Abstract
Background
The rOmpA vaccine has been shown to protect mice from lethal infection caused by extreme-drug-resistant (XDR) Acinetobacter baumannii. The role of dose in immunology of the rOmpA vaccine was explored.
Methods
Mice were vaccinated with various doses of rOmpA plus aluminum hydroxide (Al(OH)3) adjuvant. The impact of dose on antibody titers, cytokine production, and immunodominant epitopes were defined.
Results
Anti-rOmpA IgG and IgG subtype titers were higher at larger vaccine doses (30 and 100 µg vs. 3 µg). The 3 µg dose induced a balanced IFN-γ-IL-4 immune response while the 100 µg dose induced a polarized IL-4/Type 2 response. Epitope mapping revealed distinct T cell epitopes that activated IFN-γ-, IL-4-, and IL-17-producing splenocytes. Vaccination with the 100 µg dose caused epitope spreading among IL-4-producing splenocytes, while it induced fewer reactive epitopes among IFN-γ-producing splenocytes.
Conclusions
Vaccine dose escalation resulted in an enhanced Type 2 immune response, accompanied by substantial IL-4-inducing T cell epitope spreading and restricted IFN-γ-inducing epitopes. These results inform continued development of the rOmpA vaccine against A. baumannii, and also are of general importance in that they indicate that immune polarization and epitope selectivity can be modulated by altering vaccine dose.
Keywords: Acinetobacter baumannii, OmpA, vaccine, type 1/type 2 immunity, epitope spreading
Introduction
Acinetobacter baumannii has emerged as one of the most common and highly antibiotic-resistant pathogens in the United States (US) and throughout the world [1–3]. The majority of such strains are now carbapenem resistant [4–11], and there is increasing resistance even to colistin and tigecycline [3, 12–18]. Such pan-drug resistant (PDR) A. baumannii strains are resistant to every FDA approved antibiotic, and are hence untreatable. New means to prevent such infections are critically needed.
In the absence of effective antibiotics, vaccination is a promising strategy to reduce the frequency of A. baumannii infections. A rational discovery program identified recombinant OmpA as a highly effective vaccine, protecting mice from otherwise lethal A. baumannii infection [19]. The current study was conducted to define the impact of dose on the immunology of the vaccine, and to elucidate immunodominant T and B cell epitopes. The impact of vaccine antigen dose on the nature of the cytokine response and the selectivity of immunodominant epitopes has not been well described. Thus, we also sought to determine if dose modulation would alter the nature of the cytokine response to the vaccine antigen and alter the epitopes triggering specific cytokine responses.
Materials and Methods
rOmpA Production and Immunization
His-tagged rOmpA (amino acids 2 to 347) was produced in an Escherichia coli pQE-32 expression system (Qiagen) as previously described [20, 21]. Briefly, ompA was amplified from A. baumannii 17978 genomic DNA with primers:
OmpA-F CATCACCATGGGATCCTTGTTGCTGCTCCATTAGCT and
OmpA-R CTAATTAAGCTTGGCTGCAGTTATTGAGCTGCTGCAGGA
and cloned into BamHI and Pst I sites of QE-32 by using In-Fusion 2.0 Dry-Down PCR Cloning Kit, per the manufacturer’s instructions (Clontech Laboratories). The 6×-His tagged protein was purified over a Ni-agarose affinity column according to the manufacturer’s instructions (Qiagen). Balb/c mice were immunized by subcutaneous injection of 3 µg of rOmpA in 0.1% Al(OH)3 (Alhydrogel, Brenntag Biosector, Frederikssund, Denmark) in phosphate buffered saline (PBS). Control mice received adjuvant alone on the same schedule. Mice were immunized and boosted at 3 weeks, and serum and splenocytes were harvested 2 weeks after boosting. All animal experiments were approved by the Institutional Committee on the Use and Care of Animals at the Los Angeles Biomedical Research Institute.
ELISAs
A previously published ELISA [22–25] was adapted for detection of antibodies against A. baumannii cell membrane preparations and rOmpA. In brief, ELISA plates were coated with 100 µl per well of 5 µg/ml of rOmpA or cell membrane preparation. Coated wells were blocked with bovine serum albumin, incubated with mouse sera, washed, and stained with goat anti-mouse secondary antibody conjugated with horseradish peroxidase. Wells were washed again and incubated with o-phenylenediamine substrate with H2O2. The color was allowed to develop for 20 min after which the reaction was terminated by adding equal volume of 3N HCl and the optical density (OD) was determined at 490 nm in a microtiter plate reader. Negative control wells received an irrelevant isotype control monoclonal antibody rather than mouse serum. The ELISA titer was taken as the reciprocal of the last serum dilution with an OD reading ≥ (mean OD of negative control samples + (standard deviation * 2)).
ELISpot Assay
Splenocytes were harvested through 70 µm filters and plated at 5×105 cells per well in 100 µl of complete media on IFN-γ, IL-17, or IL-4 ELISpot kits (eBiosciences). Cells were stimulated with rOmpA (5 µg/ml) or media alone. In some experiments, splenocytes were exposed to 15 mer peptides overlapping by 5 amino acids, spanning the length of the rOmpA protein (1 peptide per well at 5 µg/ml, peptides from Sigma). After 48 h of incubation, the plates were processed per the manufacturer’s instructions, and read using a Biosys Bioreader 5000. Spot frequency in stimulated wells was corrected by subtracting background signal from wells with cells plus media alone, and was normalized per 105 cells.
B Cell Epitope Mapping and Homology Modeling
Epitope mapping and homology modeling were conducted as previously described [26–30]. In brief, overlapping 13-mer peptides spanning the rOmpA sequence, offset by three amino acids, were covalently bound at the C terminus to a Whatman 50 cellulose membrane (SPOTs membrane, Sigma Aldrich, The Woodlands, TX), and directly probed with the anti-OmpA immune serum. The membranes were then washed four times in T-TBS (TBS containing 0.05% Tween 20), and incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated Protein G (Bio-Rad, Hercules, CA), which binds to the Fc domain of the immune serum, in blocking buffer. The membranes were processed for chemiluminescent detection with an Amersham™ ECL Plus Western Blotting Detection System kit (Piscataway, NJ). Images were generated with the Fotodyne Luminary/FX System in combination with the Foto/Analyst® PC Image software V10.40. rOmpA was modeled in silica by the the SWISS-MODEL automated protein structure homology modeling server (available at http://swissmodel.expasy.org) [31–33]. The model was optimized by energy minimization using Discovery Studio version 3.1 (Accelrys, San Diego, CA). The minimization was performed in several steps, using a steepest descendent and conjugate gradient algorithm to reach the minimum convergence (0.02 kcal mol−1 A−1). The stereochemical quality of the proposed model was assessed with Procheck and the packing quality with Anolea.
Statistics
ELISpot results and antibody titers were compared with the Wilcoxon Rank Sum test for unpaired comparisons. All statistics were run using Kyplot. Differences were considered significant if the p value was < 0.05.
Results
The Impact of Vaccine Dose on Immunogenicity
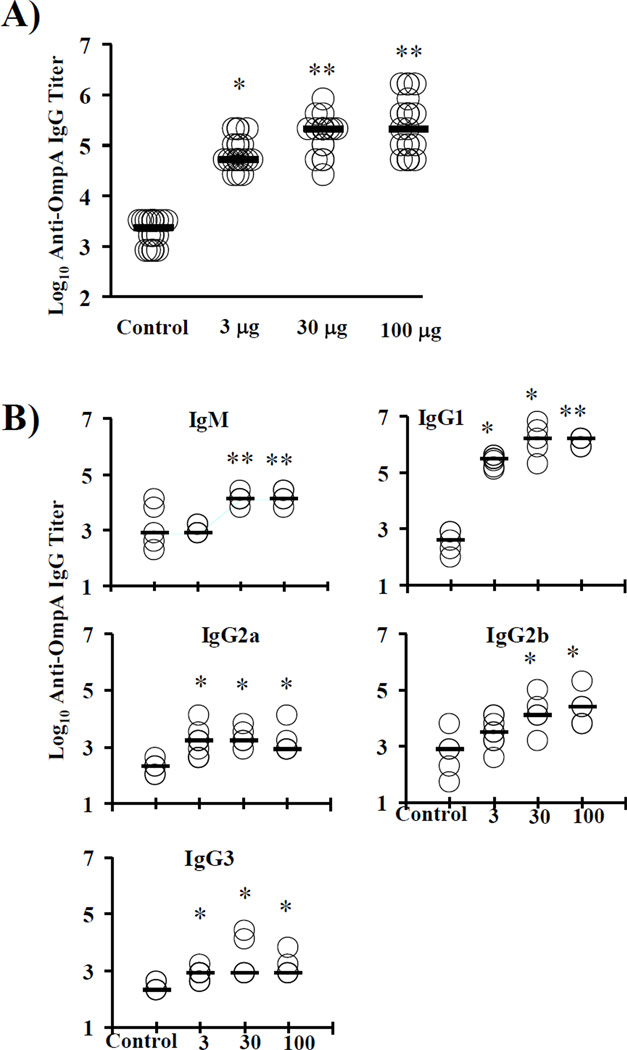
The impact of vaccine dose on the nature of the immune response to the rOmpA vaccine was explored. Based on our previous vaccine protection experiments, which used a 3 µg dose [19], mice were vaccinated with 3, 30, or 100 µg of protein plus Al(OH)3 adjuvant and boosted three weeks later. Two weeks after the boost, serum and splenocytes were harvested. Median [interquartile ranges] antibody titers for control, 3, 30, and 100 µg dose vaccinated mice were 2,400 [800–3,200], 51,200 [51,200–102,400], 204,800 [102,400–204,800], and 204,800 [89,600–512,000], respectively (p < 0.001 for all vaccinated doses vs. control and < 0.05 for both 30 and 100 µg dose vs. 3 µg dose) (Figure 1A).
Figure 1. Antibody titers induced by various doses of rOmpA or adjuvant alone.
A) Balb/c mice (n = 11 per group from 3 separate experiments) were vaccinated with one of 3 doses of vaccine or adjuvant alone. IgG titers from individual mice and the median titers (horizontal bars) for each group are shown. B) IgM and IgG subtype titers measured by ELISA from vaccinated or control mice. *p < 0.05 vs. adjuvant alone; **p < 0.05 vs. adjuvant alone and vs. 3 µg dose.
IgM responses were substantially higher in response to the 30 and 100 µg doses than the 3 µg dose (median titer 1:12,000 for both larger doses vs. 1:800 for the 3 µg dose and adjuvant control mice, p < 0.05) (Figure 1B). IgG1 was the predominant Ig subtype found, with median titers of 1:320,000 to 1:1,600,000 for vaccinated mice vs. 1:400 for control mice (p < 0.05 for all vs. control). IgG1 titers were significantly higher for mice vaccinated with 100 µg than 3 µg (p = 0.02). Median IgG2a and 2b titers were substantially lower than IgG1 titers but still significantly above the titers in control mice (Figure 1B). IgG3 titers were much lower, with median titers of 1:800 for all three vaccinated groups, but still significantly higher than control mice (median 1:200).
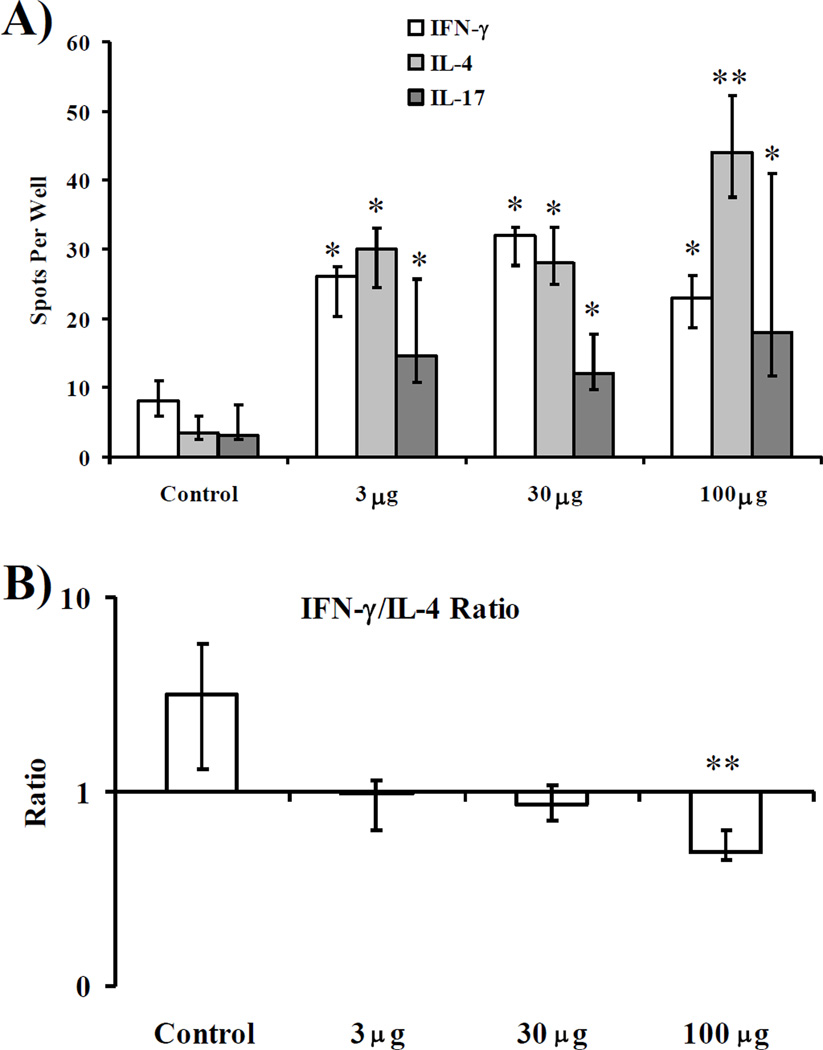
Similarly to antibody responses, all doses of vaccine mediated significant increases in IFNγ, IL-4, and IL-17 production by splenocytes, versus splenocytes from control mice (Figure 2A). IL-4 production was maximal at the largest (100 µg) dose of vaccine. Compared to the baseline IFNγ-predominant IFNγ:IL-4 ratio after stimulation with control (unvaccinated) splenocytes by rOmpA, all doses of vaccines mediated lower IFNγ:IL-4 ratios (i.e., more IL-4 relative to IFN-γ) (median [interquartile] ratios = 3.2 [1.3–5.8] for control vs. 1.0 [0.8–1.3], 0.9 [0.7–1.1], and 0.5 [0.5–0.7] for control vs. 3, 30, and 100 µg doses, respectively). The Th1:Th2 ratio was significantly lower for the 100 µg dose than for all other groups (p < 0.02 for all comparisons).
Figure 2. Splenocyte cytokine production stimulated by rOmpA.
A) IFN-γ, IL-4, or IL-17A production by splenocytes from vaccinated or control mice (n = 8 per group from 2 experiments) stimulated for 48 h with rOmpA measured by ELISpot. B) Ratio of IFN-γ:IL-4 produced by splenocytes from individual mice. Median and interquartile ranges are shown. *p < 0.05 vs. adjuvant control. **p < 0.05 vs. 3 and 30 µg dose, and vs. adjuvant control.
Vaccine Dose and Immunodominant T cell Epitopes
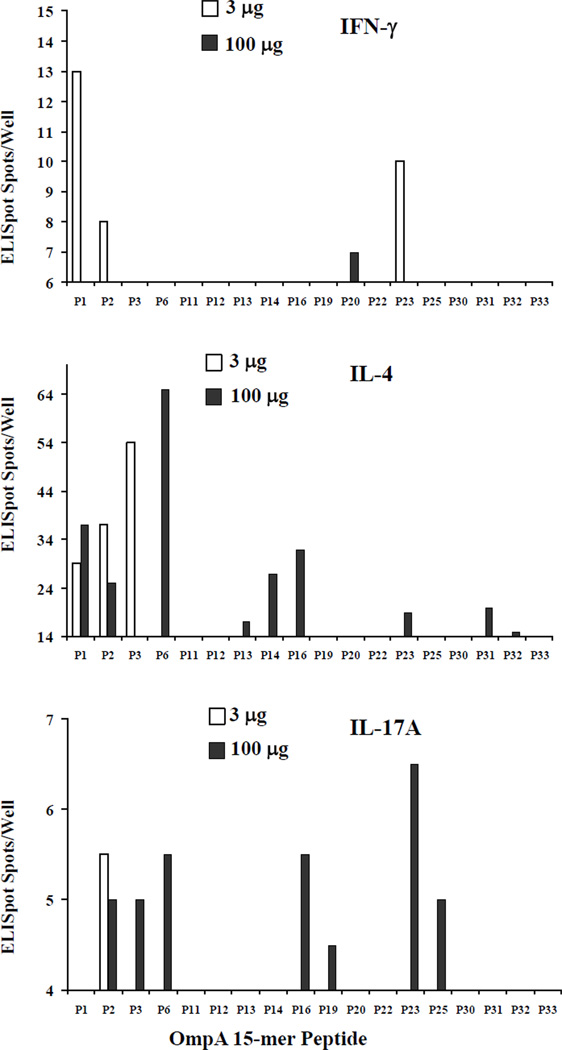
An initial T cell epitope screen was conducted in mice vaccinated with 3 µg of vaccine protein. In a pilot study, splenocytes were harvested from mice vaccinated with 3 or 100 µg and stimulated in vitro with the overlapping 15-mer peptides that spanned the length of the OmpA protein. Based on the initial reactivity, a subset of peptides that showed signal were selected for focused investigation. Using the selected peptides, splenocytes from mice vaccinated with 3 µg of rOmpA had broader epitope-reactivity of IFN-γ-producing cells than from mice vaccinated with the higher 100 µg dose (Figure 3). Conversely, IL-4-producing cells had broader epitope reactivity from high dose than low dose vaccinated mice. Thus, high-dose vaccination induced a broader epitope-based Th2-polarized response than low dose vaccination. In addition, IL-17A-producing splenocytes demonstrated epitope spreading with the largest dose of vaccine.
Figure 3. Vaccine dose altered epitope immunodominance.
Splenocytes were harvested from Balb/c mice vaccinated with 3 or 100 µg of rOmpA. The splenocytes were stimulated with 5 µg/ml of individual, overlapping 15mer peptides for 48 hours in ELISpot plates. Graphed are the means of 2 mice per group each run in triplicate. The lower bound of the Y axis is set at the third quartile of responses across all peptides in order to focus on immunodominant epitopes.
Vaccine Dose and Immunodominant B cell Epitopes
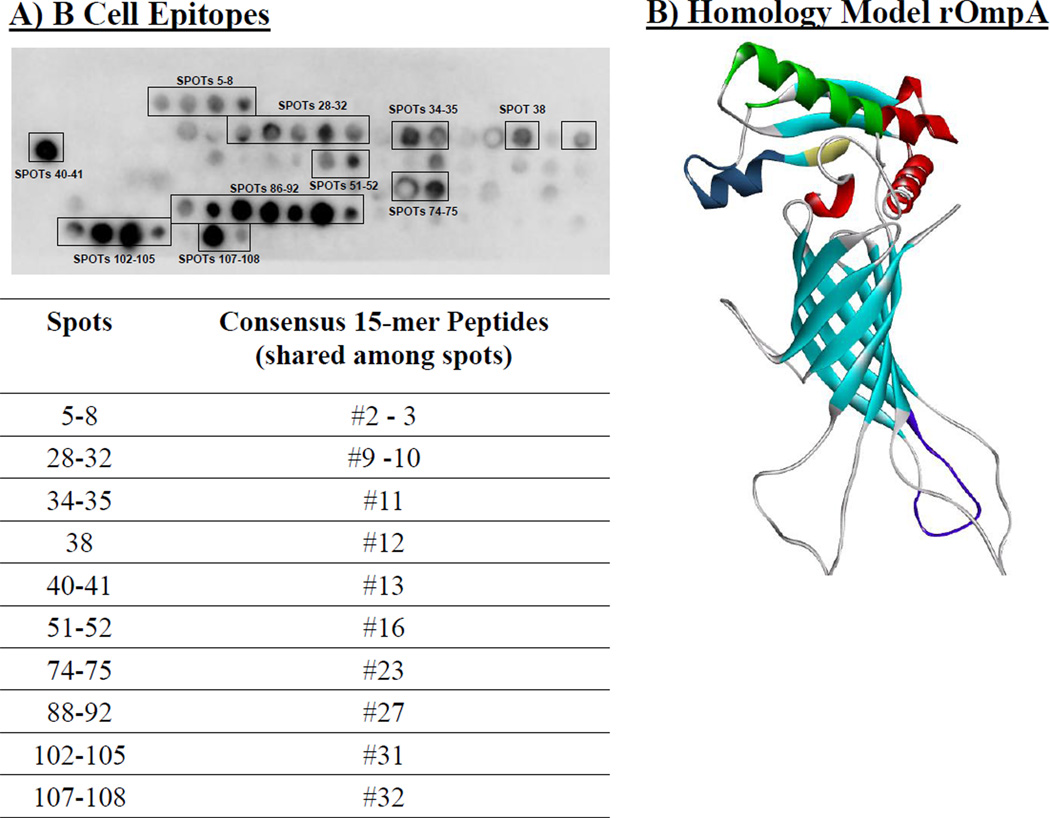
To identify B cell epitopes, SPOT blot analysis was conducted using control and immune serum and membranes containing overlapping peptides of the OmpA protein. A number of specific B cell epitopes were identified (Figure 4A). Only 3 peptides were found to represent both B cell and T cell epitopes (2, 16, and 23). Each of these peptides preferentially induced IL-4 as opposed to IFN-γ from splenocytes (Figure 3). Homology modeling revealed that the predominant B cell epitopes were localized to surface exposed α helices and β sheets, although there was also a dominant B cell epitope on the cytoplasmic face of the protein at a hairpin loop structure (Figure 4B).
Figure 4. B cell epitopes recognized by immune serum from vaccinated mice.
A) SPOT synthesis membrane containing overlapping 13-mer peptides spanning the rOmpA sequence, offset by three amino acids, were probed with pooled immune serum from mice vaccinated with 3 µg of rOmpA. B) In silica homology model of rOmpA was built using the Swiss-Model automated protein structure homology-modeling server (http://swissmodel.expasy.org). Major immunogenic epitopes are color-coded. Red = spots 86–92 from Figure 4, dark blue = spots 102–105 from Figure 4; yellow = spots 107–108 from Figure 4; green (bottom right) = spots 40–41 from Figure 4.
Discussion
rOmpA was identified as a highly promising candidate for active and passive immunization based on humoral immunodominance during infection in mice [19]. The current studies were conducted to assess the impact of vaccine dose on immunogenicity and to define T cell and B cell epitopes of the protein. All three vaccine doses stimulated high antibody titers, with larger doses inducing higher titers. The predominant IgG subtype generated was IgG1 rather than IgG2a or IgG2b, consistent with a Type 2 immune response. All three vaccine doses stimulated antigen-specific IFN-γ-, IL-4-, and IL-17-producing splenocytes. The lower vaccine dose induced a balanced IFN-γ-IL-4 response, while the largest vaccine dose significantly increased Th2 frequency out of proportion, leading to a significantly lower Th1:Th2 ratio, indicative of a predominant Type 2 immune response.
The precise role of Type 1/Type 2 immunity in host defense against A. baumannii infection is poorly defined. Several studies have confirmed the critical role of neutrophil recruitment to host defense against A. baumannii infection in mice [34, 35], suggesting that neutrophil-regulatory cytokines may be important in host defense. However, a recent study found that the key neutrophil regulatory cytokines, IL-17 and KC, did not play a major role in host defense against systemic A. baumannii infection mice [35]. Furthermore, a model of pulmonary infection in mice found that more severe infection was associated with higher levels of Type 1 inflammatory cytokines (IL-12 and IL-23) and lower levels of IL-10, suggesting that Type 2 immune responses may be protective [36]. In our previous study establishing the efficacy of rOmpA as a vaccine against A. baumannii, antibody titers correlated with protection, and immune serum passively transferred protection, also suggesting that Type 2-drive antibody-based mechanisms are protective [19]. Nevertheless, the precise roles of IL-4 vs. IFN-γ in host defense against the organism remains uncertain and merit further investigation.
T cell epitope mapping demonstrated a diverse set of immunodominant peptides, and of interest, the peptides tended to induce preferential cytokine responses, rather than all peptides inducing all cytokines. Furthermore, peptides induced distinct cytokine responses when splenocytes were harvested from mice given different doses of vaccine. At the larger dose, a broader range of peptides were found to be immunodominant for IL-4 and IL-17A production, consistent with epitope spreading induced by larger vaccine doses. In contrast, at the larger dose a narrower set of epitopes induced IFN-γ-production by splenocytes, consistent with a preferential Th2 response. Linear B cell epitopes were generally distinct from T cell epitopes. There were several peptides, however, that were immunodominant for both B and T cells. These peptides favored stimulation of IL-4 production more than IFN-γ production by splenocytes.
Larger doses of vaccines are known to be capable of inducing enhanced immunity [37–39], particularly in immunocompromised patients [40–42]. Nevertheless, the reason for enhanced immunogenicity of larger dose vaccines has not been elucidated, and to our knowledge, vaccine dose has not been previously associated with epitope-spreading. Antigen competition is known to drive selection of immunodominant epitopes and affinity maturation of B cells during an immune response, with B and T cells expressing higher affinity antigen-specific receptors preferentially receiving proliferation and survival signals from antigen presenting cells [43–45]. Thus, epitope spreading may occur at higher doses because there is greater quantity of processed antigen available in lymph node germinal centers, enabling B and T cells with receptors that have lower affinity to bind to the antigen and receive survival and proliferative signals. The dynamics of antigen availability in germinal centers based on antigen dose is an important area of future investigation.
Aluminum-based adjuvants have been shown to drive immune responses by activating the Nalp3 inflammasome [46]. Typically, aluminum-based adjuvants are considered Type 2-priming adjuvants. Nevertheless, we found evidence of IFN-γ-priming, particularly at lower antigen dose, indicating that depending on the protein with which the adjuvant is combined, aluminum hydroxide can help induce polarization to a Type 1 response. Additional study is required to determine the impact of dose with other adjuvants which are more prone to induce IFN-γ-based Type 1 immune responses.
The fact that epitope spreading occurs at higher doses, as does an enhanced Th2 response, suggests that higher doses may be favorable for future development. Thus, these results inform the continued pre-clinical development of the rOmpA vaccine targeting A. baumannii. Furthermore, they indicate that the immunogenicity, breadth of epitope coverage, and nature of the cytokine response to vaccines can be modified by altering vaccine dosage.
Highlights.
Higher doses of the rOmpA anti-A. baumannii vaccine induced higher vaccine titers
Higher dose of the rOmpA vaccine induced more polarized Type 2 immune response
Distinct rOmpA peptides preferentially induced IFN-γ or IL-4 T cell responses
Higher vaccine dose induced epitope spreading for IL-4 producing lymphocytes
Higher vaccine dose induced epitope restriction for IFN-γ producing lymphocytes
Acknowledgments
Funding
Financial support from PHS R01 AI081719. R. A. B. is funded by The Veterans Affairs Merit Review Program, the National Institutes of Health (RO1AI063517, RO1AI072219) and VISN 10 Geriatric Research Education and Clinical Center (GRECC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
LL and BS have filed a patent for the rOmpA vaccine.
References
- 1.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007 Oct;51(10):3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010 Feb;65(2):233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 3.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. Extensively drug-resistant Acinetobacter baumannii. Emerging infectious diseases. 2009 Jun;15(6):980–982. doi: 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003–2008, issued June 2009. American journal of infection control. 2010 Mar;38(2):95–104 e2. doi: 10.1016/j.ajic.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Perez F, Hujer AM, Hulten EA, Fishbain J, Hujer KM, Aron D, et al. Antibiotic resistance determinants in Acinetobacter spp and clinical outcomes in patients from a major military treatment facility. American journal of infection control. 2010 Feb;38(1):63–65. doi: 10.1016/j.ajic.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann MS, Eber MR, Laxminarayan R. Increasing resistance of acinetobacter species to imipenem in United States hospitals, 1999–2006. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2010 Feb;31(2):196–197. doi: 10.1086/650379. [DOI] [PubMed] [Google Scholar]
- 7.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2008 Nov;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, et al. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2009 Dec;30(12):1186–1192. doi: 10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 9.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug Resistance among Gram-Negative Pathogens Causing Healthcare-Associated Infections Reported to the National Healthcare Safety Network, 2006–2008. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2010;31:528–531. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 10.Dizbay M, Tunccan OG, Sezer BE, Hizel K. Nosocomial imipenem-resistant Acinetobacter baumannii infections: Epidemiology and risk factors. Scandinavian journal of infectious diseases. 2010 May 26;42:741–746. doi: 10.3109/00365548.2010.489568. [DOI] [PubMed] [Google Scholar]
- 11.Mera RM, Miller LA, Amrine-Madsen H, Sahm DF. Acinetobacter baumannii 2002–2008: Increase of Carbapenem-Associated Multiclass Resistance in the United States. Microb Drug Resist. 2010 Aug 14;16:209–215. doi: 10.1089/mdr.2010.0052. [DOI] [PubMed] [Google Scholar]
- 12.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, et al. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother. 2009 Sep;53(9):3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008 Nov;32(5):450–454. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Park YK, Jung SI, Park KH, Cheong HS, Peck KR, Song JH, et al. Independent emergence of colistin-resistant Acinetobacter spp. isolates from Korea. Diagn Microbiol Infect Dis. 2009 May;64(1):43–51. doi: 10.1016/j.diagmicrobio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, Fernandez-Cuenca F, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2009 Mar;30(3):257–263. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 16.Hernan RC, Karina B, Gabriela G, Marcela N, Carlos V, Angela F. Selection of colistin-resistant Acinetobacter baumannii isolates in postneurosurgical meningitis in an intensive care unit with high presence of heteroresistance to colistin. Diagn Microbiol Infect Dis. 2009 Oct;65(2):188–191. doi: 10.1016/j.diagmicrobio.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Livermore DM, Hill RL, Thomson H, Charlett A, Turton JF, Pike R, et al. Antimicrobial treatment and clinical outcome for infections with carbapenem- and multiply-resistant Acinetobacter baumannii around London. Int J Antimicrob Agents. 2010 Jan;35(1):19–24. doi: 10.1016/j.ijantimicag.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang YF, Dowzicky MJ. In vitro activity of tigecycline and comparators on Acinetobacter spp. isolates collected from patients with bacteremia and MIC change during the Tigecycline Evaluation and Surveillance Trial, 2004 to 2008. Diagn Microbiol Infect Dis. 2010 Sep;68(1):73–79. doi: 10.1016/j.diagmicrobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, et al. Active and Passive Immunization Protects against Lethal, Extreme Drug Resistant-Acinetobacter baumannii Infection. PLoS One. 2012;7(1):e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spellberg B, Ibrahim AS, Yeaman M, Lin L, Fu Y, Avanesian V, et al. The anti-fungal rAls3p-N vaccine protects mice against the bacterium Staphylococcus aureus. Infection and immunity. 2008;76:4574–4580. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010 Jun 1;201(11):1718–1728. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JEJ. The anti-Candida rAls1p-N vaccine is broadly active against disseminated candidiasis. Infection and immunity. 2006;74:3039–3041. doi: 10.1128/IAI.74.5.3039-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE., Jr Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infection and immunity. 2005 Feb;73(2):999–1005. doi: 10.1128/IAI.73.2.999-1005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006 Jul 15;194(2):256–260. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 25.Spellberg BJ, Ibrahim AS, Avenissian V, Filler SG, Myers CL, Fu Y, et al. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infection and immunity. 2005 Sep;73(9):6191–6193. doi: 10.1128/IAI.73.9.6191-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hujer AM, Bethel CR, Bonomo RA. Antibody mapping of the linear epitopes of CMY-2 and SHV-1 beta-lactamases. Antimicrob Agents Chemother. 2004 Oct;48(10):3980–3988. doi: 10.1128/AAC.48.10.3980-3988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drawz SM, Babic M, Bethel CR, Taracila M, Distler AM, Ori C, et al. Inhibition of the class C beta-lactamase from Acinetobacter spp.: insights into effective inhibitor design. Biochemistry. 2010 Jan 19;49(2):329–340. doi: 10.1021/bi9015988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papp-Wallace KM, Taracila M, Hornick JM, Hujer AM, Hujer KM, Distler AM, et al. Substrate selectivity and a novel role in inhibitor discrimination by residue 237 in the KPC-2 beta-lactamase. Antimicrob Agents Chemother. 2010 Jul;54(7):2867–2877. doi: 10.1128/AAC.00197-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drawz SM, Taracila M, Caselli E, Prati F, Bonomo RA. Exploring sequence requirements for C(3) /C(4) carboxylate recognition in the Pseudomonas aeruginosa cephalosporinase: Insights into plasticity of the AmpC beta-lactamase version 2. Protein Sci. 2011 Mar 14;20:941–958. doi: 10.1002/pro.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hujer AM, Page MG, Helfand MS, Yeiser B, Bonomo RA. Development of a sensitive and specific enzyme-linked immunosorbent assay for detecting and quantifying CMY-2 and SHV beta-lactamases. J Clin Microbiol. 2002 Jun;40(6):1947–1957. doi: 10.1128/JCM.40.6.1947-1957.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006 Jan 15;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 32.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic acids research. 2003 Jul 1;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997 Dec;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 34.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infection and immunity. 2007 Dec;75(12):5597–5608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breslow JM, Meissler JJ, Jr, Hartzell RR, Spence PB, Truant A, Gaughan J, et al. Innate Immune Responses to Systemic Acinetobacter baumannii infection in Mice: Neutrophils, but not IL-17, Mediate Host Resistance. Infection and immunity. 2011 May 16; doi: 10.1128/IAI.00069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Breij A, Eveillard M, Dijkshoorn L, van den Broek PJ, Nibbering PH, Joly-Guillou ML. Differences in Acinetobacter baumannii strains and host innate immune response determine morbidity and mortality in experimental pneumonia. PLoS One. 2012;7(2):e30673. doi: 10.1371/journal.pone.0030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fife KH, Wheeler CM, Koutsky LA, Barr E, Brown DR, Schiff MA, et al. Dose-ranging studies of the safety and immunogenicity of human papillomavirus Type 11 and Type 16 virus-like particle candidate vaccines in young healthy women. Vaccine. 2004 Jul 29;22(21–22):2943–2952. doi: 10.1016/j.vaccine.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 38.Harro C, Betts R, Orenstein W, Kwak EJ, Greenberg HE, Onorato MT, et al. Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from the first study of the vaccine dose range in humans. Clinical and vaccine immunology : CVI. 2010 Dec;17(12):1868–1874. doi: 10.1128/CVI.00356-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006 Mar 30;354(13):1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005 Apr 22;23(22):2902–2908. doi: 10.1016/j.vaccine.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 41.Treanor JJ, Schiff GM, Couch RB, Cate TR, Brady RC, Hay CM, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006 May 1;193(9):1223–1228. doi: 10.1086/503050. [DOI] [PubMed] [Google Scholar]
- 42.Aziz A, Aziz S, Li DS, Murphy L, Leone N, Kennedy M, et al. Efficacy of repeated high-dose hepatitis B vaccine (80 microg) in patients with chronic liver disease. J Viral Hepat. 2006 Apr;13(4):217–221. doi: 10.1111/j.1365-2893.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 43.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000 Oct 16;192(8):1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007 Jan 26;315(5811):528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 45.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. 2003 Feb;15(1):120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 46.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 Jun 19;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]