Abstract
Following antibiotic treatment for Lyme disease, some patients report persistent or relapsing symptoms of pain, fatigue, and/or cognitive deficits. Factors other than active infection, including immune abnormalities, have been suggested, but few clues regarding mechanism have emerged. Furthermore, the effect of antibiotic treatment on immune response in affected individuals remains unknown. In this study, a longitudinal analysis of specific immune markers of interest was carried out in patients with a history of Lyme disease and persistent objective memory impairment, prior to and following treatment with either ceftriaxone or placebo. IFNα activity was measured by detection of serum-induced changes in specific target genes, using a functional cell-based assay and quantitative real-time PCR. Level and pattern of antibody reactivity to brain antigens and to Borrelia burgdorferi proteins were analyzed by ELISA and immunoblotting. Sera from the patient cohort induced significantly higher expression of IFIT1 and IFI44 target genes than those from healthy controls, indicating increased IFNα activity. Antibody reactivity to specific brain and borrelial proteins was significantly elevated in affected patients. IFNα activity and antibody profile did not change significantly in response to ceftriaxone. The heightened antibody response implies enhanced immune stimulation, possibly due to prolonged exposure to the organism prior to the initial diagnosis and antibiotic treatment of Lyme disease. The increase in IFNα activity is suggestive of a mechanism contributing to the ongoing neuropsychiatric symptoms.
Keywords: Lyme disease, Borrelia burgdorferi, Post-Lyme disease syndrome, Chronic Lyme, IFNα, Cognitive dysfunction, Antibody
1. Introduction
Lyme borreliosis is a multisystem infection that is caused by spirochetes of the Borrelia burgdorferi sensu lato genospecies complex and transmitted by Ixodes ticks (Stanek and Strle, 2003). It is endemic to North America, Europe, and Asia (Stanek and Strle, 2008; Steere, 2001). The early phase of the infection is usually associated with a characteristic skin lesion, known as erythema migrans (EM) (Bratton et al., 2008). Extracutaneous manifestations of Lyme disease may affect the joints, heart, and/or the nervous system (Steere, 2001; Wormser et al., 2006). Neurologic complications include lymphocytic meningitis, cranial neuropathy, peripheral neuropathy, and encephalopathy characterized by deficits in cognitive functioning (Halperin, 2008). Although these complications typically respond well to currently standard antibiotic therapy, some Lyme disease patients report persistent symptoms despite treatment (Feder et al., 2007; Marques, 2008). The symptoms in affected patients include musculoskeletal pain, fatigue, and difficulties with verbal fluency and memory (Feder et al., 2007; Marques, 2008). The condition has been variably referred to as chronic Lyme disease, post-treatment Lyme disease syndrome, and post-Lyme disease syndrome (PLDS).
Despite several years of debate, few clues to the causes of the persistent symptoms following the treatment of Lyme disease have emerged and the search for effective therapeutic options has remained elusive. In addition, there is no definitive test or biomarker to link the presence of persistent symptoms in the affected patients to having been infected with B. burgdorferi in the past. A recent study demonstrated heightened levels of antibodies to brain proteins in patients with PLDS, thus for the first time demonstrating the presence of certain objective immunologic abnormalities that may be relevant to the pathogenic mechanism of the symptoms experienced (Chandra et al., 2010). Another study on the same patients showed increased antibody reactivity towards specific B. burgdorferi proteins in comparison to post-Lyme healthy individuals (Chandra et al., 2011b). Furthermore, epitope mapping of the immune response to VlsE protein of B. burgdorferi in these patients revealed elevated antibody reactivity to specific sequences in the membrane-proximal region of the protein (Chandra et al., 2011a). These findings have offered useful clues about the course of the antecedent spirochetal infection in patients with PLDS and pointed to the possibility of finding biomarkers for the condition.
A recent treatment trial evaluated the effect of a 10-week course of ceftriaxone versus placebo in borrelial seropositive patients with a history of Lyme disease and objective memory impairment (Fallon et al., 2008). Some of the patients in the trial experienced moderate short-term cognitive improvement, although the mechanism for this was not clear. In the present study we expand upon our prior work by carrying out an extensive longitudinal analysis of the level and antigenic specificity of serum anti-neural and anti-borrelia antibody reactivity in these patients throughout the course of the treatment trial. In addition, we assess serum interferon-alpha (IFNα) activity, previously implicated in other disorders with adverse neuropsychiatric manifestations and associated animal models (Crow, 2012), using a sensitive cell-based assay. The data presented here yield novel clues regarding the persistence of symptoms following antibiotic treatment of Lyme disease.
2. Materials and Methods
Study participants
Serum samples from 19 patients with a history of Lyme disease and objective memory impairment, recruited as part of a previously conducted clinical trial of treatment with ceftriaxone (Fallon et al., 2008), were utilized in this study (10 female, 9 male; mean age 42.1 ± 13.9 y (SD); mean elapsed time since the original diagnosis of Lyme disease 6.0 ± 3.7 y (SD)). Criteria for patient selection are described in detail in the original report. Briefly, patients met the following criteria: 1) history of physician-documented erythema migrans or U.S. Centers for Disease Control and Prevention (CDC)-defined manifestation of Lyme disease, 2) current positive IgG Western blot (WB) according to CDC criteria, 3) prior treatment for Lyme disease with at least 3 weeks of IV ceftriaxone, completed at least 4 months before study entry, 4) subjective memory impairment after the onset of Lyme disease, and 5) objective evidence of memory impairment as documented by the Wechsler Memory Scale–III (Wechsler, 1997) compared with age-, sex-, and education-adjusted population norms. Patients had been assigned to receive treatment with 10 weeks of either IV ceftriaxone (n=14) or IV placebo (n=5). Selection of these 19 specific patients from the original cohort was based on the availability of at least two serum samples representing a time point both before and after initiation of treatment. Specimens were from prior to initiation of treatment and spanning up to 48 weeks after the start of treatment, resulting in 58 samples.
The study also included control serum specimens from 11 borrelial IgG WB-seropositive (according to CDC algorithm) individuals who had been treated for early localized or disseminated Lyme disease associated with single or multiple EM with no post-Lyme symptoms after at least 2 years of follow-up (3 female, 8 male; mean age 49.8 ± 15.7 y (SD); mean elapsed time since the original diagnosis of Lyme disease 4.9 ± 4.2 y (SD)). The original diagnosis of acute Lyme disease in these currently healthy subjects was confirmed by recovery of B. burgdorferi in cultures of skin and/or blood. The source of samples and selection criteria were previously described (Chandra et al., 2010). In addition, serum samples from 20 healthy individuals without history or serologic evidence of Lyme disease were analyzed in the study. This study was approved by the Human Subjects Review Committee of the Institutional Review Board of Columbia University Medical Center.
Immune response to B. burgdorferi
B. burgdorferi whole cell enzyme-linked immunosorbent assay (ELISA)
IgG anti-borrelia antibody levels were determined by ELISA as previously described (Chandra et al., 2010).
B. burgdorferi Western blot assay (WB)
IgG antibody response to B. burgdorferi B31 was further characterized by WB, using commercial blots and the Euroblot automated WB instrument, according to the manufacturer's protocols (Euroimmun, Lubec, Germany). Briefly, nitrocellulose strips containing electrophoresis-separated B. burgdorferi B31 proteins were blocked and then incubated with 1.5 mL of diluted serum sample (1:50) for 30 min. Membrane strips were washed and incubated with AP-conjugated anti-human IgG antibody for 30 min. Bound antibodies were detected using the NBT/BCIP (nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indoly phosphate) staining system. Quantitative analysis of bands on each blot was carried out using the EUROLineScan software (Euroimmun). Accurate background correction and determination of cutoff values for positivity were performed by the software for the p18, p25 (OspC), p28, p30, p31 (OspA), p34 (OspB), p39 (BmpA), p41 (FlaB), p45, p58, p66, p93, and recombinant VlsE borrelial protein bands. Determination of IgG positive serology for Lyme disease was based on the CDC criteria (Aguero-Rosenfeld et al., 2005; CDC, 1995).
Antibodies to IR6 epitope of VlsE
IgG antibodies to C6, a peptide that reproduces the IR6 invariable region of the B. burgdorferi VlsE lipoprotein, was determined using an ELISA kit, according to the manufacturer's instructions (Immunetics, Boston, Mass.). Each sample was considered positive if its index value was greater than or equal to 1.10, negative if it was less than or equal to 0.90, and equivocal when it was between 0.91 and 1.09.
Anti-neural autoantibodies
Antibodies to brain proteins were detected by immunoblotting for all specimens, as was previously described (Chandra et al., 2010). Briefly, SDS-PAGE was carried out on 400 μg protein aliquots of mouse brain lysate (Alaedini et al., 2007) at 200 V in Tris-glycine-SDS buffer for 35 min using 4-15% pre-cast 2D-prep gels (Bio-Rad, Hercules, Calif.), followed by transfer to nitrocellulose membrane at 33 V in Tris-glycine buffer containing 20% methanol for 16 h. Each gel contained the Precision Plus molecular weight marker mix (Bio-Rad) in one lane. The membrane was incubated in blocking buffer (Tris-buffered saline (TBS) containing 5% milk, 0.5% BSA, and 0.05% Tween-20) for 2 h. Incubation with patient serum (1:2000 in dilution buffer containing 10% blocking buffer, 10% fetal bovine serum, and 0.05% Tween-20 in TBS) was carried out for 1 h in a Mini-PROTEAN II Multiscreen apparatus (Bio-Rad). A positive control sample was included on every membrane. HRP-conjugated sheep anti-human IgG (GE Healtcare, Piscataway, N.J.) was used as the secondary antibody. Detection of bound antibodies was by the ECL system (Millipore, Billerica, Mass.) and BioMax MR film (Kodak, Rochester, N.Y.) after 10s exposure. Each membrane was treated with stripping buffer (Thermo Fisher, Rockford, Ill.) at 58 °C for 30 min, and reblotted with HRP-conjugated rabbit anti-β tubulin antibody (Novus, Littleton, Colo.). Detection of bound antibodies was as before. Conversion of immunoblots to line graph, density analysis, and subtraction of background were performed by the Unscan-It program (Silk Scientific, Orem, Utah). Measurement of total antibody reactivity towards neural proteins in each sample was done by calculating the sum of gray-level intensities for all software-assigned and background-subtracted reactive bands. Total gray-level intensity for each specimen was corrected for 1) inconsistencies within each membrane (e.g., for variation in sample loading and efficiency of protein transfer) according to the gray-level intensity of the tubulin band for each lane, and 2) inconsistencies in experimental conditions between membranes (e.g., for variation in sample loading, efficiency of protein transfer, and autoradiography exposure time) according to the total gray-level intensity for the positive control on each membrane.
IFNα activity
WISH cell culture and stimulation
Human WISH epithelial cell line cells (product no. CCL-25; American Type Culture Collection, Manassas, Va.) were grown in minimum essential medium supplemented with L-glutamine (2 mM), HEPES (20 mM), penicillin (100 units/mL), streptomycin (100 μg/mL), and 10% fetal bovine serum at 37 °C in an atmosphere containing 5% CO2. To measure IFN-inducing activity in patient serum, WISH cells were cultured at a density of 5 × 105 mL in 96-well flat-bottom plates in medium, containing either recombinant human IFNα A (BioSource International, Camarillo, Calif.) at 100 units/mL or 50% serum. Sera were from the 19 affected patients, as well as 11 post-Lyme healthy and 17 non-Lyme healthy individuals. After 6 hours of incubation, WISH cells were lysed and stored at -80°C.
Real-time quantitative PCR
RNA was extracted from each cell lysate using the TurboCapture 96mRNA kit (Qiagen, Chatsworth, Calif.), and 0.125 μg of this RNA was reverse-transcribed to cDNA in a 10 μL reaction using TaqMan Reverse Transcription Reagents (Invitrogen, Carlsbad, Calif.). The cDNA obtained from each sample was diluted 1:10, and 4 μL was amplified in an 8 μL real-time quantitative PCR reaction using 0.4 μM sense and antisense primers and the 2× iQ SYBR Green Supermix (Bio-Rad). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene control. Primer sequences for the IFIT1, IFI44, and IFIT3 genes were as previously described (Hua et al., 2006). WISH cells cultured with medium were included in each assay to provide a basis for normalization across experiments. Results for each culture condition are reported as the relative expression compared to WISH cells cultured with medium. Details of the real-time quantitative PCR method and data analysis have been described in detail previously (Hua et al., 2006).
Data analysis
Differences between groups were analyzed by one-way analysis of variance (ANOVA) with post-hoc Dunn test (continuous data), and Fisher's exact test (nominal data). No adjustment for multiple testing was done. Differences with p values of <0.05 were considered to be statistically significant. Statistical analyses were performed with Prism 6 (GraphPad, San Diego, Calif.).
3. Results
Immune response to B. burgdorferi
B. burgdorferi whole cell ELISA
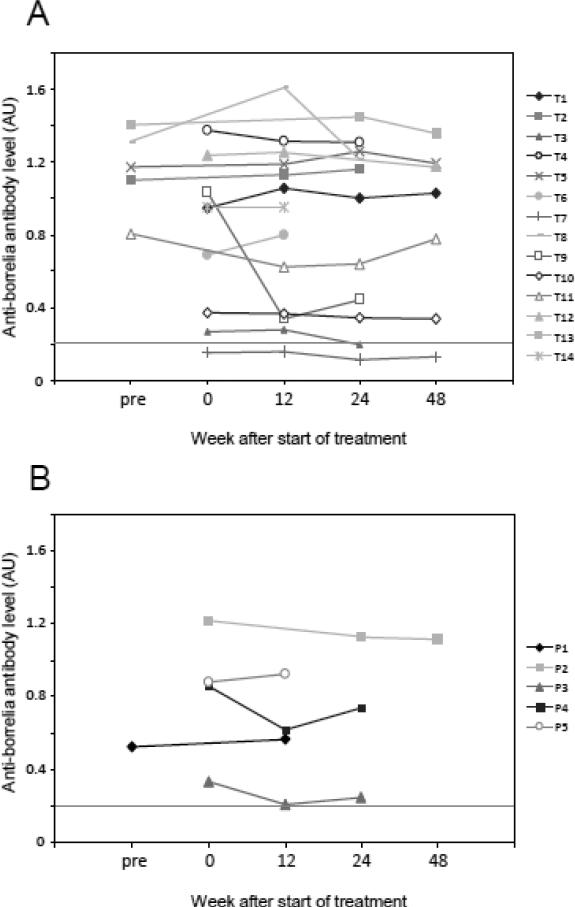
Total IgG anti-borrelia antibody levels are shown for the ceftriaxone and placebo groups in Figure 1. All except one patient were determined to be ELISA-positive prior to treatment. The mean anti-borrelia antibody level in patients did not change significantly in response to either treatment at any of the time points after the initiation of the trial. However, there was a sharp decline in the anti-borrelia antibody response for one patient (T9) following treatment. The antibody decline in this patient coincided with improvement in verbal fluency at week 12 that was sustained to week 24, and in improvement in memory and physical functioning at week 12 that was lost by week 24, as previously defined (Fallon et al., 2008).
Figure 1.
Anti-B. burgdorferi antibody levels as detected by whole-cell ELISA in the ceftriaxone treatment group (A) and the placebo group (B) at 0, 12, 24, and 48 weeks after the start of treatment. Cutoff for positivity is shown as a dashed line.
B. burgdorferi WB
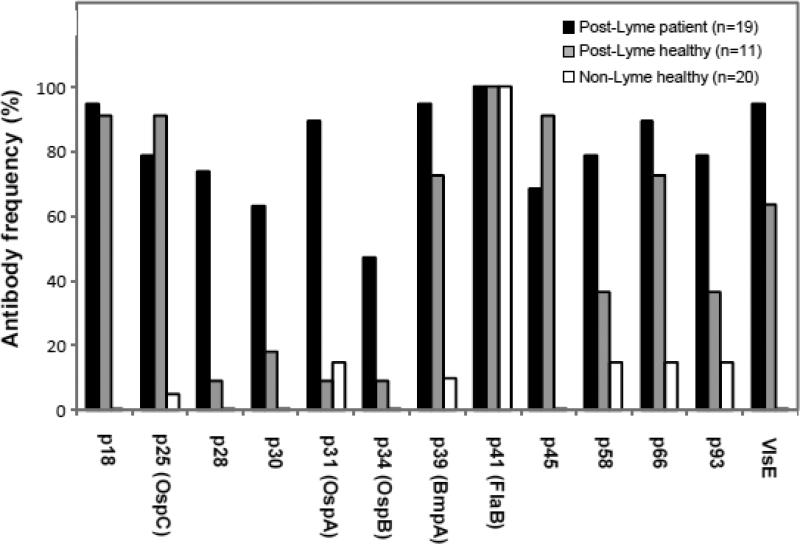
Using the CDC criteria, all post-Lyme symptomatic patients prior to antibiotic treatment and post-Lyme healthy subjects were IgG WB seropositive for anti-borrelia antibodies. None of the healthy control subjects without history of Lyme disease was positive by WB. The frequencies of antibodies to 13 protein bands (p18, p25 (OspC), p28, p30, p31 (OspA), p34 (OspB), p39 (BmpA), p41 (FlaB), p45, p58, p66, p93, and VlsE) in the analyzed specimens of the three groups are shown in Fig. 2. There was a significant difference in frequency of antibody reactivity to p28 (p <0.01) and p31 (p <0.0001) bands between post-Lyme patient and post-Lyme healthy groups. Frequencies of antibody reactivity to all bands, with the exception of p41, were significantly higher in the patient group than in the group of healthy individuals without history or serologic evidence of Lyme disease. There was not a detectable fluctuation in the level and profile of antibody reactivity to any borrelial protein bands throughout the course of treatment, except in the same patient identified in the results for whole-cell ELISA (T9). The change in the anti-borrelia antibody response for this patient at week 12 was characterized by the disappearance of reactivity to VlsE and p25 proteins and reduction in reactivity to others (Fig. 3).
Figure 2.
Frequency of antibody reactivity to immunodominant proteins of B. burgdorferi in patients and control subjects. Significantly more post-Lyme patients were found to have antibodies to p28 (p <0.01) and p31 (p <0.0001) bands than post-Lyme healthy subjects. Frequencies of antibody reactivity to all bands, with the exception of p41, were significantly higher in the patient group than in the non-Lyme healthy group.
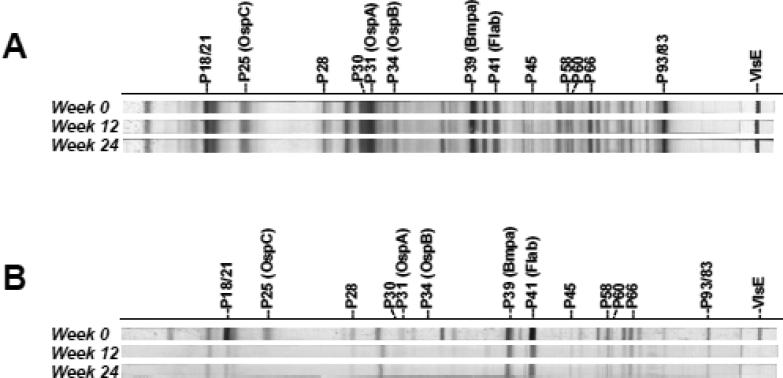
Figure 3.
Immunoblots of serum sample antibodies from 2 representative patients, indicating the extent of change in the level and profile of the anti-borrelia antibody response as a function of time after the initiation of treatment. A) The lack of any significant fluctuation in the level and pattern of anti-borrelia antibody response in this patient is representative of the majority of the study subjects. B) Only in patient T9 was there an obvious change in the level and specificity of the anti-borrelia antibody response at weeks 12-24.
Antibodies to IR6 epitope of VlsE
12 of 14 patients in the ceftriaxone group, 5 of 5 patients in the placebo group, and 7 of 11 post-Lyme healthy subjects were positive for C6 antibodies according to the specified cutoffs. The mean C6 antibody level for the affected patients did not change significantly in response to either treatment at any of the time points (Fig. 4). However, the C6 antibody for patient T9 became negative at week 12 after the start of ceftriaxone treatment. The positivity/negativity status for other patients remained constant throughout the course of treatment.
Figure 4.
Anti-IR6 (C6) antibody levels in the ceftriaxone treatment group (A) and the placebo group (B) at 0, 12, 24, and 48 weeks after start of treatment. Cutoff for positivity is shown as a dashed line.
Anti-neural autoantibodies
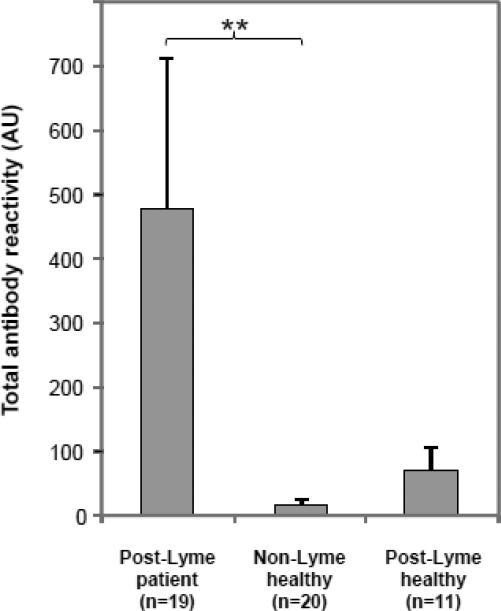
At the dilution and exposure levels examined in this study, anti-neural antibody reactivity, as represented by the presence of reactivity to one or more protein bands, was seen in serum specimens from 9 of 19 (47.4%) patients (prior to treatment), 2 of 11 (18.2%) post-Lyme healthy individuals, and 3 of 20 (15.0%) healthy subjects. There was a significant difference between the patient and the non-Lyme healthy control groups (p<0.05), but the difference between the patient and the post-Lyme healthy cohorts did not reach the level of significance. The anti-neural antibody reactivities in patients were directed at multiple antigens, as indicated by binding to different protein bands. When the number and intensity of bands (total antibody reactivity) were taken into account (measured as described in the methods section), the difference between the patient and non-Lyme healthy control groups remained significant (p<0.01) (Fig. 5).
Figure 5.
Total anti-neural antibody reactivity in patients and controls. Reactivity was significantly higher in the patient group (prior to treatment) than in the non-Lyme healthy group. Error bars represent the standard error of the mean. ** = p<0.01.
Among the affected patients, 8 of 14 in the ceftriaxone group and 1 of 5 in the placebo group were positive for anti-neural antibodies prior to treatment. Comparison of anti-neural antibody levels prior to, during, and following treatment did not indicate a significant change in the total antibody level or band reactivity profile in the drug and placebo groups (Fig. 6).
Figure 6.
Log of anti-neural antibody reactivity in the ceftriaxone treatment group (A) and the placebo group (B) at 0, 12, 24, and 48 weeks after the start of treatment. Reactivity for patients T3, T11, T13, and P4 was below detectable threshold. Cutoff for positivity is shown as a dashed line. C) Immunoblots of serum antibodies from two representative patients at weeks 0, 12, and 24 demonstrate the lack of substantial fluctuation in anti-neural antibodies in response to treatment. Molecular weight markers are indicated to the left of the panel (kDa).
IFNα activity
Compared with the post-Lyme healthy and non-Lyme healthy control groups, sera from patients with a history of Lyme disease and persistent symptoms induced significantly greater expression of IFIT1 and IFI44 in reporter cells (Fig. 7), indicating increased serum IFNα activity. The expression levels of IFIT3 did not vary significantly in the 3 groups. There was not a statistically significant change in levels of induction of IFIT1, IFI44, and IFIT3 in either the treatment or the placebo group throughout the course of the trial (data not shown).
Figure 7.
Induction of IFNα target gene (IFIT1, IFI44, and IFIT3) expression by serum from patients with a history of Lyme disease and persistent cognitive dysfunction, post-Lyme healthy individuals, and non-Lyme healthy control subjects. There was a significantly higher mean expression of IFIT1 and IFI44 genes in response to patient sera in comparison to post-Lyme healthy and non-Lyme healthy sera. A significant difference in IFIT3 expression between the three groups was not found. Error bars represent the standard error of the mean. * = p<0.05, ** = p<0.01, *** = p<0.001.
4. Discussion
The observation that nearly half of the patients in this study have increased serum antibody reactivity to neural antigens is similar to findings from an earlier study by our group (Chandra et al., 2010). In that study, 49% of serum samples from a cohort of patients, recruited as part of a treatment trial of patients with PLDS (Klempner et al., 2001), were found to have elevated anti-neural antibody reactivity. The heightened antibody response level in those patients was statistically similar to that in patients with systemic lupus erythematosus, a multisystem autoimmune disease. The agreement in the observed anti-neural antibody response between this study (in patients with objective evidence of memory impairment) and the earlier work (in patients with subjective symptoms of pain, cognitive impairment, or dysesthesia) points to the similarity between the two patient populations examined.
We also found higher frequencies of antibody to p28, p30, p31, and p34 borrelial proteins in the patient group compared with the post-Lyme healthy group. Previous studies have shown that IgG antibodies against p30, p31, and p34 are more frequent in later stages of active infection, which are associated with longer exposure to the pathogen (Akin et al., 1999; Das et al., 1996; Kalish et al., 1993; Ma et al., 1992; Nowalk et al., 2006). As such, the serologic pattern observed may imply increased mitogenic and/or antigenic stimulation through prolonged exposure to the organism, prior to the initial diagnosis and treatment. This would be in line with other studies showing that delayed treatment is associated with increased incidence of post-Lyme disease symptoms (Marques, 2008).
There was no significant change in anti-neural and anti-borrelia antibody reactivity in response to ceftriaxone or placebo in affected patients. We observed a substantive decline in anti-borrelia antibody level and change in profile of reactivity for only one patient among the 19 examined. The sharp decrease and disappearance of antibodies to several borrelial proteins in the relatively short period of 12 weeks after the start of treatment may be suggestive of the presence of active borrelia infection in this particular patient at week 0 or shortly before. However, our data indicate that such antibody decline is an uncommon occurrence among patients with a history of Lyme disease and persistent cognitive deficits.
The patients in this study were characterized by increased levels of IFNα in comparison to asymptomatic post-Lyme subjects and non-Lyme healthy individuals, as indicated by elevated expression of IFNα-inducible genes in a reporter cell line (WISH cells) cultured with patient sera. The utilized assay for detection of IFNα activity is more quantitative and sensitive than commercially available ELISAs that directly measure IFNα level, making it particularly suitable for this type of longitudinal analysis. It has been shown to be highly useful in a number of studies published by our group and others (Hua et al., 2006; Kariuki et al., 2008; Niewold et al., 2007; Pothlichet et al., 2011; Somers et al., 2012). The finding suggests a possible mechanism contributing to the observed ongoing neuropsychiatric symptoms in affected patients. IFNα is a cytokine that is produced in response to infection and inflammation by a variety of cells, including plasmacytoid dendritic cells, macrophages, neutrophils, T cells, astrocytes, microglia, and neurons. In addition to its immunoregulatory properties, various studies have demonstrated a correlation between increased IFN activity and cognitive impairment in humans and animal models (Fritz-French and Tyor, 2012). In chronic viral infections such as hepatitis C, treatment with IFNα is often complicated by side effects including fatigue, myalgia, and cognitive dysfunction (Lieb et al., 2006). In addition, IFN activity has been shown to be elevated in a number of conditions with adverse neuropsychiatric manifestations, including HIV-associated neurocognitive disorders, systemic lupus erythematosus, and multiple sclerosis (Fritz-French and Tyor, 2012). Furthermore, peripheral administration of IFNα in patients has been shown to cause a significant increase in cerebrospinal fluid (CSF) IFNα and concomitant elevation in CSF IL6 and MCP1, indicating that IFNα can cross the blood-brain barrier and/or activate mechanisms that affect the central nervous system (Raison et al., 2009). In a mouse model of HIV-associated neurocognitive disorders, increased IFNα correlated with a similar array of behavioral and pathological abnormalities as seen in humans (Sas et al., 2007).
Animal data support a causal role for IFNα in the associated neuropsychiatric dysfunction. Transgenic mice that overexpress IFNα from astrocytes display severe encephalitis and cognitive abnormalities (Campbell et al., 1999) and intraventricular infusion of IFNα into brain provokes behavioral alterations and concomitant changes in expression of serotonin and pro-inflammatory cytokines (Hayley et al., 2012). In addition, the blocking of IFNα with injections of IFNα neutralizing antibodies in an HIV encephalitis mouse model has been shown to improve cognitive function significantly compared to control antibody-treated mice (Sas et al., 2009). While the mechanism of IFNα neurotoxicitiy is not well understood, its effect on loss of dendritic arborization, modulation of glutamate levels causing changes in excitability, and inhibition of long-term potentiation and reduction of excitatory post-synaptic potential affecting signal transmission are likely culprits that have been demonstrated in animal models (Dafny, 1998; D'Arcangelo et al., 1991; Mendoza-Fernandez et al., 2000; Sas et al., 2009).
In patients with acute Lyme disease, greater symptom scores and elevated serum levels of IFNα have been previously shown to be associated with dissemination of infection (Salazar et al., 2003). A study of mice infected with B. burgdorferi revealed a critical role for type I IFNs in the development of arthritis, which was reduced by using type I IFN receptor-blocking antibodies (Miller et al., 2008). In a study that evaluated the inflammatory potential of various B. burgdorferi isolates in cultures of macrophages and PBMCs, RST1 strains, which are associated with increased likelihood of hematogenous dissemination and more severe disease symptoms, induced significantly greater IFNα than other isolates (Strle et al., 2011; Wormser et al., 1999). As such, the observed elevated serum IFNα activity in our study may be indicative of a more severe infection prior to antibiotic treatment in these patients. In addition, the persistence of elevated IFNα activity in the affected patients might be linked to genetic predisposition, possibly exerting its effect through the amplification of TLR signaling. For example, the high serum IFNα activity in lupus patients is associated with a polymorphism in IRF5 (Niewold et al., 2008). Considering the strong connection between IFNα and neurocognitive impairment, treatments aimed at inhibition of IFNα activity or reduction in levels may be effective at improving cognitive functioning in some of the affected individuals. It would be of interest to determine whether increased IFNα activity is unique to persistent symptoms following treatment of infection with B. burgdorferi or a common feature in post-treatment symptoms associated with other infections, which would imply relevance beyond Lyme disease.
In conclusion, this study offers further evidence for the existence of an immune-related disease process in patients with persistent symptoms following antibiotic treatment for Lyme disease. It is the first report to demonstrate increased IFNα activity in affected patients, suggesting a potential mechanism contributing to the associated ongoing neuropsychiatric symptoms. In addition, there is a significantly enhanced antibody response to autoantigens and to specific borrelial proteins, possibly indicating prolonged exposure to the organism prior to the original diagnosis and treatment of Lyme disease The data also show that additional β-lactam antibiotic therapy is not effective at modulating the activated immune response in affected patients. The study's findings point to the possibility of discovering biomarkers that could help in identification of specific subsets of patients with, or at risk for developing, persistent symptoms. In addition, they yield novel clues regarding disease mechanism that may become useful in finding safe and effective treatments for affected individuals in the future.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) [grant numbers R56 AI093763-01 and RO1 MH 071456], Lyme Research Alliance, and the Lyme and Tick-borne Diseases Research Center at Columbia University Medical Center. We would like to thank Ms. Susan Bittker, Ms. Diane Holmgren, Ms. Donna McKenna, and Mr. Fawad Viqar for their assistance with specimen collection and organization. We are grateful to all of the research participants involved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: Authors have no conflicts of interest relevant to this article to disclose.
Disclosure statement: AA: Research grants related to Lyme disease from National Institutes of Health (NIH) and Lyme Research Alliance. BAF: Director of the Lyme and Tick-Borne Diseases Research Center at Columbia University Medical Center; Research grants related to Lyme disease from NIH, Lyme Research Alliance, and Lyme Disease Association. GPW: Research grants from CDC, NIH, Immunetics, Inc., Bio-Rad, DiaSorin, Inc., and BioMerieux; Equity in Abbott; Expert witness in malpractice cases involving Lyme disease; Unpaid board member at American Lyme Disease Foundation; Expert witness regarding Lyme disease in a disciplinary action for the Missouri Board of Registration for the Healing Arts; Consultant to Baxter for Lyme vaccine development.
References
- Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin E, McHugh GL, Flavell RA, Fikrig E, Steere AC. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun. 1999;67:173–181. doi: 10.1128/iai.67.1.173-181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaedini A, Okamoto H, Briani C, Wollenberg K, Shill HA, Bushara KO, Sander HW, Green PH, Hallett M, Latov N. Immune cross-reactivity in celiac disease: anti-gliadin antibodies bind to neuronal synapsin I. J Immunol. 2007;178:6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- Bratton RL, Whiteside JW, Hovan MJ, Engle RL, Edwards FD. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566–571. doi: 10.4065/83.5.566. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Krucker T, Steffensen S, Akwa Y, Powell HC, Lane T, Carr DJ, Gold LH, Henriksen SJ, Siggins GR. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-alpha. Brain Res. 1999;835:46–61. doi: 10.1016/s0006-8993(99)01328-1. [DOI] [PubMed] [Google Scholar]
- CDC Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- Chandra A, Latov N, Wormser GP, Marques AR, Alaedini A. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin Immunol. 2011a;141:103–110. doi: 10.1016/j.clim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Wormser GP, Klempner MS, Trevino RP, Crow MK, Latov N, Alaedini A. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun. 2010;6:1018–1024. doi: 10.1016/j.bbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Wormser GP, Marques AR, Latov N, Alaedini A. Anti-Borrelia burgdorferi antibody profile in post-Lyme disease syndrome. Clin Vaccine Immunol. 2011b;18:767–771. doi: 10.1128/CVI.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res Ther 12 Suppl. 2012;1:S5. doi: 10.1186/ar2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafny N. Is interferon-alpha a neuromodulator? Brain Res Brain Res Rev. 1998;26:1–15. doi: 10.1016/s0165-0173(97)00029-5. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Grassi F, Ragozzino D, Santoni A, Tancredi V, Eusebi F. Interferon inhibits synaptic potentiation in rat hippocampus. Brain Res. 1991;564:245–248. doi: 10.1016/0006-8993(91)91459-e. [DOI] [PubMed] [Google Scholar]
- Das S, Shraga D, Gannon C, Lam TT, Feng S, Brunet LR, Telford SR, Barthold SW, Flavell RA, Fikrig E. Characterization of a 30-kDa Borrelia burgdorferi substrate-binding protein homologue. Res Microbiol. 1996;147:739–751. doi: 10.1016/s0923-2508(97)85121-2. [DOI] [PubMed] [Google Scholar]
- Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, Slavov I, Cheng J, Dobkin J, Nelson DR, Sackeim HA. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- Feder HM, Jr., Johnson BJ, O'Connell S, Shapiro ED, Steere AC, Wormser GP, Agger WA, Artsob H, Auwaerter P, Dumler JS, Bakken JS, Bockenstedt LK, Green J, Dattwyler RJ, Munoz J, Nadelman RB, Schwartz I, Draper T, McSweegan E, Halperin JJ, Klempner MS, Krause PJ, Mead P, Morshed M, Porwancher R, Radolf JD, Smith RP, Jr., Sood S, Weinstein A, Wong SJ, Zemel L. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–1430. doi: 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- Fritz-French C, Tyor W. Interferon-alpha (IFNalpha) neurotoxicity. Cytokine Growth Factor Rev. 2012;23:7–14. doi: 10.1016/j.cytogfr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Halperin JJ. Nervous system Lyme disease. Infect Dis Clin North Am. 2008;22:261–274. doi: 10.1016/j.idc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Hayley S, Scharf J, Anisman H. Central administration of murine interferon-alpha induces depressive-like behavioral, brain cytokine and neurochemical alterations in mice: A mini-review and original experiments. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki SN, Crow MK, Niewold TB. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activity and low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum. 2008;58:2818–2823. doi: 10.1002/art.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- Lieb K, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Janssen G, Schaefer M. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNalpha): results from a prospective study. Eur Psychiatry. 2006;21:204–210. doi: 10.1016/j.eurpsy.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Ma B, Christen B, Leung D, Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992;30:370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. 2008;22:341–360. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res. 2000;885:14–24. doi: 10.1016/s0006-8993(00)02877-8. [DOI] [PubMed] [Google Scholar]
- Miller JC, Ma Y, Bian J, Sheehan KC, Zachary JF, Weis JH, Schreiber RD, Weis JJ. A critical role for type I IFN in arthritis development following Borrelia burgdorferi infection of mice. J Immunol. 2008;181:8492–8503. doi: 10.4049/jimmunol.181.12.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowalk AJ, Gilmore RD, Jr., Carroll JA. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun. 2006;74:3864–3873. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothlichet J, Niewold TB, Vitour D, Solhonne B, Crow MK, Si-Tahar M. A loss-of-function variant of the antiviral molecule MAVS is associated with a subset of systemic lupus patients. EMBO Mol Med. 2011;3:142–152. doi: 10.1002/emmm.201000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JC, Pope CD, Sellati TJ, Feder HM, Jr., Kiely TG, Dardick KR, Buckman RL, Moore MW, Caimano MJ, Pope JG, Krause PJ, Radolf JD. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–2670. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29:3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson HA, Tyor WR. Cognitive dysfunction in HIV encephalitic SCID mice correlates with levels of Interferon-alpha in the brain. Aids. 2007;21:2151–2159. doi: 10.1097/QAD.0b013e3282f08c2f. [DOI] [PubMed] [Google Scholar]
- Somers EC, Zhao W, Lewis EE, Wang L, Wing JJ, Sundaram B, Kazerooni EA, McCune WJ, Kaplan MJ. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS One. 2012;7:e37000. doi: 10.1371/journal.pone.0037000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–1647. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- Stanek G, Strle F. Lyme disease: European perspective. Infect Dis Clin North Am. 2008;22:327–339. doi: 10.1016/j.idc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale Scale. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, Holmgren D, Schwartz I. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]