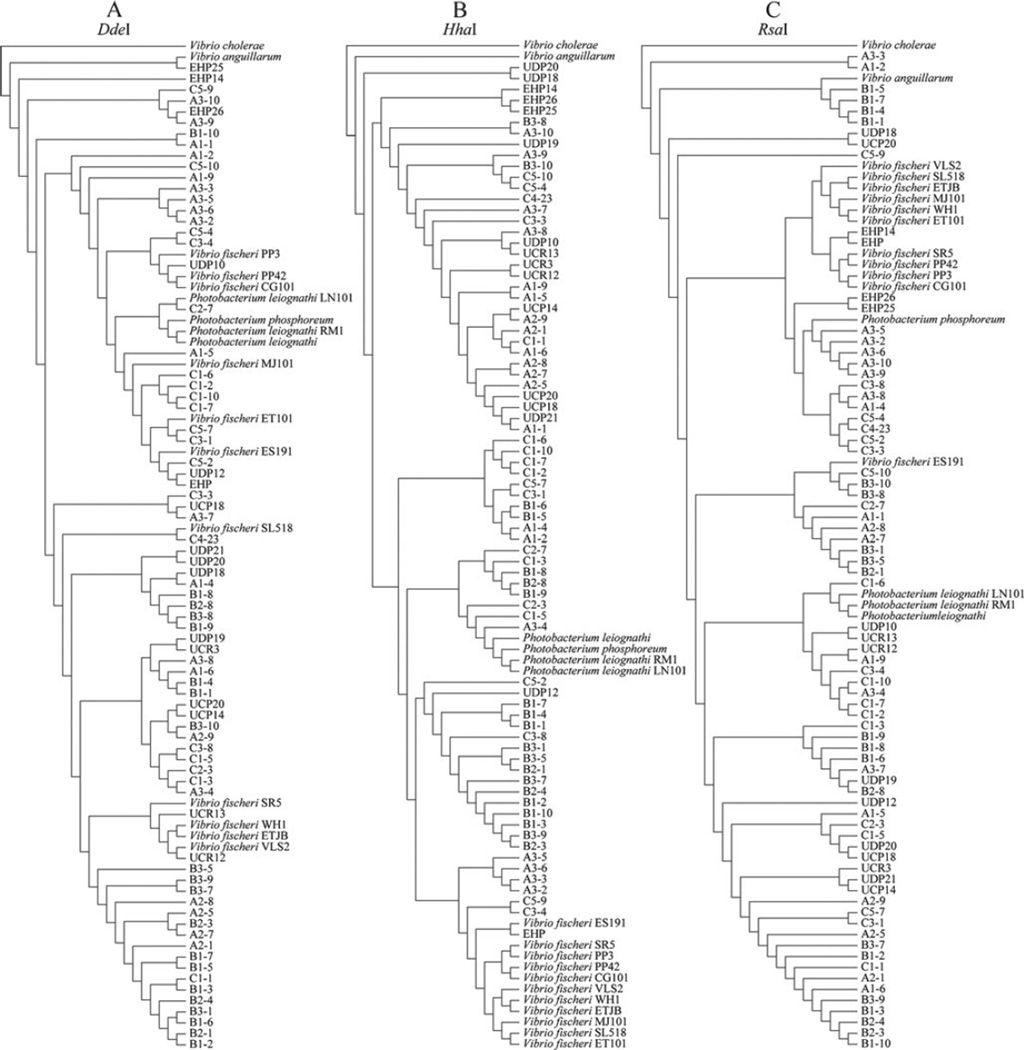
In a complementary approach to further explore the diversity of bacterial species colonizing light organs in Thai and Australian squids, we analyzed 16S rRNA-PCR/RFLPs of 92 strains including natural isolates and laboratory strains. Amplification of the 16S rRNA gene resulted in a gene product of ~1,400 bp corresponding to the predicted size for this gene amplified under the conditions presented. After digestion with three restriction enzymes, a series of fragment patterns were obtained and are schematically summarized in Fig. 2. The number of restriction banding patterns obtained for each enzyme treatment was: 25 for DdeI, 36 for HhaI, and 38 for RsaI. Fingerprints constructed with these restriction enzymes exhibited considerable variation when compared among environmental and laboratory isolates. These differences were confirmed by dendograms constructed using restriction patterns (band presence or absence) as input for the phylogenetic analysis (Fig. 3).
Figure 2.
Diagrams representing restriction patterns of 16S rRNA gene digested with DdeI (a), HhaI (b) or RsaI (c). First column in each diagram corresponds to the banding pattern for the 1 Kb ladder
Figure 3.
Dendograms built from restriction profiles using parsimony implemented in POY 4.0. Refer to Table 1 for isolates names
Analysis of strains isolated from groups A, B, and C (from Australia) indicates that bacteria colonizing loliginid light organs are represented by more than one species. Interestingly, RFLP patterns consistently grouped strains from Uroteuthis etheriogei (Group B) in clusters different than those of V. fischeri and Photobacterium species. HhaI and DdeI RFLP analyses resulted in grouping of Photobacterium isolates into their own clade (Fig. 3a, b). However, RFLP data from only HhaI restriction (independent of RsaI and DdeI) generated a phylogeny in which the genus Photobacterium grouped independently from the other Vibrio isolates (Fig. 3b), specifically V. fischeri. These results are in accordance with a study by Urakawa et al. [34], where only HhaI RFLP analysis resulted in the separation of Photobacterium from Vibrio genera. Other enzymes tested in the aforementioned study did not produce RFLP data that separated these two genera into different operational taxonomic units (OTUs). In our study, the cladogram obtained from RsaI restriction profiles neither engendered an apparent Photobacterium clade, nor put all V. fischeri strains as sister taxa (Fig. 3c). Not all mutualistic Vibrio isolates appear in this group, with free-living V. fischeri WH1 grouping separately with Thailand strains.
The distribution of isolates within and among OTUs was neither determined by geographical origin of each isolate nor by its animal host. This is an indication that host biogeography does not play a pivotal role on the phylogenetic history of bacterial populations associated with these species of squids. Australian isolates from groups A, B, and C (three different collection sites in Australia; Table 1) appear scattered throughout the dendograms, indicating no biogeographical partitioning. This lack of pattern is visible in all RFLP derived dendograms, where isolates from Thailand, Australia, Hawaii, and the Mediterranean Sea appear to group together in single clades, despite their geographical origin. This is in contrast to previous studies using more sensitive methods (sequence data) where clear delineation was apparent among V. fischeri strains that were allopatric and exhibited introgression between closely related populations [61]. Interestingly, sepiolid squids are benthic and do not move between areas as much as loliginid squids, thereby producing more fragmented populations of Vibrio bacteria.
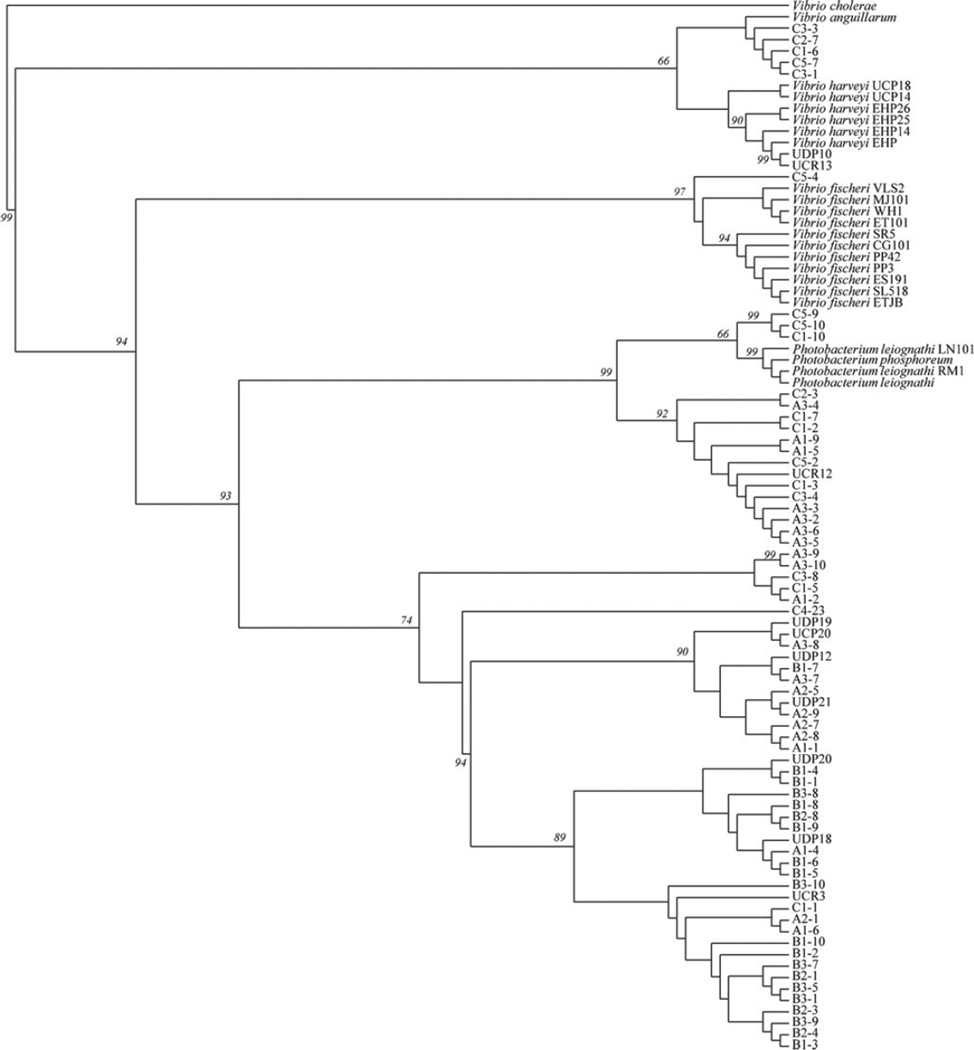
Combined Phylogenetic Analysis Using PCR/RFLP HhaI Profiles and 16S rRNA Sequence Data
Considering the recognized efficiency of using HhaI restriction profiling to distinguish between species of the genera Photobacterium and Vibrio [34, 62], and recognizing the relevance of 16S rRNA gene sequences for the construction of Vibrionaceae phylogenies and the study of the evolution of symbiotic bacteria [1, 12], our study also incorporated a combined phylogenetic approach using both sequence and HhaI restriction profile data. Figure 4 depicts the phylogenetic tree resulting from this combined approach. A combination of both data types in a single analysis yielded a distribution of taxa that restricts both the V. fischeri group (97 % jackknife support) and the Photobacterium genus (99 % jackknife support) into their individual clades. In addition, a number of loliginid squid isolates that the microbiological assays identified as V. harveyi were placed within a sole clade, adding strength to our initial conclusions. These results also provide some additional support to previous cladistic analysis, where Vibrio and Photobacterium were split into separate clades [3].
Figure 4.
Phylogenetic analysis combining PCR/RFLP data with 16S rRNA gene sequences using parsimony. Jackknife values of more than 50 % are shown as numbers on nodes. Trees were searched by TBR (tree bisection and reconnection) branch-swapping on the best of 100 replicates. One round of tree-fusing was also implemented [65]. At the same time, the command TreeView 0.4.1 was used for visualization of binary trees and PAUP 4.0.10 for consensus tree calculation
Conclusion
The use of RFLP of PCR amplified 16S rRNA genes proved to be effective for preliminary screening, evaluation, and characterization of Vibrionaceae populations of bacteria colonizing light organs in loliginid squids. 16S rRNA analysis has been used for the rapid identification of unknown bacterial isolates in samples of fisheries or aquaculture stocks, as well as natural harvests of marine organisms. A systematic development of this technique for Vibrio specific groups would contribute to the quick diagnostics of field-collected samples, with the goal of determining whether microbial pathogens (in particular Vibrio species) exist as contaminants. In addition, this research further supports that PCR/RFLP analysis is a rapid and economical tool to distinguish the genus Vibrio from other members of the family Vibrionaceae, particularly when the number of samples makes phenotypic characterization an expensive and tedious task. Finally, the combination of molecular and biochemical assays has provided additional information regarding species dynamics in Vibrio-loliginid squid symbiosis.
Our study also presents additional evidence of a newly recognized association between V. harveyi and squids of the family Loliginidae. Our findings contribute to the understanding of bacterial populations in the ocean as it demonstrates that pathogenic bacteria such as V. harveyi can also exist as partners in mutualistic associations with loliginid squids. Considering this, there may be some implications regarding the epidemiology of vibriosis in Thailand and Australian coastal areas. Species of sepiolid and loliginid squids are distributed broadly in the Andaman Sea, the Gulf of Thailand, and off the coasts of Australia, and these hosts may represent an ecological niche for pathogens of other marine organisms (including those exploited in aquaculture). V. harveyi may utilize these squids as a subtle reservoir for the maintenance of its populations during periods of quiescence. Understanding these survival strategies would better our approaches for assessment of water quality and also clarify the mechanisms of transmission of Vibrio-borne diseases and the transition between mutualistic and pathogenic life history strategies. Future studies to examine the distribution of V. harveyi throughout the Indo-west Pacific, and the possible existence of specific strains from other locations, may help provide evidence for plausible precursors of vibriosis in the marine environment.
Acknowledgements
The authors would like to thank J. Nabhitabhata for help collecting specimens in Rayong and Phuket, Thailand and Rami Al-Khatib from the Electron Microscopy facility at NMSU for help with the Electron Microscopy work. E. Shipp collaborated with DNA amplification and sequencing. Phylogenetic analyses were analyzed on the NetBSD biology computer system at NMSU. This work was supported in part by NIH- SO6 GM008136-32 S2, NIH-NIAID 1SC1AI081659, NSF IOS-0744498, and NSF-DBI-0520956 to M.K.N. Support for C. Gorman and A. Chavez was provided by the NIH-MBRS RISE (NIH-GM-61222-01) and C. Gorman was also supported by the NMSU MARC program (NIH-T34GMO7667-34). S. Willie and E. Shipp were supported by the BRIDGES to Native American Students program (NIH-R25 GM48998) at New Mexico State University.
Contributor Information
Ricardo Guerrero-Ferreira, Department of Pediatrics, Division of Pediatric Infectious Diseases, Emory University School of Medicine, Atlanta, GA 30322, USA.
Clayton Gorman, Department of Biology, New Mexico State University, Las Cruces, NM 88003-8001, USA.
Alba A. Chavez, Department of Biology, New Mexico State University, Las Cruces, NM 88003-8001, USA
Shantell Willie, Department of Biology, New Mexico State University, Las Cruces, NM 88003-8001, USA.
Michele K. Nishiguchi, Email: nish@nmsu.edu, Department of Biology, New Mexico State University, Las Cruces, NM 88003-8001, USA.
References
- 1.Nishiguchi MK, Nair VS. Evolution of symbioses in the Vibrionaceae: a combined approach using molecules and physiology. Int J Syst Evol Micr. 2003;53:2019–2026. doi: 10.1099/ijs.0.02792-0. [DOI] [PubMed] [Google Scholar]
- 2.Farmer JJ, III, Janda JM, Brenner FW, Cameron DN, Birkhead KM. Genus I. Vibrio Pacini 1854, 411AL. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey’s manual of systematic bacteriology. vol 2. New York: Springer; 2005. pp. 494–546. [Google Scholar]
- 3.Dikow RB. Systematic relationships within the Vibrionaceae (Bacteria: Gamma proteobacteria): steps toward a phylogenetic taxonomy. Cladistics. 2010;27:9–28. doi: 10.1111/j.1096-0031.2010.00312.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson FL, Hoste B, Vandemeulebroecke K, Swings J. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism. Syst Appl Microbiol. 2001;24:520–538. doi: 10.1078/0723-2020-00067. [DOI] [PubMed] [Google Scholar]
- 5.Jones BW, Nishiguchi MK. Counter illumination in the bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda) Mar Biol. 2004;144:1151–1155. [Google Scholar]
- 6.Dunlap PV, McFall-Ngai MJ. Initiation and control of the bioluminescent symbiosis between Photobacterium leiognathi and the leiognathid fishes. In: Lee JJ, Frederick JF, editors. Proceedings of the New York Academy of Sciences Symposium on Endosymbiosis; 1987. pp. 269–283. [DOI] [PubMed] [Google Scholar]
- 7.Fidopiastis PM, Boletzky Sv, Ruby EG. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J Bacteriol. 1998;180:59–64. doi: 10.1128/jb.180.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colwell RR, Huq A. Vibrios in the environment: viable but nonculturable Vibrio cholerae. In: Wachsmuth IK, Blake PA, Olsvik O, editors. Vibrio cholerae and cholera: molecular and global perspectives. Washington, D.C.: American Society of Microbiology Press; 1994. pp. 117–133. [Google Scholar]
- 9.Yumoto II, Iwata H, Sawabe T, Ueno K, Ichise N, Matsuyama H, Okuyama H, Kawasaki K. Characterization of a facultatively psychrophilic bacterium, Vibrio rumoiensis sp. nov., that exhibits high catalase activity. Appl Environ Microbiol. 1999;65:67–72. doi: 10.1128/aem.65.1.67-72.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruby EG, Morin JG. Luminous enteric bacteria of marine fishes: a study of their distribution, densities, and dispersion. Appl Environ Microbiol. 1979;38:406–411. doi: 10.1128/aem.38.3.406-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruby EG, Nealson KH. Symbiotic associations of Photobacterium fischeri with the marine luminous fish Monocentris japonica: a model of symbiosis based on bacterial studies. Biol Bull. 1976;151:574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero-Ferreira RC, Nishiguchi MK. Biodiversity among luminescent symbionts from squid of the genera Uroteuthis, Loliolus and Euprymna (Mollusca: Cephalopoda) Cladistics. 2007;23:497–506. doi: 10.1111/j.1096-0031.2007.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stabili L, Gravili C, Piraino S, Boero F, Alifano P. Vibrio harveyi associated with Aglaophenia octodonta (Hydrozoa, Cnidaria) Microb Ecol. 2006;52:603–608. doi: 10.1007/s00248-006-9010-7. [DOI] [PubMed] [Google Scholar]
- 14.Foster JS, Boletzky Sv, McFall-Ngai MJ. A comparison of the light organ development of Sepiola robusta Naef and Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Bull Mar Sci. 2002;70:141–153. [Google Scholar]
- 15.Dalsgaard A, Serichantalergs O, Forslund A, Lin W, Mekalanos J, Mintz E, Shimida T, Wells AJG. Clinical and environmental isolates of Vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J Clin Microbiol. 2001;39:4086–4092. doi: 10.1128/JCM.39.11.4086-4092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePaola A, Ulaszek J, Kaysner CA, Tenge BJ, Nordstrom JL, Wells J, Puhr N, Gendel SM. Molecular, serological, and virulence characteristics of Vibrio parahaemolyticus isolated from environmental, food, and clinical sources in North America and Asia. Appl Environ Microbiol. 2003;69:3999–4005. doi: 10.1128/AEM.69.7.3999-4005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcaide E, Amaro C, Todoli R, Oltra R. Isolation and characterization of Vibrio parahaemolyticus causing infection in Iberian toothcarp Aphanius iberus. Dis Aquat Organ. 1999;35:77–80. doi: 10.3354/dao035077. [DOI] [PubMed] [Google Scholar]
- 19.Alcaide E, Gil-Sanz C, Sanjuan E, Esteve D, Amaro C, Silveira L. Vibrio harveyi causes disease in seahorse, Hippocampus sp. J Fish Dis. 2001;24:311–313. [Google Scholar]
- 20.Alcaide E, Sanjuan E, del Ganaa F, Garcia-Gomez A. Susceptibility of Amberjack (Seriola dumerili) to bacterial fish pathogens. Bull Eur Ass Fish Pathol. 2000;20:153–156. [Google Scholar]
- 21.Banin E, Israely T, Kushmaro A, Loya Y, Orr E, Rosenberg E. Penetration of the coral-bleaching bacterium Vibrio shiloi into Oculina patagonica. Appl Environ Microbiol. 2000;66:3031–3036. doi: 10.1128/aem.66.7.3031-3036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Haim Y, Zicherman-Keren M, Rosenberg E. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol. 2003;69:4236–4242. doi: 10.1128/AEM.69.7.4236-4242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stelma GN, Jr, Reyes AL, Peeler JT, Johnson CH, Spaulding PL. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Envrion Microbiol. 1992;52:2776–2782. doi: 10.1128/aem.58.9.2776-2782.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aznar R, Ludwig W, Amann RI, Schleifer KH. Sequence determination of rRNA genes of pathogenic Vibrio species and whole-cell identification of Vibrio vulnificus with rRNA-targeted oligonucleotide probes. Int J Syst Bacteriol. 1994;44:330–337. doi: 10.1099/00207713-44-2-330. [DOI] [PubMed] [Google Scholar]
- 25.Owens L, Busico-Salcedo N. Vibrio harveyi: pretty problems in paradise. In: Thompson FL, editor. The biology of vibrios. Washington, D.C.: ASM Press; 2006. pp. 266–280. [Google Scholar]
- 26.Vidgen M, Carson J, Higgins M, Owens L. Changes to the phenotypic profile of Vibrio harveyi when infected with the Vibrio harveyi myovirus-like (VHML) bacteriophage. J Appl Microbiol. 2006;100:481–487. doi: 10.1111/j.1365-2672.2005.02829.x. [DOI] [PubMed] [Google Scholar]
- 27.Dunlap PV, Davis KM, Tomiyama S, Fujino M, Fukui A. Developmental and microbiological analysis of the inception of bioluminescent symbiosis in the marine fish Nuchequula nuchalis (Perciformes: Leiognathidae) Appl Environ Microbiol. 2008;74:7471–7481. doi: 10.1128/AEM.01619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu WT, Marsh TL, Cheng H, Forney LJ. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516–4522. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward DM, Weller R, Bateson MM. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990;345:63. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero-Ferreira RC, Nishiguchi MK. Ultrastructure of light organs of loliginid squids and their bacterial symbionts: a novel system for the study of marine symbioses. Vie et Milieu. 2009;59:307–313. [PMC free article] [PubMed] [Google Scholar]
- 32.Jamann S, Fernandez MP, Normand P. Typing method for N2-fixing bacteria based on PCR-RFLP-application to the characterization of Frankia strains. Mol Ecol. 1993;2:17–26. doi: 10.1111/j.1365-294x.1993.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 33.Urakawa H, Kita-Tsukamoto K, Ohwada K. 16S rDNA genotyping using PCR/RFLP (restriction fragment length polymorphism) analysis among the family Vibrionaceae. FEMS Microbiol Lett. 1997;152:125–132. doi: 10.1111/j.1574-6968.1997.tb10418.x. [DOI] [PubMed] [Google Scholar]
- 34.Urakawa H, Kita-Tsukamoto K, Ohwada K. A new approach to separate the genus Photobacterium from Vibrio with RFLP patterns by Hha I digestion of PCR-amplified 16S rDNA. Curr Microbiol. 1998;36:171–174. doi: 10.1007/pl00006762. [DOI] [PubMed] [Google Scholar]
- 35.Urakawa H, Kita-Tsukamoto K, Ohwada K. 16S rDNA restriction fragment length polymorphism analysis of psychrotrophic vibrios from Japanese coastal water. Can J Microbiol. 1999;45:1001–1007. [PubMed] [Google Scholar]
- 36.Urakawa H, Kita-Tsukamoto K, Ohwada K. Microbial diversity in marine sediments from Sagami Bay and Tokyo Bay, Japan, as determined by 16S rRNA gene analysis. Microbiology. 1999;145(Pt 11):3305–3315. doi: 10.1099/00221287-145-11-3305. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura S, Yoshimura A, Saito A, Kishmoto N, Kawase M, Yano M, Nakagahra M, Ogawa T, Iwata N. RFLP analysis of introgressed chromosomal segments in three near-isogenic lines of rice for bacterial blight resistance genes, Xa-1, Xa-3 and Xa-4. Jpn J Genet. 1992;67:29–37. [Google Scholar]
- 38.Marhual NP, Das BK. Use of random amplified polymorphic DNA and restriction fragment length polymorphism for typing of Vibrio alginolyticus isolated from black tiger shrimp Penaeus monodon. J Pure Appl Microbiol. 2009;3:75–82. [Google Scholar]
- 39.Nishiguchi MK, Lopez JE, Boletzky Sv. Enlightenment of old ideas from new investigations: more questions regarding the evolution of bacteriogenic light organs in squids. Evol Dev. 2004;6:41–49. doi: 10.1111/j.1525-142x.2004.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler WC. Optimization alignment: the end of multiple sequence alignment in phylogenetics? Cladistics. 1996;12:1–9. [Google Scholar]
- 41.Wheeler WC. Alignment characters, dynamic programming and heuristic solutions. In: DeSalle R, Schierwater B, editors. Molecular approaches to ecology and evolution. vol 1. Basel: Birkhauser Verlag; 1998. pp. 243–251. [Google Scholar]
- 42.Wheeler WC, Gladstein D, DeLaet J. POY v. 3.0. 2002 [Google Scholar]
- 43.Wheeler WC. Sequence alignment, parameter sensitivity, and the phylogenetic analysis of molecular data. Syst Biol. 1995;44:321–331. [Google Scholar]
- 44.Alsina M, Blanch AR. Improvement and update of a set of keys for biochemical identification of Vibrio species. J Appl Bacteriol. 1994;77:719–721. doi: 10.1111/j.1365-2672.1994.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 45.Allen RD, Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971;107:295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumann P, Baumann L, Bang SS, Woolkalis MJ. Reevaluation of the taxonomy of Vibrio, Beneckea, and Photobacterium: abolition of the genus Beneckea. Curr Microbiol. 1980;4:127–132. [Google Scholar]
- 47.Kidron H, Repo S, Johnson MS, Salminen TA. Functional classification of amino acid decarboxylases from the alanine racemase structural family by phylogenetic studies. Mol Biol Evol. 2007;24:79–89. doi: 10.1093/molbev/msl133. [DOI] [PubMed] [Google Scholar]
- 48.Patel CN, Wortham BW, Lines JL, Fetherston JD, Perry RD, Oliveira MA. Polyamines are essential for the formation of plague biofilm. J Bacteriol. 2006;188:2355–2363. doi: 10.1128/JB.188.7.2355-2363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karatan E, Duncan TR, Watnick PI. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholera biofilm formation by norspermidine. J Bacteriol. 2005;187:7434–7443. doi: 10.1128/JB.187.21.7434-7443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones BW, Nishiguchi MK. Differentially expressed genes reveal adaptations between free-living and symbiotic niches of Vibrio fischeri in a fully established mutualism. Can J Microbiol. 2006;52:1218–1227. doi: 10.1139/w06-088. [DOI] [PubMed] [Google Scholar]
- 51.Ariyakumar DS, Nishiguchi MK. Characterization of two host-specific genes, mannose-sensitive hemagglutinin (mshA) and uridyl phosphate dehydrogenase (UDPDH) that are involved in the Vibrio fischeri–Euprymna tasmanica mutualism. FEMS Microbiol Lett. 2009;299:65–73. doi: 10.1111/j.1574-6968.2009.01732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merrell DS, Hava DL, Camilli A. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol Microbiol. 2002;43:1471–1491. doi: 10.1046/j.1365-2958.2002.02857.x. [DOI] [PubMed] [Google Scholar]
- 53.Guerrero-Ferreira RC, Nishiguchi MK. Differential gene expression in bacterial symbionts from loliginid squids demonstrates variation between mutualistic and environmental niches. Environ Microbiol Rep. 2010;2:514–523. doi: 10.1111/j.1758-2229.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graf J. Symbiosis of Aeromonas veronii Biovar sobria and Hirudo medicinalis, the medicinal leech: a novel model for digestive tract associations. Infect Immun. 1999;67:1–7. doi: 10.1128/iai.67.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddall ME, Worthen PL, Johnson M, Graf J. Novel role for Aeromonas jandaei as a digestive tract symbiont of the North American medicinal leech. Appl Environ Microbiol. 2007;73:655–658. doi: 10.1128/AEM.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hickman-Brenner FW, MacDonald KL, Steigerwalt AG, Fanning GR, Brenner DJ, Farmer JJ., III Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J Clin Microbiol. 1987;25:900–906. doi: 10.1128/jcm.25.5.900-906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarter LL. Motility and chemotaxis. In: Thompson FL, Austin B, Swings J, editors. The biology of vibrios. Washington, D.C.: ASM Press; 2006. p. 423. [Google Scholar]
- 58.Pollock FJ, Wilson B, Johnson WR, Morris PJ, Willis BL, Bourne DG. Phylogeny of the coral pathogen Vibrio coralliilyticus. Environ Microbiol Rep. 2010;2:172–178. doi: 10.1111/j.1758-2229.2009.00131.x. [DOI] [PubMed] [Google Scholar]
- 59.Bleicher A, Neuhaus K, Scherer S. Vibrio casei sp. nov., isolated from the surface of two French red smear soft cheeses. Int J Syst Evol Microbiol. 2010;60:1745–1749. doi: 10.1099/ijs.0.016493-0. [DOI] [PubMed] [Google Scholar]
- 60.Thompson FL, Gevers D, Thompson CC, Dawyndt P, Naser S, Hoste B, Munn CB, Swings J. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl Environ Microbiol. 2005;71:5107–5115. doi: 10.1128/AEM.71.9.5107-5115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones BW, Lopez JE, Huttenburg J, Nishiguchi MK. Population structure between environmentally transmitted vibrios and bobtail squids using nested clade analysis. Mol Ecol. 2006;15:4317–4329. doi: 10.1111/j.1365-294X.2006.03073.x. [DOI] [PubMed] [Google Scholar]
- 62.Kita-Tsukamoto K, Wada M, Yao K, Kamiya A, Yoshizawa S, Uchiyama N, Kogure K. Rapid identification of marine bioluminescent bacteria by amplified 16S ribosomal RNA gene restriction analysis. FEMS Microbiol Lett. 2006;256:298–303. doi: 10.1111/j.1574-6968.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 63.Visick KL, Ruby EG. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J Bacteriol. 1998;180:2087–2092. doi: 10.1128/jb.180.8.2087-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ast JC, Cleenwerck I, Engelbeen K, Urbanczyk H, Thompson FL, De Vos P, Dunlap PV. Photobacterium kishitanii sp. nov., a luminous marine bacterium symbiotic with deep-sea fishes. Int J Syst Evol Microbiol. 2007;57:2073–2078. doi: 10.1099/ijs.0.65153-0. [DOI] [PubMed] [Google Scholar]
- 65.Goloboff PA. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics. 1999;15:415–428. doi: 10.1111/j.1096-0031.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]