Abstract
Purpose
Determine the relationship between diet and metabolic abnormalities among adult survivors of childhood acute lymphoblastic leukemia (ALL).
Methods
We surveyed 117 adult survivors of childhood ALL using the Harvard Food Frequency Questionnaire. Physical activity energy expenditure (PAEE) was measured with the SenseWear Pro2 Armband. Insulin resistance was estimated using the Homeostasis Model for Insulin Resistance (HOMA-IR). Visceral and subcutaneous adiposity were measured by abdominal CT. Adherence to a Mediterranean diet pattern was calculated using the index developed by Trichopoulou. Subjects were compared using multivariate analysis adjusted for age and gender.
Results
Greater adherence to a Mediterranean diet pattern was associated with lower visceral adiposity (P=0.07), subcutaneous adiposity (P<0.001), waist circumference (P=0.005), and body mass index (P=0.04). For each point higher on the Mediterranean Diet Score, the odds of having the metabolic syndrome fell by 31% (OR 0.69; 95% CI 0.50, 0.94; P = 0.019). Higher dairy intake was associated with higher HOMA-IR (P =0.014), but other individual components of the Mediterranean diet, such as low intake of meat or high intake of fruits and vegetables, were not significant. PAEE was not independently associated with metabolic outcomes, although higher PAEE was associated with lower body mass index.
Conclusions
Adherence to a Mediterranean diet pattern was associated with better metabolic and anthropometric parameters in this cross-sectional study of ALL survivors.
Keywords: insulin resistance, leukemia, Mediterranean diet, obesity, survivorship
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common cancer of childhood. Between 1970 and 2006, the mortality rate for childhood leukemia dropped by over 60% (1). Today, the large majority of children with ALL are anticipated to become long-term survivors; among those diagnosed before age 15, current five-year survival exceeds 88% (1). Nevertheless, for many patients survivorship is accompanied by serious health problems (2-6). A report from the Childhood Cancer Survivor Study found that by thirty years from their diagnosis, 73% of pediatric cancer survivors will develop at least one chronic physical health condition, while in 42% the condition will be severe, life-threatening, disabling, or result in death (7).
ALL survivors are at increased risk for obesity, insulin resistance, and increased visceral and subcutaneous adiposity (8-13). We have reported insulin resistance and derangements in the leptin-adiponectin system even among normal-weight ALL survivors (11, 14). Importantly, a history of cranial radiotherapy (CRT) increases the risk of obesity and insulin resistance in this population, but obesity and insulin resistance are found even among ALL survivors who did not receive CRT (11).
The concept of the Mediterranean Diet was first described in the 1950s, when the Seven Countries Study suggested that the long life expectancy, very low prevalence of heart disease and low prevalence of certain cancers seen in the Greek island of Crete might be diet and lifestyle-related (15). Subsequent investigation has found a reduction in coronary events (16, 17), protection from obesity (18-20) and prevention of insulin resistance and the metabolic syndrome (21-25) among subjects adherent to a Mediterranean-style diet, which includes a high intake of fruits and vegetables and low intake of meat. Whether diet may contribute to insulin resistance and increased adiposity in ALL survivors is not known.
METHODS
A detailed description of the ALLIFE Study has been published elsewhere (11, 14, 26). Briefly, 118 long-term survivors of childhood acute lymphoblastic leukemia (diagnosed between 1970 and 2000) were recruited from the Dallas area. Subjects were young (median age 23, range 18-37 years) and many years from ALL treatment (mean, 17.5 years). At baseline, anthropometric measures, biochemical measures, abdominal computed tomography (CT), full-body dual-energy x-ray absorptiometry scan (DXA), physical activity energy expenditure (PAEE) and the Harvard Food Frequency Questionnaire (FFQ) were completed. The anthropometric measures included: height, weight, waist circumference. Waist circumference was measured at the level of superior iliac crest to the nearest 0.1 cm. Body mass index (BMI) was defined as weight in kilograms divided by height in meters, squared. Blood pressure was measured using an automatic oscillometric device (Series #52,000, Welch Allyn, Inc., Arden, NC). Venous blood samples were taken after a 12-hour overnight fast. Glucose was measured at the University of Texas Southwestern Medical Center GCRC Core Laboratory by the glucose oxidation methodology using an oxygen electrode. A commercial radioimmunoassay was used to measure insulin levels (Linco Research, Inc., St. Charles, MO) (intra-assay CV, 3.1%; inter-assay CV, 6.0%; detection limit, 2 μU/ml; sensitivity, 100%). Insulin resistance was estimated using the homeostasis model for assessment of insulin resistance (HOMA-IR) (27). Subjects were categorized as being insulin resistant when HOMA-IR was equal to or more than 2.86 (above the 75th percentile for HOMAIR derived from the Third National Health and Nutrition Examination Survey) (28). Total cholesterol, LDL-C, HDL-C, and triglycerides were measured through the GCRC Core laboratory using the Beckman Synchron CX9ALX system (Beckman Coulter, Fullerton, CA) (29-31). The Harvard FFQ is a well-validated and much-studied assessment tool for measuring usual food intake over the previous year (32-34). One subject who did not complete the FFQ questionnaire was excluded from this analysis.
Presence of the metabolic syndrome was defined by three or more of the following: triglycerides > 150 mg/dl; systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; fasting blood glucose ≥ 100 mg/dl; waist circumference > 102 cm in men or >88 cm in women; HDL <40 mg/dl in men or < 50 mg/dl in women (35). CT scans of the abdomen were performed on all subjects using an electron beam CT scanner (Imatron; General Electric, Milwaukee, Wisconsin) (26). Images were analyzed using specialized software (Tomovision, Montreal, Canada). A contiguous series of CT images between the L4-L5 and L3-L4 vertebral disc spaces were used to calculate visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) mass. DXA (Lunar DPX scanner, MEC, Minster, Ohio) was used to calculate total body fat mass and percent body fat.
Physical activity energy expenditure (PAEE) was objectively measured with the SenseWear Pro2 Armband (BodyMedia, Pittsburgh, PA), a device that combines a biaxial accelerometer with sensors to detect heat flux, galvanic skin response, skin temperature, and near-body temperature (36, 37). The SenseWear armband classifies activities (walking, running, resistance training, biking, riding in a car, resting) and applies algorithms to determine energy expenditure. In validation studies, physical activity and energy expenditure measures from the SenseWear armband compare favorably with those from other activity monitors, indirect calorimetry, and doubly labeled water (38-41). Participants wore the SenseWear armband on their upper right arm for seven consecutive days, removing it only for bathing and swimming. Custom channel algorithms for InnerView Research Software (version 4.0) were used to calculate physical activity duration (minutes per day) and energy expenditure (kilocalories/day and kilocalories/kilogram/day).
The Mediterranean diet is characterized by a high intake of fruits, vegetables, fish, and legumes, a low intake of meat and dairy products, and a moderate amount of alcohol. Additionally, meals are taken in a social setting. For this study, adherence to the Mediterranean diet pattern was calculated using a method described in 2003 by Trichopoulou (42) and used extensively in Mediterranean diet research (43-47). Briefly, the diet is divided into positive components (fruits, vegetables, fish, cereals, legumes, olive oil or a high ratio of monounsaturated to saturated fat) and negative components (meat and dairy). Median intake of each component is calculated. Subjects score points by being at or above the median intake of positive components and below the median intake of negative components. Subjects can score a single point for intake of alcohol within a specified range (one to six drinks per day for men and ½ to three drinks per day for women). Of note, this range of alcohol intake is the standard for calculating Mediterranean diet adherence and type of alcoholic beverage is not specified. As legume consumption was estimated with only two questions: consumption of “tofu or soybeans,” and consumption of “peas or lima beans,” in the FFQ, legumes were incorporated into vegetable consumption. Therefore, a subject who is adherent to a Mediterranean Diet pattern on all components would score 8, while a subject who was non-adherent to a Mediterranean Diet pattern on all components would score 0. Using this method, subjects within a population can be compared to one another as being more or less adherent to a Mediterranean diet, rather than to an external standard.
Medians, means, and standard deviations were calculated for dietary characteristics. As noted, median intake is used to calculate the Mediterranean Diet Score for each participant. Other dietary characteristics (total kilocalorie intake/day, total fat intake/day) and Mediterranean Diet Scores were compared by gender and whether or not a subject had a history of treatment with CRT using Wilcoxon rank sum testing. The relationships between the Mediterranean Diet Score and anthropometric and metabolic outcomes were tested using univariate followed by multivariate regression analysis. Variables tested, with P less than 0.05 considered significant, included physical activity energy expenditure (PAEE), demographic variables (age, age at treatment, gender, race/ethnicity, education), and other treatment variables (dexamethasone, anthracyclines, and others). Finally, analyses were performed with all subjects combined and then by separating subjects based on gender and whether or not a subject had a history of treatment with CRT.
To further investigate the effect of increasing adherence to a Mediterranean diet pattern, logistic regression was used to calculate odds ratios. Subjects were grouped by Mediterranean Diet Score (0-3, 4-5, or 6-8). Subjects in then least adherent group (score 0-3) were considered a reference group. Multivariate logistic regression, including adjustment for age and gender, was used to test the odds of having a specific metabolic or anthropometric abnormality by Mediterranean Diet Score group (4-5 or 6-8). Comprehensive model testing was performed to check for other significant demographic or treatment variables, with a P less than 0.05 considered significant. Variables tested in this way included physical activity energy expenditure (PAEE), other demographic variables (age, age at diagnosis, race/ethnicity, education), and other treatment variables (dexamethasone, anthracyclines, and others). A multivariate linear regression model including age, gender, and the Mediterranean Diet Score was used to calculate the P for trend for each metabolic or anthropometric abnormality by Mediterranean Diet Score. All calculations were completed with STATA (College Station, Texas). All subjects provided written informed consent and the study was approved by the institutional review boards at The University of Texas Southwestern Medical Center and the Cooper Institute.
RESULTS
The majority (56%) of subjects were female; 25% were of minority race or Hispanic. A history of CRT was present in 15 (29%) men and 25 (38%) women. (Table 1) The average body mass index (BMI) among all men was 26.6 kg/m2 and 27.5 kg/m2 among all women. Total reported caloric intake varied widely but averaged 1872 kilocalories/day (standard deviation (SD), 1115) among men and 1562 kilocalories/day (SD, 852) among women (P = 0.02). Further, mean total fat intake was 68 grams/day among men and 55.4 grams/day among women (P = 0.05). Subjects with a history of CRT had higher total body fat, waist circumference, and body mass index, and lower lean body mass, yet the reported dietary intake did not differ by history of treatment with CRT. (Table 2) Alcohol intake was low overall; mean intake among men over the age of 21 was 1 glass per day and among women over the age of 21 years was 1 glass per week. Interestingly, many subjects (>25%) reported no alcohol intake at all. No subject achieved a Mediterranean Diet Score of 8. The mean (standard deviation) Mediterranean Diet Score among women was 4.5 (1.6) and among men was 3.9 (1.8); P = 0.09 for difference between genders. (Table 2)
Table 1.
Characteristics of 117 survivors of childhood acute lymphoblastic leukemia.
| All (117) | Men (52) | Women (65) | |
|---|---|---|---|
| Age at diagnosis, years (mean, SD) | 24.3 (4.9) | 23.9 (4.8) | 24.6 (5.0) |
| Age at time of diagnosis, years (mean, SD) | 6.7 (4.3) | 7.2 (4.3) | 6.3 (4.3) |
| Time since cancer, years (mean, SD) | 17.6 (6.0) | 16.7 (5.8) | 18.3 (6.2) |
| Ethnicity, n (%) | |||
| African American | 13 (11%) | 4 (8%) | 9 (14%) |
| White, non-Hispanic | 84 (72%) | 41 (79%) | 43 (83%) |
| Hispanic | 15 (13%) | 4 (8%) | 11 (17%) |
| Cranial radiotherapy, n (%) | 40 (34%) | 15 (29%) | 25 (38%) |
| Anthracycline chemotherapy, n (%) | 84 (72%) | 42 (81%) | 42 (65%) |
| Dexamethasone, n (%) | 13 (11%) | 4 (8%) | 9 (14%) |
Table 2.
Insulin resistance, anthropometric measurements, and reported dietary intake among 117 survivors of childhood leukemia, by gender and history of treatment with cranial radiotherapy (CRT).
| Whole Cohort | P | Women N= 65 | P | Men N=52 | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| CRT | No CRT | CRT | No CRT | CRT | No CRT | ||||
| Number | 40 | 77 | 25 | 40 | 15 | 37 | |||
| Glucose (mg/dl; mean, SD) | 95.7 (23.0) | 89.3 (7.3) | 0.02 | 96.5 (28.7) | 87.4 (6.8) | 0.02 | 94.3 (7.9) | 91.5 (7.4) | 0.2 |
| Insulin (μU/ml; mean, SD) | 22.1 (12.4) | 16.4 (8.1) | 0.008 | 23.0 (11.1) | 16.4 (8.2) | 0.006 | 20.5 (14.5) | 16.4 (8.0) | 0.5 |
| HOMA-IR* (mean, SD) | 5.28 (5.3) | 3.67 (2.0) | 0.005 | 5.53 (3.1) | 3.60 (3.6) | 0.003 | 4.8 (3.6) | 3.7 (2.0) | 0.5 |
| Total body fat kg; mean, SD) | 29.2 (13.0) | 22.1 (12.0) | 0.002 | 32.5 (13.1) | 26.0 (12.7) | 0.02 | 23.9 (11.4) | 18.0 (9.9) | 0.05 |
| Lean body mass (kg; mean, SD) | 47.8 (12.4) | 52.7 (11.0) | 0.04 | 42.2 (9.5) | 44.6 (6.5) | 0.2 | 56.9 (11.1) | 60.9 (8.20 | 0.2 |
| Body mass index (kg/m2; mean, SD) | 30.2 (8.2) | 26.7(6.0) | 0.008 | 31.6 (8.5) | 27.2 (7.1) | 0.01 | 27.8 (7.5) | 26.2 (4.5) | 0.5 |
| Waist circumference (cm; mean, SD) | 95.8 (16.1) | 89.2 (13.6) | 0.03 | 96.4 (16.3) | 89.1 (15.1) | 0.07 | 94.6 (16.3) | 89.2 (12.1) | 0.2 |
| Physical activity (kcal/kg/day; mean, SD) | 10.7 (8.5) | 12.2 (8.3) | 0.3 | 9.8 (6.6) | 8.0 (4.3) | 0.4 | 12.3 (11.1) | 16.8 (9.2) | 0.03 |
| Total daily kilocalories (mean, SD**) | 1936 (929) | 1893 (1030) | 0.8 | 1973 (1120) | 1588 (601) | 0.3 | 1876 (494) | 2223 (1277) | 0.6 |
| Total daily carbohydrates (grams; mean, SD) | 255 (141) | 232 (143) | 0.2 | 262(172) | 191 (83) | 0.1 | 244 (65) | 276 (179) | 0.9 |
| Total daily fat (grams; mean, SD) | 67 (34.0) | 69 (38.2) | 0.9 | 67.7 (39.5) | 59.2 (25.1) | 0.7 | 66.6 (23.6) | 79.7 (46.6) | 0.6 |
| Total daily protein (grams; mean, SD) | 83.6 (48.6) | 82.5 (42.5) | 0.9 | 87.9 (58.2) | 71.5 (27.2) | 0.4 | 76.4 (26.3) | 94.4 (52.2) | 0.3 |
| Daily vegetable intake (median servings)† | 1 to 3 | 1 to 3 | 0.5 | 1 to 3 | 1 to 3 | 0.09 | 1 to 3 | 1 to 3 | 0.3 |
| Daily fruit intake (median servings)† | 0.5 to 1.5 | 0.5 to 1.5 | 0.2 | 0.5 to 1.5 | 0.5 to 1.5 | 0.02 | 0.5 to 1.5 | 0.5 to 1.5 | 0.5 |
| Daily dairy intake (median servings)† | 0 to 0.5 | 0 to 0.5 | 0.06 | 0 to 0.5 | 0 to 0.5 | 0.3 | 0 to 0.5 | 0 to 0.5 | 0.1 |
| Daily whole grain intake (median servings)† | <0.5 | <0.5 | 0.9 | <0.5 | <0.5 | 0.9 | 0.5 to 1 | <0.5 | 0.6 |
| Daily fish intake (median servings)† | <0.2 | <0.2 | 0.8 | <0.2 | <0.2 | 0.7 | <0.2 | <0.2 | 0.9 |
| Ratio of monounsaturated: saturated fat | 1.8 | 1.7 | 0.9 | 1.8 | 1.7 | 0.9 | 1.5 | 1.7 | 0.9 |
| Daily meat intake (median servings)† | 0.5 to 1 | 0.5 to 1 | 0.9 | 0.5 to 1 | 0.5 to 1 | 0.2 | 0.3 to 1 | 0.5 to 1 | 0.2 |
| Weekly alcohol intake (median drinks)†,†† | 1 | 2 to 4 | 0.3 | 0 to 1 | 0 to 1 | 0.8 | 5 to 6 | 7 | 0.2 |
| Mediterranean diet score (median, SD) | 4.5 | 4 | 0.9 | 5 | 4.5 | 0.6 | 4 | 4 | 0.5 |
HOMA-IR: Homeostasis model of insulin resistance
SD: standard deviation
Median of categorical response options
Age over 21 years only
As expected, the odds of having the metabolic syndrome were increased by male gender (OR 5.3; 95% CI: 1.8, 15.8; P = 0.003) and with higher BMI (OR for each one-point increase in BMI 1.1; 95% CI: 1.0, 1.2; P = 0.005). Other demographic and treatment variables were not significantly associated with the anthropometric or metabolic outcomes.
Among all subjects, multivariate regression adjusted for age and gender revealed that greater adherence to a Mediterranean diet pattern was associated with lower visceral adiposity (β= -0.3; P=0.007) and subcutaneous adiposity (β= -0.11; P<0.001), smaller waist circumference (β= -2.17; P=0.005), and lower body mass index (β= -1.05; P=0.004). When the Mediterranean Diet Score was individually adjusted for daily total caloric intake results did not change direction or differ in strength (data not shown).
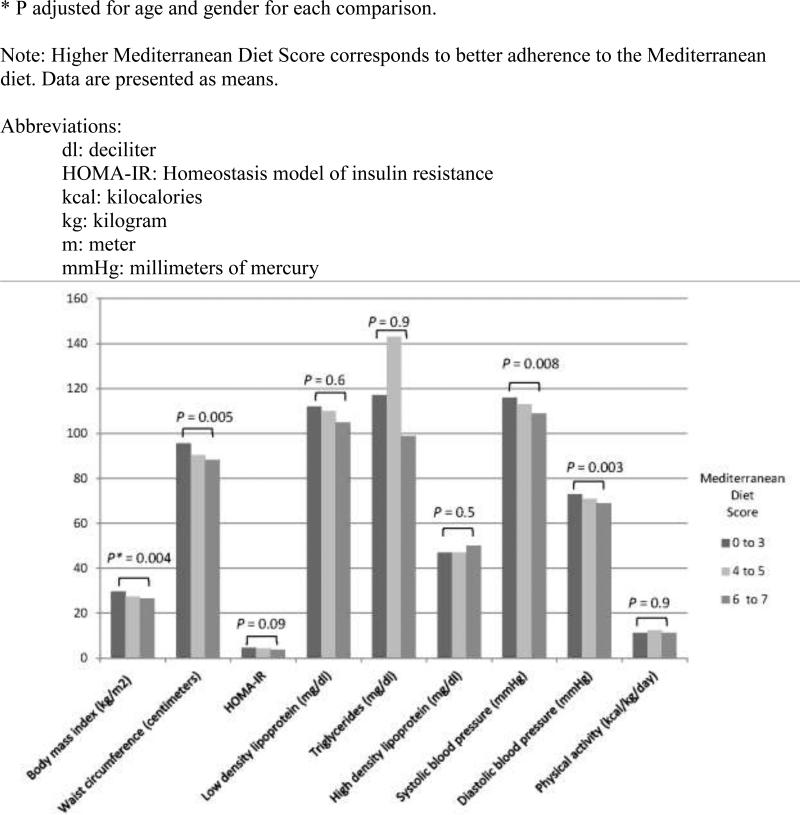
The relationships between the Mediterranean diet and components of the metabolic syndrome were evaluated. With higher Mediterranean Diet Score, average systolic and diastolic blood pressure were lower (adjusted for age and gender; P = 0.008 and 0.003, respectively; Figure 1). Additionally, better adherence to the Mediterranean diet was associated with a lower odds of having a waist circumference greater than 88cm for women or 102cm for men; multivariate odds ratio in the healthiest diet group was 0.2 (adjusted for age and gender; 95% CI: 0.1, 0.7; P for trend = 0.003; Table 3) when compared to the lower two groups. Among those eating the most Mediterranean-style diet (Mediterranean Diet Score 6-8), compared to those eating the least Mediterranean-style diet (Mediterranean Diet Score 0-3), the odds ratio for presence of the metabolic syndrome was 0.1 (adjusted for age and gender; 95% CI: 0.01, 0.9; P = 0.004; Table 3). For each point higher on the Mediterranean Diet Score, the odds of having the metabolic syndrome fell by 31% (OR 0.69, for each point higher on the Mediterranean Diet Score, adjusted for age and gender; 95% CI: 0.50, 0.94; P = 0.019). Higher dairy intake was associated with higher HOMA-IR (insulin resistance, [β= -1.06; P=0.029]; adjusted for age and gender), but other individual components of the Mediterranean diet, such as low intake of meat, moderate intake of alcohol, or high intake of fruits and vegetables, when tested individually, were not significantly associated with any anthropometric or metabolic outcome.
Figure 1.
The relationships between adherence to a Mediterranean diet, measured by the Mediterranean Diet Score, and characteristics of obesity and the metabolic syndrome among 117 survivors of childhood leukemia.
Table 3.
The relationship between adherence to a Mediterranean diet, measured by the Mediterranean Diet Score, and characteristics of obesity and the metabolic syndrome among 117 survivors of childhood leukemia, using logistic regression to calculate odds ratios (OR).
| Mediterranean Diet Score | |||||||
|---|---|---|---|---|---|---|---|
| 0 - 3 | 4 - 5 | 6 - 8 | |||||
| N = 36 | N = 51 | N = 30 | |||||
| Anthropometric/metabolic parameters | N | OR (95% CI)† | N | OR (95% CI) | N | OR (95% CI) | P for trend |
| Waist circumference > 88 cm in women, > 102 cm in men | 20 | - | 17 | 0.4 (0.1, 1.0) | 7 | 0.2 (0.1, 0.7) | 0.003 |
| Systolic blood pressure ≥ 130 mmHg | 5 | - | 3 | 0.4 (0.1, 1.8) | 0 | - | 0.03 |
| Diastolic blood pressure ≥ 85 mmHg | 4 | - | 2 | 0.3 (0.1, 1.8) | 0 | - | 0.028 |
| Glucose ≥ 100 mg/dl | 3 | - | 11 | 3.5 (0.9, 14.5) | 2 | 0.7 (0.1, 4.6) | 0.96 |
| High density lipoprotein < 40 mg/dl in women, < 50 mg/dl in men | 20 | - | 19 | 0.5 (0.1, 1.5) | 6 | 0.2 (0.1, 0.8) | 0.01 |
| Triglycerides ≥ 150 mg/dl | 9 | - | 13 | 1.1 (0.4, 3.1) | 5 | 0.6 (0.2, 2.2) | 0.5 |
| Presence of the metabolic syndrome* | 9 | - | 11 | 0.9 (0.3, 2.7) | 1 | 0.1 (0.01, 0.9) | 0.04 |
| Body mass index ≥ 25 kilograms/meter2 | 28 | - | 31 | 0.3 (0.1, 0.9) | 18 | 0.3 (0.1, 1.1) | 0.04 |
| HOMA-IR** ≥ 2.86 | 22 | - | 37 | 1.5 (0.6, 3.8) | 15 | 0.6 (0.2, 1.6) | 0.36 |
Presence of the metabolic syndrome was defined by three or more of the following: triglycerides > 150 mg/dl; systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; fasting blood glucose ≥ 100 mg/dl; waist circumference > 102 cm in men or > 88 cm in women; HDL <40 mg/dl in men or < 50 mg/dl in women (35).
HOMA-IR: Homeostasis model of insulin resistance; subjects were categorized as being insulin resistant when HOMA-IR was equal to or more than 2.86 (above the 75th percentile for HOMA-IR derived from the Third National Health and Nutrition Examination Survey) (28).
Odds ratios were adjusted for age and gender.
Abbreviations:
cm: centimeter
dl: decileter
mg: milligram
mmHg: millimeters of mercury
Although we tested extensively for an effect of physical activity on metabolic and anthropometric outcomes in this population of ALL survivors, we did not find substantial associations. Importantly, higher physical activity energy expenditure (PAEE) was associated with lower percent body fat (β= -0.007; P<0.001). But, PAEE was not associated with lower body mass index, smaller waist circumference, less insulin resistance, or the presence or absence of the metabolic syndrome. PAEE was not independently associated with metabolic or anthropometric outcomes in the multivariate model that included gender and age. Furthermore, inclusion of PAEE in the individual logistic regression models of dietary intake and metabolic parameters did not alter our findings.
CONCLUSIONS
In this study of 117 young adult survivors of childhood ALL, greater adherence to a Mediterranean diet pattern was associated with improved anthropometric and metabolic measures. While no single subject reported complete adherence to the Mediterranean diet (characterized by high intake of fruits, vegetables, fish, cereals, and legumes, modest intake of alcohol, and low intake of meat and dairy), subjects whose reported intake was more consistent with a Mediterranean diet pattern had lower body mass index, a decreased risk of the metabolic syndrome, and lower measures of visceral and subcutaneous adiposity. Importantly, for this population, changes of a single point in the Mediterranean Diet Score were relevant. In other words, differences such as an additional 0.5 serving of vegetables per day, or 1 more serving of fish per week, were associated with significantly improved body mass index, adiposity, and blood pressure, and lower risk of the metabolic syndrome.
ALL is the most common cancer of childhood and accounts for a large proportion of current survivors. This population has been shown repeatedly to be at increased risk for insulin resistance, the metabolic syndrome, derangements of leptin and adiponectin and cardiovascular death (3, 10, 11, 14). Treatment factors, especially a history of cranial radiotherapy (CRT), appear to be particularly relevant to the metabolic derangements, but insulin resistance has been described even among normal weight individuals or after adjustment for body mass index (10, 11). One prior evaluation of adult lymphoblastic leukemia (ALL) survivors, reporting from the Childhood Cancer Survivor Study (CCSS), reported that survivors were unlikely to follow dietary recommendations (48). In that analysis, the diets of 72 ALL survivors from the CCSS were compared with the 2007 World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention recommendations, the Dietary Approaches to Stop Hypertension (DASH) diet, and the 2005 United States Department of Agriculture Food Guide. Fruit and vegetable consumption was typically adequate, but otherwise subjects were not adherent to any of the dietary guidelines. Importantly, this study did not find an association between compliance with a recommended diet and body mass index or waist circumference, and associations between diet and the metabolic syndrome were not evaluated. Therefore, whether differences in dietary intake might be relevant to metabolic and anthropometric abnormalities in the ALL survivor population has not been previously reported.
Interestingly, while diet did not differ substantially between men and women or between those with a history of cranial radiotherapy and those without, better adherence to a Mediterranean diet pattern was associated with lower visceral and subcutaneous adiposity as well as a lower risk of the metabolic syndrome in this study, even after adjustment for age, gender, physical activity, and total caloric intake, and despite the fact that subjects were not eating a low-calorie or a lowfat diet. These findings suggest that while body weight is important, ALL survivors who eat more fruits, vegetables, grains, and fish, and less meat and dairy, may benefit metabolically even if they do not lose weight, and regardless of their treatment history. Our results are supported by work in the non-cancer population; a meta-analysis of more than 10,000 subjects reported that adherence to a Mediterranean diet was associated with a significantly lower risk of the metabolic syndrome (log-hazard ratio, -0.69; 95% CI: -1.24, -1.16) (23). Other studies suggest that when calorie-restricted, the Mediterranean diet may be effective for weight loss (49).
As noted, we did not find a strong effect of physical activity on metabolic or anthropometric outcomes, apart from waist circumference, in this study of ALL survivors. Nonetheless, physical activity remains an important component of health recommendations for this population. A recent report from the CCSS states that the risk of obesity was decreased among survivors who met guidelines for physical activity (50); while ALL survivors were not presented separately in that manuscript, increasing physical activity is likely to be beneficial in this subpopulation.
While our findings are important given the lack of literature in this area and its relevance to the cancer survivor community, this study does have some limitations. Importantly, diet was self-reported (via the Harvard Food Frequency Questionnaire (FFQ)) rather than directly measured, and subjects may have under-reported caloric intake (1872 kilocalories/day among men and 1562 kilocalories/day among women). Nonetheless, dietary reporting is not likely subject to differential reporting by variables such as blood pressure or cancer treatment history. Prior studies have found that most subjects report diet relatively accurately, with underreporting of caloric intake in both obese and non-obese subjects (51, 52). Furthermore, the Harvard FFQ has been used extensively in dietary research and is considered reliable and reproducible (32, 33).
Also of note, adjustment for total caloric intake did not change the impact of adherence to the Mediterranean diet on metabolic or anthropometric outcomes. Another limitation is that this study did not include a non-cancer (control) population. Future studies of the effect of diet on metabolic and anthropometric parameters in the ALL survivor population could address this comparison. Finally, while this study remains the largest dietary study to date in the ALL survivor population (48), sample size limitations did preclude some analyses.
In conclusion, in this cross-sectional study of adult survivors of childhood leukemia, subjects who reported a more Mediterranean-style diet had lower body mass index, lower levels of body fat, and a lower risk of the metabolic syndrome. Although further research is needed, these findings suggest that leukemia survivors should consider adopting the Mediterranean diet, including higher intake of fruits and vegetables and lower intake of meat and dairy.
ACKNOWLEDGEMENTS
Designed research: Oeffinger, Tonorezos, Moskowitz, Robien, Ross, Church
Conducted research: Oeffinger, Tonorezos, Eshelman-Kent, Ross, Church
Statistical analysis: Tonorezos
Wrote paper: Tonorezos, Robien, Eshelman-Kent, Moskowitz, Ross, Church
Primary responsibility for final content: Oeffinger
Sources of support: This work was supported by research grants from the National Institutes of Health (R01-CA 100474 and K05-CA165702), the Howard J. and Dorothy Adleta Foundation, and the Donald W. Reynolds Cardiovascular Research Center at Dallas, the General Clinical Research Center (Grant M01-RR-00633 and CTSA UL1-RR-024982), and an American Cancer Society Cancer Control Career Development Award.
Abbreviations
- ALL
acute lymphoblastic leukemia
- BMI
body mass index
- CRT
cranial radiotherapy
- FFQ
food frequency questionnaire
- HOMA-IR
homeostasis model for insulin resistance
- PAEE
physical activity energy expenditure
Footnotes
All authors read and approved the final manuscript. None of the authors has a possible conflict of interest to declare.
REFERENCES
- 1.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–34. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–38. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh JM, Nekhlyudov L, Goldie SJ, Mertens AC, Diller L. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152:409–17. W131–8. doi: 10.1059/0003-4819-152-7-201004060-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner R, Wallace WH, Levitt GA. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7:489–98. doi: 10.1016/S1470-2045(06)70724-0. [DOI] [PubMed] [Google Scholar]
- 6.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–9. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 8.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–45. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–12. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–65. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 11.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–9. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 13.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169:1381–8. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonorezos ES, Vega GL, Sklar CA, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer. 2012;58:31–6. doi: 10.1002/pbc.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–15. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 16.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–9. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 17.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–61. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 18.Romaguera D, Norat T, Vergnaud AC, et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am J Clin Nutr. 2010;92:912–21. doi: 10.3945/ajcn.2010.29482. [DOI] [PubMed] [Google Scholar]
- 19.Panagiotakos DB, Chrysohoou C, Pitsavos C, Stefanadis C. Association between the prevalence of obesity and adherence to the Mediterranean diet: the ATTICA study. Nutrition. 2006;22:449–56. doi: 10.1016/j.nut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Tyrovolas S, Bountziouka V, Papairakleous N, et al. Adherence to the Mediterranean diet is associated with lower prevalence of obesity among elderly people living in Mediterranean islands: the MEDIS study. Int J Food Sci Nutr. 2009:1–14. doi: 10.1080/09637480903130546. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez Leon EE, Henriquez P, Serra-Majem L. Mediterranean diet and metabolic syndrome: a cross-sectional study in the Canary Islands. Public Health Nutr. 2006;9:1089–98. doi: 10.1017/S1368980007668487. [DOI] [PubMed] [Google Scholar]
- 22.Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nunez-Cordoba JM, Martinez-Gonzalez MA. Mediterranean diet inversely associated with the incidence of metabolic syndrome: the SUN prospective cohort. Diabetes Care. 2007;30:2957–9. doi: 10.2337/dc07-1231. [DOI] [PubMed] [Google Scholar]
- 23.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Ciotola M, Giugliano D. Mediterranean diet and the metabolic syndrome. Mol Nutr Food Res. 2007;51:1268–74. doi: 10.1002/mnfr.200600297. [DOI] [PubMed] [Google Scholar]
- 25.Salas-Salvado J, Fernandez-Ballart J, Ros E, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168:2449–58. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- 26.Janiszewski PM, Oeffinger KC, Church TS, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92:3816–21. doi: 10.1210/jc.2006-2178. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960–5. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26:787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni KR, Garber DW, Jones MK, Segrest JP. Identification and cholesterol quantification of low density lipoprotein subclasses in young adults by VAP-II methodology. J Lipid Res. 1995;36:2291–302. [PubMed] [Google Scholar]
- 31.Aubourg A, Benboubker L, Picon L, Goupille P, Maillot F. Successful autologous stem cell transplantation in Gaucher disease patient with multiple myeloma. Am J Hematol. 2011;86:529–30. doi: 10.1002/ajh.22028. [DOI] [PubMed] [Google Scholar]
- 32.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 33.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 34.Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95:1182–9. doi: 10.3945/ajcn.111.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adult Treatment Panel III. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults. JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 36.Andre D, Pelletier R, Farringdon J, et al. The development of the SenseWear armband, a revolutionary energy assessment device to assess physical activity and lifestyle. BodyMedia, Inc. 2006 [Google Scholar]
- 37.Liden CB, Wolowicz M, Stivoric J, et al. Characterization and implications of the sensors incorporated into the SenseWear armband for energy expenditure and activity detection. BodyMedia, Inc. 2006 [Google Scholar]
- 38.Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband (TM) to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36:897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 39.King GA, Torres N, Potter C, Brooks TJ, Coleman KJ. Comparison of activity monitors to estimate energy cost of treadmill exercise. Med Sci Sports Exerc. 2004;36:1244–51. doi: 10.1249/01.mss.0000132379.09364.f8. [DOI] [PubMed] [Google Scholar]
- 40.Welk GJ, McClain JJ, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity. 2007;15:918–28. doi: 10.1038/oby.2007.624. [DOI] [PubMed] [Google Scholar]
- 41.St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742–9. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- 42.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 43.Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 44.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 45.Trichopoulou A, Orfanos P, Norat T, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330:991. doi: 10.1136/bmj.38415.644155.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maillot M, Issa C, Vieux F, Lairon D, Darmon N. The shortest way to reach nutritional goals is to adopt Mediterranean food choices: evidence from computer-generated personalized diets. Am J Clin Nutr. 2011;94:1127–37. doi: 10.3945/ajcn.111.016501. [DOI] [PubMed] [Google Scholar]
- 47.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93:601–7. doi: 10.3945/ajcn.110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:815–22. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Met Syn Rel Dis. 2011;9:1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 50.Green DM, Cox CL, Zhu L, et al. Risk factors for obesity in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30:246–55. doi: 10.1200/JCO.2010.34.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braitman LE, Adlin EV, Stanton JL., Jr Obesity and caloric intake: the National Health and Nutrition Examination Survey of 1971-1975 (HANES I). J Chron Dis. 1985;38:727–32. doi: 10.1016/0021-9681(85)90114-6. [DOI] [PubMed] [Google Scholar]
- 52.Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327:1893–8. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]