Abstract
The corpus callosum (CC) has been implicated in the pathogenesis of schizophrenia, and CC deficits have been reported in adults with schizophrenia. We explored the developmental trajectory of the corpus callosum in Childhood-onset schizophrenia (COS) patients, their healthy siblings and healthy volunteers. We obtained 235 anatomic brain MRI scans from 98 COS patients, 153 scans from 71 of their healthy siblings, and 253 scans from 100 age and gender matched healthy volunteers, across ages 9–30 years. The volumes of 5 sub-regions of the CC were calculated using FreeSurfer, and summed to give the total volume. Longitudinal data were examined using mixed model regression analysis. There were no significant differences for the total or sub-regional CC volumes between the three groups. There were also no significant differences between the groups for developmental trajectory (slope) of the CC. This is the largest longitudinal study of CC development in schizophrenia and the first COS study of the CC to include healthy siblings. Overall, CC volume and growth trajectory did not differ between COS patients, healthy siblings, or healthy volunteers. These results suggest that CC development, at least at a macroscopic level, may not be a salient feature of schizophrenia.
Keywords: Corpus Callosum, MRI, Neuroimaging, Development
1. Introduction
The corpus callosum (CC) is the largest connective structure in the brain, made up of over 190 million axons, which transfer information between the left and right brain integrating the activities of the two cerebral hemispheres (Giedd et al., 1999b; Paul et al., 2007). The corpus callosum has been implicated in the pathogenesis of schizophrenia, and patients with complete or partial CC deficits have shown disorganized speech, difficulty with logic and social understanding (Paul et al., 2007), psychosis (David, 1994), and schizophrenia (Paul et al., 2007).
CC involvement in schizophrenia may be mediated by a reduction in communication between the two hemispheres. CC volume reductions have been associated with decreased interhemispheric transmission times and reduced interhemispheric communication in recent onset psychosis patients (Chaim et al., 2010). A meta-analysis published in 2008 of 28 anatomic MRI studies showed smaller CC mid-sagital area in adults with schizophrenia compared to healthy volunteers (Arnone et al., 2008). However, most studies have been cross sectional with relatively smaller sample sizes, and to-date there has been only a single adult longitudinal study, which showed smaller callosal mid-sagital area at baseline, and a more pronounced decline in absolute CC size for patients with schizophrenia over 4 years (Mitelman et al., 2009).
The fibers of the corpus callosum are thought to take the shortest route and roughly maintain a topographical organization. The genu connects the frontal cortices, the body and truncus connect the middle cortical areas, and the splenium connects the posterior cortical areas (Giedd et al., 1999b). This has led to subregional analyses of the CC in schizophrenia, with inconclusive results. Here too, most studies to-date have been cross sectional, with relatively smaller sample sizes. Various studies of CC sub-regional area and volume have found decreases in almost every region, from the anterior (Keshavan et al., 2002; Hulshoff Pol et al., 2004; Venkatasubramanian et al., 2010), to the body (Hulshoff Pol et al., 2004; Venkatasubramanian et al., 2010), to the posterior (Keshavan et al., 2002; Bersani et al., 2010). The only longitudinal study to address this question (Mitleman et al., 2009) found a smaller CC in all subregions except the posterior body.
The age of the subject may be another important factor in assessing total and sub-regional CC size. In a study of 50 adult males with schizophrenia and 50 healthy controls, the CC splenium was smaller in 36–45 year olds, while the CC anterior midbody area was smaller in 18–25 year olds, after controlling for age of onset and illness duration (Bersani et al., 2010). Thus it may be important to look at the longitudinal CC development, especially from early ages through adulthood.
Childhood onset schizophrenia (COS), defined by onset of psychosis before age 13, is clinically and neurobiologically continuous with later onset schizophrenia (Frazier et al., 1996; Jacobsen and Rapoport, 1998; Rapoport et al., 2005; Gogtay, 2008), and these patients have a more severe course with poorer outcomes (Watkins et al., 1988; Asarnow, 1994). COS shows profound and global loss of cortical gray matter (GM) relative to healthy controls and adult onset patients (Gogtay, 2008), which appears to spread dynamically in the parieto-frontal direction (Thompson et al., 2001) eventually merging into an adult pattern by late adolescence (Greenstein et al., 2006). A single previous study of overall WM growth abnormalities using tensor-based morphometry of COS patients revealed 2.2% per year slower overall WM growth, particularly in the right hemisphere (Gogtay et al., 2008).
There are few studies of the CC on either early onset or at risk populations. Our earlier smaller study in COS, which is the only longitudinal study of the CC in early onset schizophrenia, found normal total CC area. However, the trajectory of the splenium in COS decreased nonlinearly from controls by age 22, while the splenium of controls continued to show a linear age related increase (Keller et al., 2003). High risk studies have also found decreased total CC volume, a decrease in the mid-posterior region, and the absence of an age related increase in the splenium (Sismanlar et al., 2010; Francis et al., 2011). Another study of ultra-high risk (UHR) patients found that total area, length and curvature of the CC did not differ between UHR patients and controls, but the group of UHR individuals who went on to develop psychosis had significant regional reductions in the anterior genu of the CC compared to both UHR subjects that did not progress to psychosis and controls (Walterfang et al., 2008).
Examining the volume and trajectory of CC in non psychotic COS full siblings could also help address whether structural abnormalities of the CC are trait markers or disease related. To our knowledge, the volume and developmental trajectory of the corpus callosum has never been studied in healthy siblings of childhood onset schizophrenia patients. In previous NIMH COS imaging studies, healthy siblings of COS probands were found to initially have gray matter deficits that normalized by age 17 (Gogtay et al., 2007). A previous study of healthy relatives of adult schizophrenia patients showed that the relatives had CC volumes intermediate between the patients and controls in the genu, isthmus and splenium, with a significant decrease from controls in the posterior genu and isthmus (Knochel et al., 2012).
In the present study we examined the longitudinal development of CC volumes in a large sample of childhood-onset schizophrenia probands, their unaffected siblings, and matched healthy comparison subjects, all examined prospectively using anatomic MRI scans to determine the total and sub-regional CC volumes. To explore group differences in CC development over time, we applied mixed effect regressions to model CC volume growth trajectories in COS patients, siblings, and healthy controls from age 9 to 30. We then tested the hypotheses that the COS and sibling groups would show differences in their developmental CC growth curves and/or volumes relative to controls. In particular, based on the results of previous cross-sectional studies of the CC and a previous study showing delayed overall WM development in COS (Gogtay et al., 2008), we predicted that COS patients and their siblings would show reduced total CC volume and delayed growth of the CC. Finally, we also hypothesized that if regional reductions in CC are present, they would be most pronounced for the splenium, as was seen in the previous study of CC mid-sagital area in COS patients.
2. Methods
2.1. Subjects
Childhood-onset schizophrenia patients were recruited nationally and were diagnosed after an inpatient observation usually lasting 2–3 months, which in most cases included a medication free observation period lasting approximately 1–3 weeks in order to achieve diagnostic clarity. Patients were diagnosed with DSM-IV criteria for the diagnosis of schizophrenia with onset of psychosis prior to age 13. Exclusionary criteria were medical or neurobiological illness, history of head trauma, or premorbid IQ below 70. Subjects were also excluded for any lifetime history of substance abuse, assessed during clinical interview at admission and follow-up using the Kiddie Sads Present and Lifetime Version (K-SADS-PL). All patients along with their full siblings were followed prospectively with anatomic rescans at 2-year intervals. The study group of 98 COS patients consisted of 41 girls and 57 boys, for whom at least one scan was available, and included a total of 235 scans. Thirty-two of the COS subjects had only one scan.
Seventy-one healthy full siblings of COS patients for whom at least one scan was available were included in the study, including 38 females and 33 males. This included a total of 153 scans, with 30 siblings having only a single scan. Siblings were interviewed using structured psychiatric interviews for axis I and II diagnoses. Siblings were included only if they were free of any schizophrenia spectrum diagnoses, including schizophrenia, schizoaffective disorder, or any psychotic illness on axis I or paranoid, schizotypal, schizoid, or avoidant personality disorders on axis II.
One hundred healthy comparison subjects, including 41 females and 59 males, were selected from a larger prospective study of normal brain development and were matched for age, sex, and scan interval to the COS patients and healthy siblings. A total of 253 scans from the healthy volunteers were used, with 29 control subjects having only a single scan. Comparison subjects were free of lifetime medical or psychiatric disorders, which was determined through clinical examination and standardized interview. Psychiatric illness in a first degree relative was also exclusionary. Further details have been described previously (Giedd et al., 1999a).
The research protocol was approved by the National Institute of Mental Health (NIMH) institutional review board. Written informed consent was obtained from parents and controls and patients older than 18 years, and written informed assent was obtained from minors.
2.2. Imaging processing and analysis
All scans were obtained using a previously published sequence (Mattai et al., 2011). T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using a three-dimensional spoiled gradient recalled echo sequence in the steady state. Imaging parameters were as follows: echo time=5 msec, repetition time=24 msec, flip angle=45°, acquisition matrix=256×192, number of excitations=1, and field of view=24 cm. Head placement was standardized as previously described (Castellanos et al., 2001).
The image files in DICOM (Digital Imaging and Communications in Medicine) format were transferred to a Linux workstation for analysis. Subcortical volumes were measured automatically with the FreeSurfer (version 5.1) image analysis suite, which is documented and available online (http://surfer.nmr.mgh.harvard.edu/). Prior to processing, all scans were visually examined for motion artifacts or other distortions by a trained rater (LC), and only scans with no visible distortion were included in the sample. The automated procedures for subcortical volume measurements of different brain structures have been described previously (Fischl et al., 2002; Fischl et al., 2004). This procedure automatically provides segments and labels for many brain structures and assigns a neuroanatomical label to each voxel in magnetic resonance imaging volume on the basis of probabilistic information estimated automatically from a manually labeled training set. Briefly, this process includes motion correction and averaging of multiple volumetric T1-weighted images (when more than one is available), removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including the hippocampus, amygdala, caudate, putamen, and ventricles) (Fischl et al., 2002; Fischl et al., 2004), intensity normalization, tessellation of the gray-white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007) and surface deformation following intensity gradients to optimally place the gray-white matter and gray matter/CSF borders at the location where the greatest shift in intensity defines the transition to the other tissue class.
The segmentation uses the following data to disambiguate labels: the prior probability of a given tissue class at a specific atlas location, the likelihood of the image intensity given the tissue class, and the probability of the local spatial configuration of labels given the tissue class. This technique has previously been shown to be comparable in accuracy to manual labeling (Fischl et al., 2002) and has been demonstrated to show good test-retest reliability across scanner manufacturers and field strengths (Han et al., 2006).
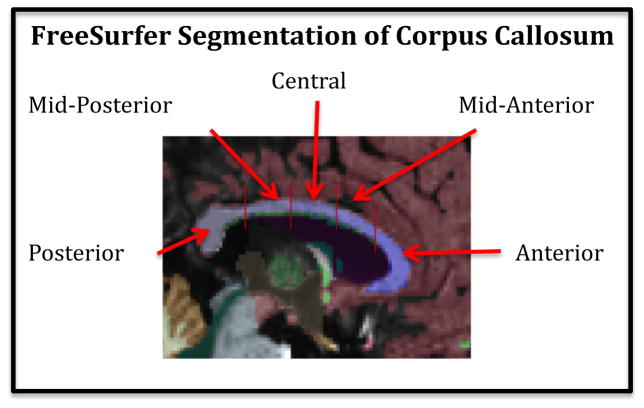
The corpus callosum was automatically identified and segmented by the FreeSurfer processing software. Within the FreeSurfer processing pipeline, the default lateral thickness of the CC was 5mm (a 1mm sagittal slice at midline and 2 1mm slices to either side). This extended sampling laterally of the CC mitigated against any residual misalignment along the midline after registration to the template. Once the corpus callosum was identified, a line was drawn down the center of the structure and it was divided into five segments of equal length, which were labeled Anterior, Mid-anterior, Central, Mid-posterior, and Posterior (See Figure 1). Total corpus callosal volume was calculated as the sum of these five segment volumes for each study participant.
Figure 1.
Subject MRI scan identifying the sub-regions of the corpus callosum.
The FreeSurfer-based CC parcellation, which closely approximates the widely-accepted schemes of Witelson (1989) and Hofer and Frahm (2006) has been previously used successfully in a study on normal aging (Salat et al., 2005). We also have further confidence in this methodology, because we have unpublished data that shows a high correlation (about r=.95) between FreeSurfer and manual measurements in a group of young normals and OCD patients.
Three participants, two from the COS group and one healthy sibling, had CC regional volumes calculated by FreeSurfer, that were identified as outliers and omitted from statistical analysis due to having erroneously large or small values (more than 4 standard deviations away from the group mean volume). The scans with volumes that were identified as outliers (in graphs) were examined for accuracy of registration and segmentation. Because we ruled out the possibility that the outliers were due to scanning or registration issues, we did not delete all the measurements for these scans when one region was an outlier. One of these subjects was omitted from the analysis for 2 (out of 5) of the sub-regions, and two were omitted from the analysis for 1 (out of 5) of the subregions. These three subjects were also excluded from the analysis for total CC volume.
2.3. Statistical analysis
Demographic differences between groups were tested using analysis of variance (ANOVA) for age and chi-square tests of independence for sex and handedness. We initially included handedness as a covariate in all models. However, it was not a significant effect in any model (P > 0.14) and as such, we omitted handedness from the final models. To examine group differences in the developmental trajectories of posterior, mid-posterior, central, mid-anterior, anterior, and total corpus callosal volume measures, we used mixed-effect regression models. We applied the false discovery rate (FDR) (Benjamini and Hochberg, 1995) to account for multiple tests, and we set q=0.05, thereby limiting the expected rate of false positives among all positives to 0.05%. The FDR procedure is reported to control q when test statistics are positively correlated (for example, multiple endpoints) (Benjamini and Yekutieli, 2001). The dependent variables were individual corpus callosum volumes; fixed effects were age (centered at the sample average age of 17.31 years [SD=4.66]), group (COS, NV, and SIB), group-by-age, intracranial volume, and sex. Random effects included an intercept per family (to account for within-family dependence) and an intercept for a person nested within a family (to account for within-person dependence) for subjects with multiple scans. Polynomial age terms and their respective group interactions were also included if F tests indicated that they significantly contributed to the explanatory power of the model. We tested group differences in intercept (at the average age) and trajectory using F tests. Trajectories are visually represented in graphs of the fitted regression lines for the middle 80% of the age range.
3. Results
The three study groups were well matched with respect to age and sex. The age range was from 9.02–30.44 years. See Table 1 for demographic information, and Table 2 for clinical information about the Childhood-onset Schizophrenia patients, including severity of symptoms as assessed by ratings on the Scale for the Assessment of Positive Symptoms (SAPS), Scale for the Assessment of Negative Symptoms (SANS), and the Children’s Global Assessment Scale (CGAS), taken while the patients were hospitalized on our inpatient unit and free of medications. We selected these ratings in order to control for variability in response to medication, number of medications, environmental supports and stressors.
Table 1.
Demographics of Childhood-Onset Schizophrenia Probands (COS), Healthy Comparison Subjects (NV), and Healthy Siblings (SIB)
| COS (N =98) | NV (N =100) | SIB (N =71) | Statistics (df) | P value | |
|---|---|---|---|---|---|
| Sex | 41 F; 57 M | 41 F; 59 M | 38 F; 33 M | x 2 =3.019 (2) | P =0.221 |
| Handedness | 11 L; 13 M; 73 R | 2 L; 8 M; 90 R | 2 L; 5 M; 61 R | x 2 =12.368 (4) | P =0.015 |
| Mean Age Scan 1 (SD) | 14.6(2.4) [N =98] | 14.5(4.4) [N =100] | 15.8(5.3) [N =71] | f =2.389 (266) | P =0.094 |
| Mean Age Scan 2 (SD) | 17.1(2.8) [N =66] | 16.6(3.6) [N =71] | 17.8(4.6) [N =41] | f =1.366 (175) | P =0.258 |
| Mean Age Scan 3 (SD) | 19.7(3.1) [N =43] | 19.4(3.8) [N =45] | 20.0(4.7) [N =28] | f =0.208 (113) | P =0.813 |
| Mean Age Scan 4 (SD) | 22.2(2.9) [N =17] | 22.1(2.9) [N =25] | 21.4(2.7) [N =9] | f =0.229 (48) | P =0.796 |
| Mean Age Scan 5 (SD) | 24.5(2.8) [N =6] | 24.0(2.2) [N =9] | 23.6(2.6) [N =4] | f =0.170 (16) | P =0.845 |
| Mean Age Scan 6 (SD) | 25.9(1.6) [N =4] | 27.0(1.6) [N =3] | - | f =0.012 (5) | P =0.917 |
| Mean Age Scan 7 (SD) | 30.4 [N =1] | - | - | - | - |
| Overall Mean Age (SD) | 17.3(4.0) [N =235] | 17.2(4.9) [N =253] | 17.6(5.2) [N =153] | f =0.407 (638) | P =0.666 |
Table 2.
Clinical Measures of Childhood-Onset Schizophrenia Patients
| Mean (years) | SD | N | |
|---|---|---|---|
| Age onset of psychosis | 10.03571 | 1.894 | 98 |
| Years ill at first scan | 4.539898 | 2.284 | 98 |
| Mean Score | SD | N | |
| Scale for the Assessment of Positive Symptoms (SAPS)* | 48.60494 | 21.73 | 81 |
| Scale for the Assessment of Negative Symptoms (SANS)* | 61.06024 | 28.33 | 83 |
| Children’s Global Assessment Scale (CGAS)* | 24.62651 | 13.44 | 83 |
Ratings taken when medication free or closest available to medication free
The CC volume results are summarized in Table 3 and the pair-wise group comparisons for volume and developmental trajectory are shown in Table 4. There were no significant differences for the volume (at the mean age) of the total CC, or any of the CC sub-regions between any of the groups to survive FDR. There were also no significant differences between the groups for developmental trajectory (slope) of the total CC or any of the CC sub regions to survive FDR correction. There were also no significant group differences in volume unadjusted for ICV that survived FDR correction.
Table 3.
Magnetic Resonance Imaging Callosal Volumes (mm3) Among Childhood-Onset Schizophrenia Probands (COS), Healthy Comparison Subjects (NV), and Healthy Siblings (SIB)
| Adjusted (for ICV) Volume at the average age | Unadjusted (for ICV) Volume at the average age | |||||
|---|---|---|---|---|---|---|
| COS Mean (SE) | NV Mean (SE) | SIB Mean (SE) | COS Mean (SE) | NV Mean (SE) | SIB Mean (SE) | |
| CC Posterior | 268.47 (60.02) | 269.21 (60.90) | 244.98 (62.26) | 867.36 (15.94) | 880.25 (15.79) | 858.71 (20.14) |
| CC Mid-Posterior | 76.16 (59.79) | 95.90 (60.89) | 107.42 (61.78) | 417.58 (11.12) | 443.77 (11.16) | 456.74 (14.46) |
| CC Central | 112.34 (66.04) | 148.87 (67.05) | 131.11(68.08) | 467.54 (12.38) | 509.93 (12.41) | 496 (16.14) |
| CC Mid-Anterior | 98.22 (50.64) | 107.79 (50.86) | 100.75 (51.92) | 465.34 (11.42) | 478.02 (11.38) | 471.34 (14.55) |
| CC Anterior | 195.76 (64.25) | 175.86 (65.21) | 174.3 (66.56) | 867.26 (15.89) | 858.12 (15.71) | 859.26 (19.97) |
| CC Total | 618.57 (212.72) | 653.13 (215.86) | 612.47 (220.31) | 3092.18 (52.65) | 3173.29 (52.23) | 3140.62 (66.81) |
COS= Childhood-Onset Schizophrenia probands; NV= healthy comparison subjects; SIB= healthy siblings of Childhood-Onset Schizophrenia probands Data were covaried for gender at the mean centered age (17.31 years [SD=4.66]). Adjusted values were covaried for intracranial volume.
Table 4.
Pairwise Group Differences in Callosal Volume Among Childhood-Onset Schizophrenia Probands (COS), Healthy Comparison Subjects (NV), and Healthy Siblings (SIB)
| Pairwise Group differences in volume (height) at average age (17.31) | Pairwise Group differences in trajectory (slope) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COS vs. SIB | COS vs. NV | SIB vs. NV | COS vs. SIB | COS vs. NV | SIB vs. NV | |||||||||||||
| F | df | P | F | df | P | F | df | P | F | df | P | F | df | P | F | df | P | |
| CC Posterior | 1.89 | 1, 364 | 0.17 | 0 | 1, 364 | 0.969 | 1.26 | 1, 364 | 0.263 | 0.39 | 2, 364 | 0.677 | 1.26 | 2, 364 | 0.285 | 0.2 | 2, 364 | 0.822 |
| CC Mid-Posterior | 4.66 | 1, 362 | 0.032* | 1.91 | 1, 362 | 0.168 | 0.48 | 1, 362 | 0.49 | 0.85 | 3, 362 | 0.468 | 3.56 | 3, 362 | 0.015* | 0.36 | 3, 362 | 0.778 |
| CC Central | 1.28 | 1, 365 | 0.259 | 5.28 | 1, 365 | 0.022* | 0.92 | 1, 365 | 0.338 | 3.12 | 2, 365 | 0.045* | 2.12 | 2, 365 | 0.121 | 0.45 | 2, 365 | 0.64 |
| CC Mid-Anterior | 0.04 | 1, 368 | 0.845 | 0.47 | 1, 368 | 0.494 | 0.2 | 1, 368 | 0.656 | 0.1 | 1, 368 | 0.747 | 0.72 | 1, 368 | 0.396 | 0.11 | 1, 368 | 0.744 |
| CC Anterior | 1.65 | 1, 365 | 0.199 | 1.21 | 1, 365 | 0.272 | 0.01 | 1, 365 | 0.94 | 0.26 | 2, 365 | 0.768 | 2.83 | 2, 365 | 0.06 | 1.33 | 2, 365 | 0.265 |
| CC Total | 0.01 | 1, 362 | 0.916 | 0.297 | 1, 362 | 0.586 | 0.32 | 1, 362 | 0.575 | 1.9 | 2, 415 | 0.151 | 3.52 | 2, 390 | 0.03* | 0.09 | 2, 417 | 0.911 |
COS= Childhood-Onset Schizophrenia probands; NV= healthy comparison subjects; SIB= healthy siblings of Childhood-Onset Schizophrenia probands
Data were covaried for intracranial volume and gender at the mean centered age (17.31 years [SD=4.66]).
None of the results survived correction for multiple comparisons
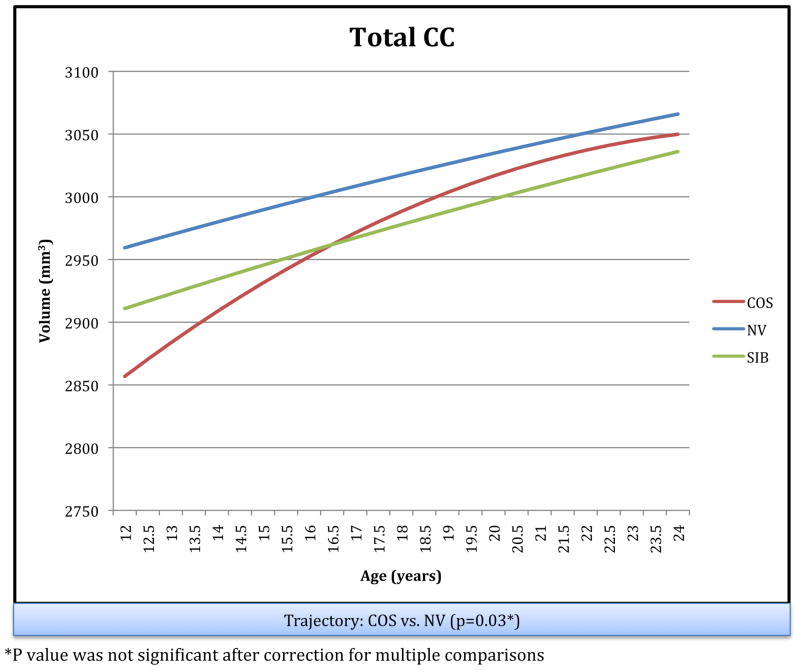
The longitudinal trajectories of the total CC for the three groups are shown in Figure 2.
Figure 2.
Schizophrenia Probands (COS), Their Healthy Siblings (SIB), and Healthy : Longitudinal Trajectories of Total CC Volume in Childhood Onset Comparison Subjects (NV)
4. Discussion
COS patients did not differ significantly from controls or their healthy siblings for either the volume or developmental trajectory of the total corpus callosum or any of its sub-regions, using anatomic measures. Although the volumes and developmental trajectories for total, mid posterior, and central CC regions appeared to be qualitatively different than controls, the results did not survive correction for multiple analyses.
Unexpectedly, and in contradiction to our a priori hypothesis, we found no significant differences that survived statistical testing for total corpus callosal volume or sub regional volumes between any of our groups. Although this is in line with one prior study that did not find any difference in the mid-sagittal area of the corpus callosum between schizophrenic patients, their relatives and normal controls (Chua et al., 2000), it is not in agreement with the majority of previous reports with respect to adult patients (Keshavan et al., 2002; Hulshoff Pol et al., 2004; Arnone et al., 2008; Mitelman et al., 2009; Bersani et al., 2010; Chaim et al., 2010; Venkatasubramanian et al., 2010; Rao et al., 2011) healthy, high-risk offspring and first-degree relatives of schizophrenia patients (Sismanlar et al., 2010; Francis et al., 2011; Knochel et al., 2012) and the previous finding of a significantly different developmental trajectory of the splenium in COS patients (Keller et al., 2003). Of note, while Keller et al. (2003) reported a slope difference, they did not find a significant difference for cross sectional area in their sample, and when they adjusted for multiple comparisons, the slope difference was no longer significant. A previous study of general WM growth found COS patients had slower overall WM growth, and significantly slower growth in the left frontal, right frontal, parietal, and occipital lobes, and anterior cingulate region bilaterally. However, on careful examination of the brain maps, the COS patients did not appear to show a deficit or growth rate difference from controls in the CC (Gogtay et al., 2008), which is confirmed by our current findings.
Consistent with prior longitudinal studies of CC development which show an age related increase in mid-sagital area of the CC, we also found an age related increase in total CC volume in each of our three groups during adolescence (Giedd et al., 1999b; Giedd et al., 2008). In our study, the total CC trended toward a significant difference in trajectory between COS patients and healthy controls. The COS patients exhibited a steeper increase in total CC volume at younger ages, which leveled off as they aged, becoming similar to healthy controls in later adolescence. This may suggest that COS patients have a deficit in total CC volume at an even younger age, possibly due to a delay in myelination, which then normalizes by late adolescence. Given the known overall delay in whole brain WM growth (Gogtay et al., 2008), the trend toward a delay in CC development at younger ages suggests that further investigation of the CC in younger COS patients may reveal more subtle deficits.
On the other hand, it is possible that COS patients have aberrant white matter connectivity that is not reflected in gross structural change, and can only be seen with more subtle modalities such as diffusion tensor imaging (DTI), which shows the organization of fibers in white matter tracts. DTI studies have shown decreased fractional anisotropy (FA), which can be considered evidence of structural damage and disorganization of tracts (Foong et al., 2000), in the CC of adult schizophrenia patients (Patel et al., 2011; Wang et al., 2011) and adolescent-onset schizophrenia patients (Douaud et al., 2007; Davenport et al., 2010), and lower FA in the CC has been correlated with increased symptom scores (Michael et al., 2008). Interestingly, a study that looked at CC size using mid-sagital area in addition to DTI, found no difference in CC size between patients and controls, but significant differences in FA in the CC (Kubicki et al., 2008). Further study of white matter tracts of COS patients is warranted, and a COS study utilizing DTI to look at whole-brain WM connectivity is currently underway.
However, given the profound cortical GM abnormalities in this population, the lack of major anatomic corpus callosum abnormalities suggests that the CC is not a pathophysiological locus at least for very early onset schizophrenia. Furthermore, CC volumes of the healthy siblings were not abnormal, suggesting CC abnormalities, even the subtle ones, are not a trait marker.
The major strengths of our study include the large sample size, longitudinal design, and inclusion of matched COS patients, healthy siblings, and controls. However, there are some important limitations. Due to the large size of our sample, we used fully automated software to assess the volume of the total and sub-regional corpus callosum, rather than using manual tracings of the corpus callosum. FreeSurfer based CC parcellation has been previously used successfully, such as in a study of normal aging (Salat et al., 2005), however it is possible that the lack of differences between the groups in our study could be due to errors in registration or parcellation occurring in the automated processing of the scans. Also, our COS sample represents a more severe form of illness, which may not be generalizable to later onset schizophrenia, although one would have expected more robust CC abnormalities with such a severe phenotype. It remains difficult to quantify the effects of antipsychotic medications, as all of our patients have been exposed to large doses of antipsychotics prior to enrollment but we do not calculate the lifetime neuroleptic dose of medications for the patients at each scan. Neuroleptic medications can lead to volume changes in gray and white matter brain structures (Bartzokis et al., 2007; Okugawa et al., 2007; Navari and Dazzan, 2009) thus medication effect to normalize the CC volume, though unlikely, cannot be ruled out. However, despite these limitations, this is the largest study of the CC in schizophrenia and the first longitudinal one to span such a wide age range. Thus the negative findings in this study, in addition to clarifying some inconsistencies in the literature, also suggest that the anatomic CC volume may not be an important candidate to focus on in further exploration of the pathophysiology of schizophrenia.
Supplementary Material
Acknowledgments
This study was funded by the Intramural Division of the National Institute of Health (NIH). Participation of SJ was made possible through the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc). None of the other authors have any financial disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2008;101:124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Asarnow Cognitive/neuropsychological studies of children with a schizophrenic disorder. Schizophrenia Bulletin. 1994;20:647–669. doi: 10.1093/schbul/20.4.647. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, Lieu C, Altshuler LL, Mintz J. Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophrenia Research. 2007;93:13–22. doi: 10.1016/j.schres.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. Control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- Bersani G, Quartini A, Iannitelli A, Paolemili M, Ratti F, Di Biasi C, Gualdi G. Corpus callosum abnormalities and potential age effect in men with schizophrenia: an MRI comparative study. Psychiatry Research. 2010;183:119–125. doi: 10.1016/j.pscychresns.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Vaituzis AC, Blumenthal JD, Nelson J, Bastain TM, Zijdenbos A, Evans AC, Rapoport JL. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- Chaim TM, Schaufelberger MS, Ferreira LK, Duran FL, Ayres AM, Scazufca M, Menezes PR, Amaro E, Jr, Leite CC, Murray RM, McGuire PK, Rushe TM, Busatto GF. Volume reduction of the corpus callosum and its relationship with deficits in interhemispheric transfer of information in recent-onset psychosis. Psychiatry Research. 2010;184:1–9. doi: 10.1016/j.pscychresns.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Chua SE, Sharma T, Takei N, Murray RM, Woodruff PW. A magnetic resonance imaging study of corpus callosum size in familial schizophrenic subjects, their relatives, and normal controls. Schizophrenia Research. 2000;41:397–403. doi: 10.1016/s0920-9964(99)00081-x. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research. 2010;181:193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS. Schizophrenia and the corpus callosum: developmental, structural and functional relationships. Behavioural Brain Research. 1994;64:203–211. doi: 10.1016/0166-4328(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain : A Journal of Neurology. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. Journal of Neurology, Neurosurgery & Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis AN, Bhojraj TS, Prasad KM, Kulkarni S, Montrose DM, Eack SM, Keshavan MS. Abnormalities of the corpus callosum in non-psychotic high-risk offspring of schizophrenia patients. Psychiatry Research. 2011;191:9–15. doi: 10.1016/j.pscychresns.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Archives of General Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999a;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuropsychopharmacology & Biological Psychiatry. 1999b;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N. Trajectories of anatomic brain development as a phenotype. Novartis Foundation Symposia. 2008;289:101–195. doi: 10.1002/9780470751251.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophrenia Bulletin. 2008;34:30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of General Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, Thompson PM. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, Rapoport J, Gogtay N. Childhood onset schizophrenia: cortical brain abnormalities as young adults. Journal of Child Psychology and Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited -comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RCW, Cahn W, Collins DL, Evans AC, Kahn RS. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. NeuroImage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Rapoport JL. Research update: childhood-onset schizophrenia: implications of clinical and neurobiological research. Journal of Child Psychology and Psychiatry. 1998;39:101–113. [PubMed] [Google Scholar]
- Keller A, Jeffries NO, Blumenthal J, Clasen LS, Liu H, Giedd JN, Rapoport JL. Corpus callosum development in childhood-onset schizophrenia. Schizophrenia Research. 2003;62:105–114. doi: 10.1016/s0920-9964(02)00354-7. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72:757–760. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, Haenschel C, Uhlhaas P, Pantel J, Hampel H, Linden DE. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. NeuroImage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, Kikinis R, McCarley RW, Shenton ME. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophrenia Research. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattai A, Hosanagar A, Weisinger B, Greenstein D, Stidd R, Clasen L, Lalonde F, Rapoport J, Gogtay N. Hippocampal volume development in healthy siblings of childhood-onset schizophrenia patients. American Journal of Psychiatry. 2011;168:427–435. doi: 10.1176/appi.ajp.2010.10050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AM, Calhoun VD, Pearlson GD, Baum SA, Caprihan A. Correlations of diffusion tensor imaging values and symptom scores in patients with schizophrenia. Conference Proceedings IEEE Engineering in Medicine and Biology Society. 2008;2008:5494–5497. doi: 10.1109/IEMBS.2008.4650458. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Nikiforova YK, Canfield EL, Hazlett EA, Brickman AM, Shihabuddin L, Buchsbaum MS. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophrenia Research. 2009;114:144–153. doi: 10.1016/j.schres.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological Medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Takase K, Saito Y, Yoshimura M, Kinoshita T. Olanzapine increases grey and white matter volumes in the caudate nucleus of patients with schizophrenia. Neuropsychobiology. 2007;55:43–46. doi: 10.1159/000103575. [DOI] [PubMed] [Google Scholar]
- Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2011;129:149–155. doi: 10.1016/j.schres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nature Reviews Neuroscience. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Rao NP, Venkatasubramanian G, Arasappa R, Gangadhar BN. Relationship between corpus callosum abnormalities and schneiderian first-rank symptoms in antipsychotic-naive schizophrenia patients. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:155–162. doi: 10.1176/jnp.23.2.jnp155. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Current Psychiatry Reports. 2005;7:81–82. doi: 10.1007/s11920-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sismanlar SG, Anik Y, Coskun A, Agaoglu B, Karakaya I, Yavuz CI. The volumetric differences of the fronto-temporal region in young offspring of schizophrenic patients. European Child & Adolescent Psychiatry. 2010;19:151–157. doi: 10.1007/s00787-009-0052-5. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Diana Rosas H, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Reddy VV, Reddy US, Gangadhar BN, Keshavan MS. Corpus callosum deficits in antipsychotic-naive schizophrenia: evidence for neurodevelopmental pathogenesis. Psychiatry Research. 2010;182:141–145. doi: 10.1016/j.pscychresns.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Yung A, Wood AG, Reutens DC, Phillips L, Wood SJ, Chen J, Velakoulis D, McGorry PD, Pantelis C. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophrenia Research. 2008;103:1–10. doi: 10.1016/j.schres.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Wang Q, Deng W, Huang C, Li M, Ma X, Wang Y, Jiang L, Lui S, Huang X, Chua SE, Cheung C, McAlonan GM, Sham PC, Murray RM, Collier DA, Gong Q, Li T. Abnormalities in connectivity of white-matter tracts in patients with familial and non-familial schizophrenia. Psychological Medicine. 2011;41:1691–1700. doi: 10.1017/S0033291710002412. [DOI] [PubMed] [Google Scholar]
- Watkins JM, Asarnow RF, Tanguay PE. Symptom development in childhood onset schizophrenia. Journal of Child Psychology and Psychiatry. 1988;29:865–878. doi: 10.1111/j.1469-7610.1988.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.