Abstract
Chronic exposure to elevated levels of manganese (Mn2+) causes neuronal injury and inflammatory activation of glia. Astrocytes selectively accumulate Mn2+, which inhibits mitochondrial respiration and increases production of reactive oxygen species. We previously reported that sub-acute exposure to low micromolar levels of Mn2+ in primary astrocytes inhibited ATP-induced calcium (Ca2+) signaling, associated with decreased levels of endoplasmic reticulum Ca2+ and increased mitochondrial Ca2+ loads. In the present studies, we postulated that the mechanism underlying the capacity of Mn2+ to inhibit these purinergic signals in astrocytes could be due to competition with Ca2+ for entry through a plasma membrane channel. These data demonstrate that acutely applied Mn2+ rapidly inhibited ATP-induced Ca2+ waves and transients in primary striatal astrocytes. Mn2+ also decreased influx of extracellular Ca2+ induced by 1-oleoyl-2-acetyl-sn-glycerol (OAG), a direct activator of the transient receptor potential channel, TRPC3. The TRPC3 inhibitor, pyrazole-3, prevented ATP- and OAG-dependent transport of Mn2+ from extracellular stores, demonstrated by a dramatic reduction in the rate of fluorescence quenching of Fura-2. These data indicate that Mn2+ can acutely inhibit ATP-dependent Ca2+ signaling in astrocytes by blocking Ca2+ entry through the receptor-operated cation channel, TRPC3. Loss of normal astrocytic responses to purinergic signals due to accumulation of Mn2+ could therefore comprise critical homeostatic functions necessary for metabolic and trophic support of neurons.
Keywords: Astrocytes, Calcium, TRP channels, Manganese, ATP
1. Introduction
Manganese (Mn2+) is essential for normal development and physiological function but high-level exposure leads to a progressive neurodegenerative condition in the basal ganglia, as well as other sub-cortical and cortical structures, that is accompanied by neuroinflammatory activation of glial cells. Manganese is required for a number of critical enzymes in the CNS including Mn-superoxide dismutase and glutamine synthetase, the latter of which is exclusively expressed in astrocytes and accounts for 80% of total brain Mn2+ (Norenberg and Martinez-Hernandez, 1979, Wedler and Denman, 1984). Elevated levels of Mn2+ accumulate in the basal ganglia, particularly in astrocytes, resulting in phenotypic changes characteristic of inflammatory activation (Hazell, 2002). Astrocytes take up Mn2+ through high-affinity transport systems such as the divalent metal transporter 1 (DMT-1) and transferrin receptor (Aschner et al., 1992, Erikson and Aschner, 2006). Additional membrane channels are also implicated in Mn2+ transport in astrocytes (Aschner et al., 1992, Crossgrove and Yokel, 2005, Tjalkens et al., 2006, Yokel, 2009), suggesting a complex mechanism for maintaining Mn2+ homeostasis that likely relies on multiple specific and general transporters.
Previous studies from our laboratory reported that sub-acute exposure to low micromolar levels of Mn2+ in primary cultured astrocytes inhibited ATP-induced calcium (Ca2+) signaling (Tjalkens et al., 2006). The observed inhibition of Ca2+ waves and transients was associated with a depletion of thapsigargin-sensitive intracellular stores, implicating inhibition of store-operated channels as possible mechanism in the decreased Ca2+ response to ATP. ATP is a major paracrine signaling factor that mediates the transmission of intercellular Ca2+ waves between astrocytes that are important for regulation of synaptic function, metabolism, and cerebral blood flow (Haydon and Carmignoto, 2006). Astrocytic responses to ATP are mediated by P2Y and P2X purinergic receptors; P2Y metabotropic receptors are activated by low physiological levels of extracellular ATP (0.1 - 10 μM), whereas P2X ionotropic receptors are activated at high levels of ATP (>100 μM) that are often associated with neurological injury (James and Butt, 2002). Activation of P2Y receptors is coupled to phospholipase C (PLC)-mediated generation of inositol triphosphate (IP3), which causes release of Ca2+ from the endoplasmic reticulum, as well as generation of diacylglycerol (DAG), which stimulates Ca2+ entry from extracellular stores.
Because DAG is a known activator of the transient receptor potential (TRP) channel TRPC3 (Hofmann et al., 1999), we postulated that this plasma membrane channel could be a site of inhibition of Ca2+ influx by Mn2+. It has been reported that TRPC3 in astrocytes mediates Ca2+ entry (Grimaldi et al., 2003) and high levels of TRPC3 mRNA are expressed in the basal ganglia in both rodent (Grimaldi et al., 2003, Kunert-Keil et al., 2006) and human (Riccio et al., 2002). Furthermore, studies in vascular smooth muscle cells reported that TRPC3 activation is associated with stimulation of pyrimidine receptors (Reading et al., 2005) and is likely a common pathway in for Ca2+ entry via PLC-coupled channels (Hofmann et al., 1999, Lintschinger et al., 2000). Thus, the capacity of Mn2+ to inhibit ATP-induced Ca2+ signaling in astrocytes could be mediated by competition for Ca2+ entry through TRP channels, thereby decreasing the amplitude of evoked Ca2+ waves and transients. Mn2+ can substitute for Ca2+ in a number of transport processes due to similarities in both valence and atomic radius but can also inhibit critical Ca2+ transporters (Chance, 1965, Gunter et al., 2006).
In contrast to previous studies using longer exposures to Mn2+, we set out to identify whether acutely administered Mn2+ could rapidly block ATP-activated Ca2+ signaling in astrocytes by inhibiting Ca2+ influx across the plasma membrane. We observed that Mn2+ rapidly inhibited mechanically-induced Ca2+ waves in astrocytes and similarly decreased the amplitude of ATP-induced Ca2+ transients. Mn2+ also decreased Ca2+ transients in astrocytes induced by the DAG mimic, 1-oleoyl-2-acetyl-sn-glycerol (OAG), a direct activator of TRPC3. Using the Fura-2 quenching technique, we demonstrated that both ATP and OAG caused entry of extracellular Mn2+ into astrocytes that rapidly quenched Fura-2 fluorescence. Mn-dependent quenching of Fura-2 in response to ATP or OAG was prevented by the TRPC3 inhibitor, pyrazole-3 (Pyr3). Collectively, these data suggest that TRPC3 is a receptor-operated plasma membrane channel involved in ATP-induced Ca2+ signaling in astrocytes which may be a novel target of Mn2+ that broadly decreases the response to purinergic signals.
2. Material and Methods
Mice (Mus musculus, C57Bl/6J) were obtained from the Jackson Laboratory (Bar Harbor, ME), cell culture media supplemented with Earle's Salts and L-glutamine was purchase from Hylcone (Logan, UT), Hank's Balanced Salt Solution from GIBCO (Grand Island, NY), and fetal bovine serum and penicillin-streptomycin-neomycin were purchases from Invitrogen (Carlsbad, CA). Fluo-4 AM and Fura-2 dyes were purchased from Molecular Probes (Eugene, OR) and OAG was from Millipore (Bedford, MA). All other chemical reagents were ordered form Sigma Aldrich (St. Louis, MO) unless otherwise stated.
2.1. Cell culture
Primary striatal astrocytes were isolated from 1 - 3 day old C57Bl/6J mice, as previously described (Aschner and Kimelberg, 1991, Moreno et al., 2008). Striatal hemispheres were rapidly dissected, extracted, and maintained in Minimal Essential Media (MEM) supplemented with Earle's Salts and L-glutamine, with 10% Fetal Bovine Serum and 1% penicillin-streptomycin-neomycin (Life Technologies, Carlsbad, CA). Cells were grown to confluence at 37°C, 5% CO2 in a humid atmosphere for approximately three weeks. Cells were sub-cultured onto 4-well poly-D-lysine-coated cover glass chambered slides (Nalgene-Nunc, Rochester, NY) and allowed to grow to semi-confluence. In our laboratory, cultures consistently yield >98% astrocytes as determined by immunofluorescence staining for glial fibrillary acidic protein (Tjalkens et al., 2006). All procedures involving animals were conducted under a protocol approved by the Animal Care and Use Committee at Colorado State University according to the guidelines of the National Institutes of Health.
2.2. Calcium Imaging
Astrocytes were sub-cultured to approximately 75% confluency on 4-well chamber slides and incubated with 2 μM of Fluo-4 AM (ex: 490 nm, em: 515 nm) for 15 minutes at 37°C prior to imaging. Cells were imaged in media (MEM, without phenol red or sodium bicarbonate) supplemented with 10 mM HEPES buffer (pH 7.4) at 25 °C. The composition of inorganic salts in the media is: 1.8 mM CaCl2, 0.8 mM MgSO4, 5.3 mM KCl, 117 mM NaCl, and 1 mM NaPO4. Groups of approximately 15-30 contiguous cells per field of view were identified for imaging. Cells were stimulated with 1 μM ATP to selectively activate G protein-coupled (GPCR) metabotropic purinergic receptors rather than ionotropic receptors, (James and Butt, 2002) or with 1-oleoyl-2-acetyl-sn-glycerol (OAG; 100 μM), a selective TRPC channel agonist. Mn2+ was added 30 seconds prior to each agonist and images of Fluo-4 fluorescence were collected every 500 milliseconds for 120 seconds with camera binning set at 4×4 pixels with an exposure time of approximately 20 milliseconds. To observe recovery of Ca2+ transients three additions of 1 μM ATP we applied and washed out with imaging media after each application using a continuous flow cell. Prior to the second ATP addition Mn2+ (10 μM) was added to the imaging media for 30 seconds. Images were collected on a Zeiss Axiovert 200M microscope equipped with a Hammatsu ORCA-ER cooled charge-coupled device camera. Fluorescent intensity was expressed relative to the baseline image (F/F0), where F0 is the fluorescence level prior to stimulation. Datasets were analyzed using Slidebook software (v5.0; Intelligent Imaging Innovations, Inc., Denver, CO).
2.3. Mechanically-induced calcium waves
For Ca2+ wave propagation studies, astrocytes were sub-cultured onto poly-D-lysine coated 30 mm round glass coverslips and placed in a flow chamber (POCmini, Carl Zeiss, New York, NY). After collection of baseline Fluo-4 intracellular Ca2+ intensity for 10 seconds, Ca2+ waves were mechanically-induced with a 5 μm diameter drawn glass micropipet using a micromanipulator. Mn2+ was added approximately 30 seconds prior to stimulation and images were acquired every 500 milliseconds for 60 seconds. Fluorescent intensity was expressed relative to the baseline image (F/F0) in all cells within the wave activation site. Wave amplitude and distance were determined using Slidebook software (v5.0; Intelligent Imaging Innovations, Inc., Denver, CO).
2.4. Fura-2 quenching
Studies examining fluorescence quenching of the dye Fura-2 by Mn2+ were performed as previously described (Grimaldi, 2006), with slight modifications. Primary astrocytes subcultured into 4-well chambered slides were loaded for 25 minutes at 25 °C with 4 μM Fura-2 AM in Ca2+ containing HEPES-buffered Hank's Balanced Salt Solution (HBSS; Life Technologies, Carlsbad, CA). Cells were then rinsed with fresh buffer and incubated for an additional 25 minutes with either the TRPC3 specific inhibitor, Pyr3 (10 μM) or DMSO (0.1%) in Ca2+ containing HEPES-buffered HBSS at 37 °C. Prior to Fura-2 imaging the cells were rinsed with Ca2+-free HEPES-buffered HBSS. Images of Fura-2 fluorescence were collected every second for 15 minutes at ex: 340 nm, em: 510 nm, in order to detect quenching of the Fura-2 signal. Baseline fluorescence was established for the first 60 seconds and the cells were then stimulated with either 1 μM ATP or 100 μM OAG for an additional 6 minutes followed by 100 μM Mn2+ for the remaining 8 minutes. Fluorescence intensity was expressed relative to baseline (F/F0).
2.5. Statistical analysis
Comparisons three or more means with one independent variable was performed using one-way analysis of variance (ANOVA) followed by the Tukey's multiple post hoc test using Prism software (v4.0c, Graphpad Software, Inc., San Diego, CA). Comparison of two-group comparisons were analyzed using the Student's t-test. The rate of Fura-2 quenching was determined through non-linear regression. Results are expressed as the mean ± SEM from a minimum of 3 independent studies and for all experiments, p<0.05 was considered significant.
3. Results
3.1. Acute Mn2+ exposure decreases physiological glial-glial communication in striatal astrocytes
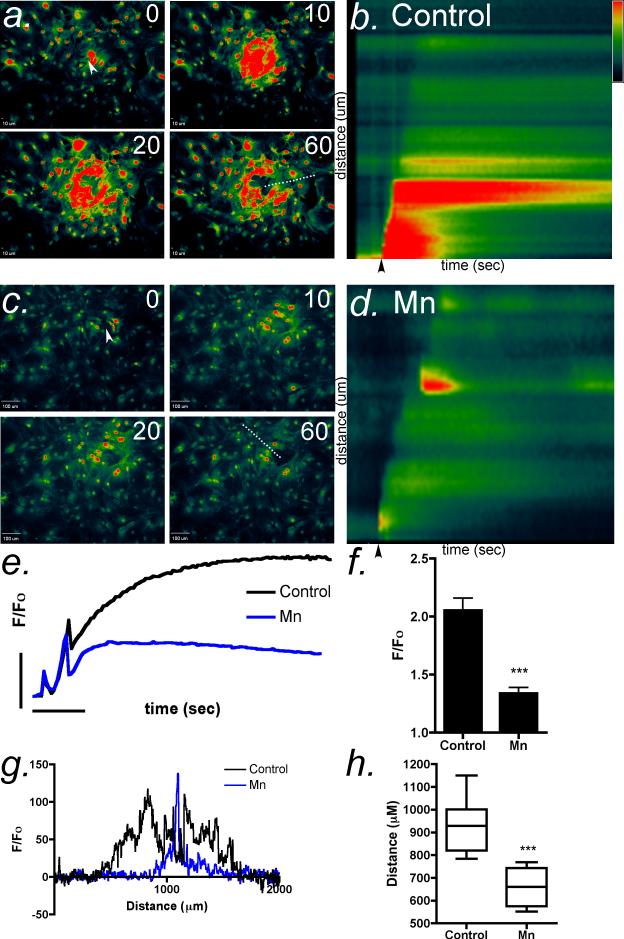
To examine the effect of Mn2+ on intercellular Ca2+ wave propagation in astrocytes, we mechanically stimulated a single cell in the center of a confluent field with a 0.5 μm glass micropipet to initiate ATP-dependent Ca2+ waves (Newman, 2001). The response was recorded using the Ca2+-sensitive dye, Fluo-4 AM, which does not significantly interact with Mn2+ (Bird et al., 2008, Johnson, 2010). Mechanical stimulation of control cells elicited robust intercellular Ca2+ waves that diminished in intensity approximately 500 μm away from the site of activation (Figure 1 a,b). Following the acute application of 10 μM Mn2+ to the extracellular media, the maximum amplitude of the intercellular Ca2+ transients from mechanically-induced intercellular waves was significantly decreased (Figure 1 c,d). Kymograph images of the Ca2+ wavefront indicated that acute exposure to Mn2+ decreased the intensity of the wave uniformly throughout the astrocyte syncytium (Figure 1 b,d). Similarly, the average intensity and distance of propagation of Ca2+ waves was decreased following acute application of Mn2+ to the imaging medium (Figure 1 e-h).
Figure 1. Manganese acutely inhibits calcium wave propagation in striatal astrocytes.
(a) Calcium waves were initiated in confluent cultures of striatal astrocytes loaded with Fluo-4 AM (2 μM) using a glass micropipette to stimulate a single astrocyte in the center of the field (white arrow). Images were collected every 500 msec for 60 sec. (b) Kymograph images were generated from the fluorescence intensity of Fluo-4 along a representative line drawn from the point of stimulation to the terminus of the Ca2+ wave across across the field of astrocytes (a, dotted line). Black arrows denote the point of stimulation. (c,d) The extent and intensity of Ca2+ waves is sharply diminished by acute application of Mn2+. (e,f) The mean intensity of intracellular Ca2+ responses in the cells acutely exposed to Mn2+ is significantly decreased compared to control. The vertical bar in (e) denotes 1 relative fluorescent unit; the horizontal bar denotes 10 sec. (g) Representative traces of the total distance of the wave front in control and Mn-treated cells. (h) Quantitative analysis of Ca2+ wave propagation indicates that Mn2+ decreases the distance traveled in striatal astrocytes by >70% relative to control (n=3 waves analyzed per group for each experiment over 3-4 independent experiments in separate cultures of striatal astrocytes; *** p<0.001).
3.2. Acute application of Mn2+ decreases ATP-induced Ca2+ transients
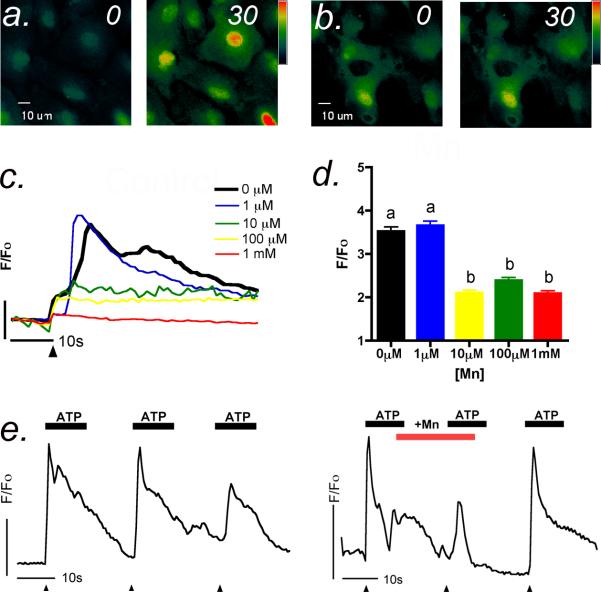
Activation of metabotropic purinergic receptors with physiological levels of ATP resulted in rapid increases in intracellular Ca2+ that were inhibited by acute application of Mn2+ (Figure 2 a,b). The shape of the ATP-induced transient reflects biphasic Ca2+ release, first from intracellular stores of the endoplasmic reticulum followed by an influx of Ca2+ from the extracellular space driven by the electrochemical gradient of Ca2+ through activation of channels in the plasma membrane. However, when Mn2+ was acutely added to the extracellular medium, the Ca2+ response was dose-dependently inhibited by low levels of Mn2+, with maximal inhibition observed as low as 10 μM Mn2+ (Figure 2 c,d). The concentration of Mn2+ added was only slightly above physiologic levels in the CNS, which are normally 2-8 μM (Pal et al., 1999). Representative traces and quantitative analysis of the average amplitude revealed a very steep dose-response curve that indicates a low threshold concentration at which Mn2+ acutely inhibits purinergic Ca2+ signaling in striatal astrocytes (Figure 2 c,d). Using a flow-cell chamber to examine the reversibility of the acute Mn2+ effect on ATP-induced Ca2+ transients (Figure 2 e,f), we found that control cells responded to ATP with repeated elevations in intracellular Ca2+ that were reversibly inhibited by Mn2+ at concentrations as low as 10 μM.
Figure 2. ATP-induced calcium transients are reversibly inhibited by low concentrations of manganese.
(a) Application of 1 μM ATP resulted in robust Ca2+ transients in striatal astrocytes that persisted for greater than 30 sec. (b) Addition of Mn2+ to the imaging media at concentrations as low as 10 μM acutely suppressed ATP-induced Ca2+ transients. (c) Representative traces of the astrocytic response to 1 μM ATP (black arrowhead indicates time of ATP addition) in the presence of increasing concentrations of Mn2+ (1 - 1000 μM) indicate a dose-dependent suppression of intracellular Ca2+ transients. (d) Quantitation of the maximum astrocytic Ca2+ response to 1 μM ATP in the presence of increasing concentrations of Mn2+. (e) Repeated additions of 1 μM ATP to striatal astrocytes using a flow chamber induce multiple Ca2+ transients that are reversibly inhibited by acute addition (f) of 10 μM Mn2+ to the flow cell. Vertical bars denote 1 relative fluorescent unit; horizontal bars denote 10 sec (n= 50-60 cells group; *** p<0.001).
3.3. Mn2+ interferes with OAG-induced Ca2+ entry through plasma membrane channels in striatal astrocytes
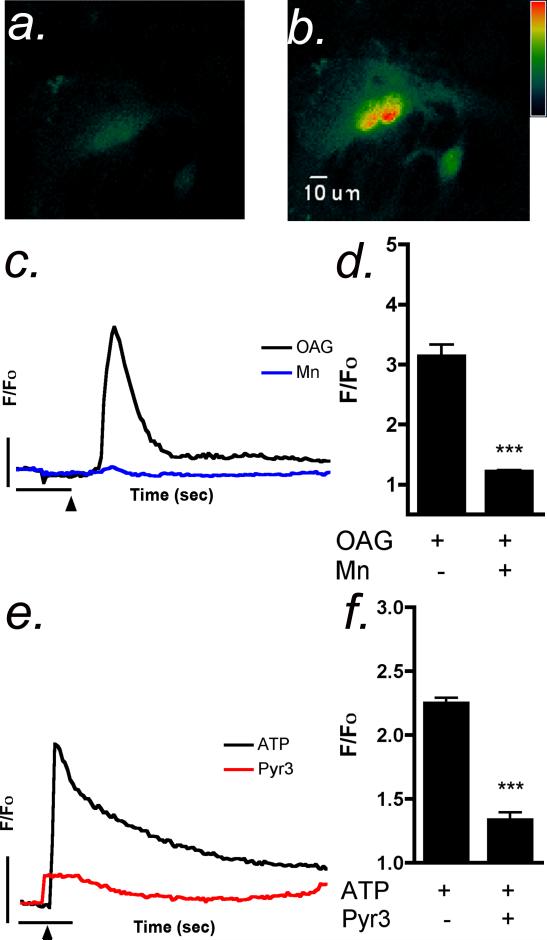
We postulated that the acute inhibitory effect of Mn2+ could be due to interference with Ca2+ influx into the astrocyte from extracellular stores. The non-selective cation channel TRPC3 is found in high abundance in the mid-brain and striatum of mice (Kunert-Keil et al., 2006) and could therefore could be a target for Mn2+ in astrocytes. The TRPC agonist, OAG, is an analog of DAG that selectively activates TRPC3, TRPC6 and TRPC7 receptors (Hofmann et al., 1999). Application of 100 μM OAG caused a robust Ca2+ response in astrocytes (Figure 3 a,b) that was attenuated in the presence of Mn2+ (Figure 3 b,c). The TRPC3 antagonist pyrazole-3 (Pyr3)(Kiyonaka et al., 2009) also suppressed ATP-induced Ca2+ transients, similarly to Mn2+ (Figure 3 e,f), strongly implicating the involvement of this channel in Ca2+ entry from extracellular stores as a result of ATP-induced transients in striatal astrocytes.
Figure 3. OAG-dependent calcium influx in striatal astrocytes is suppressed by Mn2+.
(a,b) Stimulation of striatal astrocytes with the membrane permeable DAG analog, OAG (100 μM), resulted in a robust Ca2+ transient in striatal astrocytes, indicated by increases in Fluo-4 fluorescence intensity. (c) Representative traces of OAG-induced intracellular Ca2+ transients in the absence (control) and presence of 10 μM Mn2+. (d) Quantitative analysis indicates that the acute application of Mn2+ attenuates the OAG Ca2+ response in striatal astrocytes. (e,f) The TRPC3 inhibitor, pyrazole-3 (Pyr3) reduces both the peak amplitude and plateau phase of ATP-induced Ca2+ transients in striatal astrocytes. Vertical bars denote 1 relative fluorescent unit; horizontal bars denote 10 sec (n=250-400 cells per group; *** p-<0.001).
3.4. Mn2+ influx occurs through TRPC3 in striatal astrocytes
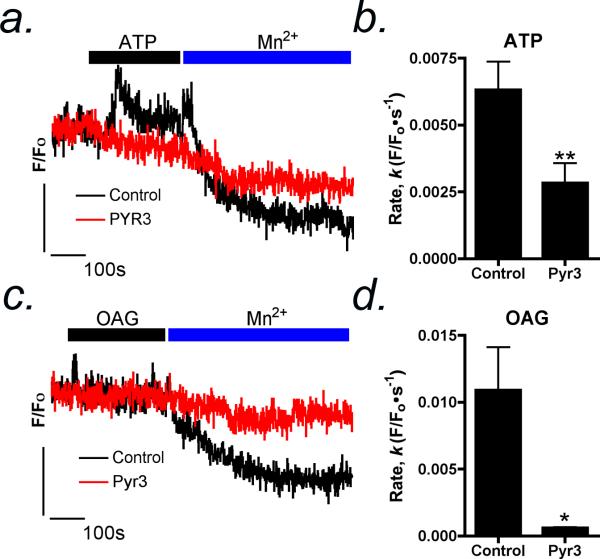
To examine the site of Mn2+ inhibition of OAG-induced Ca2+ influx across the plasma membrane in astrocytes, we utilized the ability of Mn2+ to quench the fluorescence signal of the Ca2+ indicator Fura-2 (Figure 4). Stimulation of astrocytes with 1 μM ATP caused a robust increase in intracellular Ca2+ followed by a secondary plateau phase of extracellular Ca2+ entry through receptor operated channels. Addition of 100 μM Mn2+ after stimulation with ATP resulted in rapid quenching of the Fura-2 fluorescence signal (Figure 4 a,b), plotted as the first order rate of decay (k, F/F0•sec-1), indicating Mn2+ entry into the cell. Likewise, application of Mn2+ after OAG caused rapid quenching of Fura-2 fluorescence (Figure 4 c,d). Selective inhibition of TRPC3 by Pyr3 significantly reduced the rate of Mn2+ mediated Fura-2 quenching following ATP- or OAG-activated Ca2+ entry by 55% and 95%, respectively (Figure 4 b,d).
Figure 4. Selective inhibition of TRPC3 reduces the rate of Mn2+-dependent Fura-2 quenching in striatal astrocytes.
(a) Following stimulation with 1 μM ATP, Mn2+ (100 μM) is rapidly taken up by striatal astrocytes, indicated by quenching of Fura-2 fluorescence intensity. (b) Mn-dependent quenching of Fura-2 fluorescence following ATP stimulation is prevented by the TRPC3 inhibitor, Pyr3. (c) 100 μM OAG induces direct influx of Mn2+ from the extracellular medium. (d) Pyr3 inhibits OAG-dependent Mn2+ influx and quenching of Fura-2 fluorescence. Data are presented as mean rate of decay of the Fura-2 signal ± SEM. Vertical bars denote 1 relative fluorescent unit; horizontal bars denote 10 sec (n=20-30 cells per group; * p<0.05, ** p<0.01).
4. Discussion
Studies examining the effects of Mn2+ on astrocyte function have largely focused on outcomes such as inhibition of mitochondrial respiration, loss of ATP, and production of reactive oxygen species (Brouillet et al., 1993, Zwingmann et al., 2003, Gunter et al., 2006). There is far less information on the ability of Mn2+ to disrupt Ca2+ signaling in astrocytes, although studies in brain mitochondria indicate that Mn2+ uptake by the outer mitochondrial membrane Ca2+ uniporter causes sustained increases in matrix Ca2+ and oxidative stress (Gavin et al., 1990). However, the identity of plasma membrane Ca2+ channels that could be targeted by Mn2+ is less clear. There are diverse transport mechanisms for Mn2+ across the plasma membrane (Aschner et al., 2005) and the present data suggest that one of these Ca2+ transporters could be competitively targeted by Mn2+. Our findings demonstrate that Mn2+ inhibits Ca2+ influx through receptor-activated cation channels in striatal astrocytes. We found that Ca2+ waves and transients stimulated by ATP-dependent purinergic signals are acutely inhibited by Mn2+, likely through competition for Ca2+ influx through TRPC3 channels. Dampening normal Ca2+ responses to purinergic stimuli in astrocytes could have dramatic consequences on neuronal function and survival, because these signaling events are critical for regulation of cerebral energy metabolism, blood flow, and synaptic function (Haydon, 2001), as well as for particular protection against excitotoxic neuronal injury (Pascual et al., 2005). Dysfunction in purinergic Ca2+ signaling has been associated with neuroinflammatory and neurodegenerative conditions such as Parkinson's and Alzheimer's disease (Iadecola, 2004, Burnstock et al., 2011) and the non-selective cation channel TRPC3 has been directly associated with pathophysiological activation of astrocytes in response to neuroinflammatory stimuli (Shirakawa, 2012). Thus, inhibition of ATP-dependent Ca2+ signaling by excessive exposure to Mn2+ could explain some of the phenotypic changes in astrocytes that lead to neuronal injury in brain regions where Mn2+ accumulates, such as the basal ganglia.
Propagation of Ca2+ waves in confluent astrocyte cultures models the pattern of intercellular Ca2+ waves in astrocytes in situ (Haydon and Carmignoto, 2006, Ullah et al., 2006). The primary gliotransmitter in astrocytes regulating intercellular Ca2+ waves is ATP, released through exocytosis via SNARE proteins (Newman, 2001, James and Butt, 2002, Pascual et al., 2005) and through connexin 43 hemichannels (Kang et al., 2008). Sub-acute exposure to low micromolar concentrations of Mn2+ are sufficient to inhibit Ca2+ wave activity (Tjalkens et al., 2006), consistent with the capacity of Mn2+ to cause mitochondrial sequestration of Ca2+ and decreases in thapsigargin-sensitive endoplasmic reticulum Ca2+ stores. Data from the present studies (Figure 1) indicated that Mn2+ applied only 30 seconds prior to stimulation of astrocytes inhibited both the intensity as well as the total distance travelled of mechanically-induced Ca2+ waves. Although these decreases in Ca2+ wave activity could result from both direct inhibition of Ca2+ channels as well as interference with intracellular second messenger signals (Barhoumi et al., 2010), the ability of similar divalent cations, such as Mg2+ and Ni2+, to inhibit Ca2+ influx across the plasma membrane (Crossgrove and Yokel, 2004, Ko et al., 2004, Lee et al., 2011) makes it much more likely that the rapid inhibition of Ca2+ waves observed with Mn2+ is mediated by direct blockade of plasma membrane Ca2+ channels in astrocytes.
Acute application of 10 μM Mn2+ or greater attenuated ATP-induced Ca2+ transients in cultured astrocytes (Figure 2). This concentration of Mn2+ is only slightly above that in extracellular fluid in the CNS (Pal et al., 1999) and similar to levels measured in rodent brain in chronic models of Mn2+ neurotoxicity (Liu et al., 2006, Aschner et al., 2009, Moreno et al., 2009), suggesting that quite low levels of exogenous Mn2+ in the CNS may subtly perturb normal homeostatic functions in astrocytes. However, intracellular Mn2+ levels in astrocytes are likely greater, given the high capacity for Mn2+ uptake (Erikson and Aschner, 2006). The reversibility of the effect of Mn2+ on ATP-induced transients (Figure 2e) strongly supports the conclusion that plasma membrane Ca2+ channels in astrocytes are an acute target of Mn2+. Moreover, extracellular Ca2+ is both a competitive and noncompetitive inhibitor of Mn2+ uptake for common non-selective sites of entry (Gavin et al., 1990, Crossgrove and Yokel, 2004), likely due to its similar size, oxidation state, and electrochemical properties. Studies in microvasular endothelial cells reported that Ca2+ concentrations negatively correlated with Mn2+ influx and that Mn2+ influx in Ca2+-free media was significantly greater than with Ca2+ present (Yokel et al., 2003), suggesting competition for entry through a common channel. Mn2+ uptake is also inhibited by the divalent cation Ni2+, which has been shown to inhibit receptor-operated and store-operated channels (Cui and Dannies, 1992, Kukkonen et al., 2001). Collectively, these data indicate that extracellular Mn2+ can acutely and reversibly inhibit Ca2+ influx in astrocytes.
Although astrocytes express both voltage-gated and receptor-operated Ca2+ channels (Barhoumi et al., 2010), we suspected that inhibition of non-selective cation channels permeable to both Ca2+ and Mn2+ could be responsible for the loss of ATP-induced Ca2+ signaling in astrocytes acutely exposed to Mn2+. Concentrations of ATP up to 10 μM activate P2Y receptors, which are metabotropic G protein-coupled receptors (GPCR) that cause rapid release of Ca2+ from intracellular stores through the PLC-IP3 pathway, with concomitant generation of DAG (James and Butt, 2002). Using the membrane-permeable analog of DAG, OAG, we determined that acutely applied Mn2+ inhibited OAG-induced Ca2+ transients in astrocytes (Figure 3). OAG activates TRPC3 channels leading to the influx of cations such as Ca2+ into astrocytes (Hofmann et al., 1999) and TRPC3 activation results in influx of both Ca2+ and Mn2+ across the plasma membrane of medullary kidney cells (Goel and Schilling, 2010). The data in Figure 3 (e,f) indicate that the TRPC3 inhibitor, Pyr3, abolished ATP-induced Ca2+ transients in striatal astrocytes, similar to Mn2+. Likewise, the rate of Mn2+-dependent quenching of Fura-2 fluorescence was significantly reduced by Pyr3 (Figure 4), strongly implicating TRPC3 as a site of Mn2+ influx in astrocytes mediating the observed reduction of Ca2+ signals during acute Mn2+ exposure. TRPC3 is selectively activated by DAG following stimulation of GPCR receptors, demonstrated by studies in which applied histamine caused rapid influx of Ca2+ that was quenched by Mn2+, further demonstrating competition for Ca2+ entry at this channel (Hofmann et al., 1999). These studies also reported that TRPC3, but not TRPC4, 5 or 6, was selectively inhibited by Mn2+ at concentrations similar to those used here, indicating that inhibition of TRPC channels by Mn2+ is highly selective for TRPC3. Of the canonical TRPC family members, it was previously reported that TRPC3 is expressed at significant levels in astrocytes (Grimaldi et al., 2003) and is activated by GPCR activation through the PLC-DAG pathway (Hofmann et al., 1999, Reading et al., 2005). Thus, the capacity of Pyr3 to decrease the rate of Mn-dependent Fura-2 strongly suggests that Mn2+ is competing with Ca2+ for entry through TRPC3 in response to ATP and OAG.
In conclusion, these studies support the hypothesis that Mn2+ acutely inhibits both mechanically stimulated Ca2+ waves and ATP-induced Ca2+ transients in astrocytes, in part, by preventing influx of extracellular Ca2+ through the transient receptor potential channel, TRPC3. The reversibility of this effect also suggests that the basis for this inhibition may be competition with Ca2+ for entry via this non-selective cation channel, although more detailed electrophysiological studies are required to determine the precise mechanism. These data indicate that in addition to inhibiting mitochondrial Ca2+ signaling (Gavin et al., 1990) and decreasing releasable pools of endoplasmic reticulum Ca2+ in astrocytes (Tjalkens et al., 2006), Mn2+ can acutely interfere with receptor-operated plasma membrane channels such as TRPC3 and thereby alter the pattern and amplitude of Ca2+ signals in response to purinergic signals. Such changes in these fundamental Ca2+ signaling pathways in astrocytes could have negative effects on neuronal function and provide additional insight into the mechanisms underlying the neurotoxicity of excess Mn2+.
Highlights (Streifel et al.).
Manganese rapidly inhibits calcium waves in astrocytes
ATP- and OAG-induced calcium transients are suppressed by manganese
The TRPC3 inhibitor pyrazole-3 prevents manganese entry in astrocytes
Acknowledgments
This work was supported by a grant from the National Institutes of Health (ES012941). We are grateful to Dr. Scott Earley for advice regarding inhibitors of TRPC3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Kimelberg HK. The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology. 1991;12:505–517. [PubMed] [Google Scholar]
- Barhoumi R, Qian Y, Burghardt RC, Tiffany-Castiglioni E. Image analysis of Ca2+ signals as a basis for neurotoxicity assays: promises and challenges. Neurotoxicol Teratol. 2010;32:16–24. doi: 10.1016/j.ntt.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GS, DeHaven WI, Smyth JT, Putney JW., Jr. Methods for studying store-operated calcium entry. Methods. 2008;46:204–212. doi: 10.1016/j.ymeth.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Chance B. The Energy-Linked Reaction Of Calcium With Mitochondria. J Biol Chem. 1965;240:2729–2748. [PubMed] [Google Scholar]
- Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25:451–460. doi: 10.1016/j.neuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier. IV. Evidence for brain influx through store-operated calcium channels. Neurotoxicology. 2005;26:297–307. doi: 10.1016/j.neuro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Cui ZJ, Dannies PS. Thyrotropin-releasing hormone-mediated Mn2+ entry in perifused rat anterior pituitary cells. Biochem J. 1992;283(Pt 2):507–513. doi: 10.1042/bj2830507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology. 2006;27:125–130. doi: 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990;266:329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M, Schilling WP. Role of TRPC3 channels in ATP-induced Ca2+ signaling in principal cells of the inner medullary collecting duct. Am J Physiol Renal Physiol. 2010;299:F225–233. doi: 10.1152/ajprenal.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M. Astrocytes refill intracellular Ca2+ stores in the absence of cytoplasmic [Ca2+] elevation: a functional rather than a structural ability. J Neurosci Res. 2006;84:1738–1749. doi: 10.1002/jnr.21064. [DOI] [PubMed] [Google Scholar]
- Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca 2+]I oscillations and is not involved in capacitative Ca 2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem Int. 2002;41:271–277. doi: 10.1016/s0197-0186(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Johnson IaTZS Michelle., editor. The Molecular Probes Handbook. Life Technologies Corporation; 2010. [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, Ishii M, Takemoto H, Ojida A, Watanabe K, Uemura A, Kurose H, Morii T, Kobayashi T, Sato Y, Sato C, Hamachi I, Mori Y. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EA, Park WS, Earm YE. Extracellular Mg(2+) blocks endothelin-1-induced contraction through the inhibition of non-selective cation channels in coronary smooth muscle. Pflugers Arch. 2004;449:195–204. doi: 10.1007/s00424-004-1319-9. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Lund PE, Akerman KE. 2-aminoethoxydiphenyl borate reveals heterogeneity in receptor-activated Ca(2+) discharge and store-operated Ca(2+) influx. Cell Calcium. 2001;30:117–129. doi: 10.1054/ceca.2001.0219. [DOI] [PubMed] [Google Scholar]
- Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Jantaratnotai N, McGeer E, McLarnon JG, McGeer PL. Mg2+ ions reduce microglial and THP-1 cell neurotoxicity by inhibiting Ca2+ entry through purinergic channels. Brain Res. 2011;1369:21–35. doi: 10.1016/j.brainres.2010.10.084. [DOI] [PubMed] [Google Scholar]
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J Neurosci Res. 2008;86:2028–2038. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol Sci. 2009;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- Shirakawa H. Pathophysiological Significance of Canonical Transient Receptor Potential (TRPC) Subfamily in Astrocyte Activation. Yakugaku Zasshi. 2012;132:587–593. doi: 10.1248/yakushi.132.587. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Zoran MJ, Mohl B, Barhoumi R. Manganese suppresses ATP-dependent intercellular calcium waves in astrocyte networks through alteration of mitochondrial and endoplasmic reticulum calcium dynamics. Brain Res. 2006;1113:210–219. doi: 10.1016/j.brainres.2006.07.053. [DOI] [PubMed] [Google Scholar]
- Ullah G, Jung P, Cornell-Bell AH. Anti-phase calcium oscillations in astrocytes via inositol (1, 4, 5)-trisphosphate regeneration. Cell Calcium. 2006;39:197–208. doi: 10.1016/j.ceca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul. 1984;24:153–169. doi: 10.1016/b978-0-12-152824-9.50021-6. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Manganese flux across the blood-brain barrier. Neuromolecular Med. 2009;11:297–310. doi: 10.1007/s12017-009-8101-2. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Crossgrove JS, Bukaveckas BL. Manganese distribution across the blood-brain barrier. II. Manganese efflux from the brain does not appear to be carrier mediated. Neurotoxicology. 2003;24:15–22. doi: 10.1016/s0161-813x(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Leibfritz D, Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab. 2003;23:756–771. doi: 10.1097/01.WCB.0000056062.25434.4D. [DOI] [PubMed] [Google Scholar]