Abstract
Distinct physiological stimuli are required for bidirectional synaptic plasticity in striatum and hippocampus, but differences in the underlying signaling mechanisms are poorly understood. We have begun to compare levels and interactions of key excitatory synaptic proteins in whole extracts and subcellular fractions isolated from micro-dissected striatum and hippocampus. Levels of multiple glutamate receptor subunits, calcium/calmodulin-dependent protein kinase II (CaMKII), a highly abundant serine/threonine kinase, and spinophilin, a F-actin and protein phosphatase 1 (PP1) binding protein, were significantly lower in striatal extracts, as well as in synaptic and/or extrasynaptic fractions, compared to similar hippocampal extracts/fractions. However, CaMKII interactions with spinophilin were more robust in striatum compared to hippocampus, and this enhanced association was restricted to the extrasynaptic fraction. NMDAR GluN2B subunits associate with both spinophilin and CaMKII, but spinophilin-GluN2B complexes were enriched in extrasynaptic fractions whereas CaMKII-GluN2B complexes were enriched in synaptic fractions. Notably, the association of GluN2B with both CaMKII and spinophilin was more robust in striatal extrasynaptic fractions compared to hippocampal extrasynaptic fractions. Selective differences in the assembly of synaptic and extrasynaptic signaling complexes may contribute to differential physiological regulation of excitatory transmission in striatum and hippocampus.
Keywords: calcium/calmodulin-dependent protein kinase II, hippocampus, protein complexes, protein phosphatase 1, spinophilin, striatum
Introduction
The striatum and hippocampus control different forms of learning (Berke et al. 2009, Amso et al. 2005). The majority (~95%) of neurons in the striatum are γ-aminobutyric acid-containing medium spiny neurons (MSNs) (Kreitzer & Malenka 2008, Huang et al. 1992), whereas glutamatergic pyramidal neurons predominate in hippocampus. Although bidirectional synaptic plasticity (i.e. long-term potentiation (LTP) and long-term depression (LTD)) is thought to play a key role in the function of both brain regions, there are substantial differences in the underlying mechanisms. For example, N-methyl-D-aspartate receptor (NMDAR)- and calcium/calmodulin-dependent protein kinase II (CaMKII)-dependent LTP has been extensively studied in hippocampal CA1 pyramidal neurons (Bear & Malenka 1994, Malenka 1994, Malenka & Bear 2004, Nicoll & Malenka 1995, Lisman et al. 2012), whereas LTP in striatal MSNs can only be reliably observed when NMDAR activity is enhanced (Jia et al. 2010, Calabresi et al. 1992). Moreover, these physiological synaptic differences between brain regions extend to pathological situations. For example, Rett Syndrome and Alzheimer disease are associated with a decrease in dendritic spine density in hippocampal neurons (Chapleau et al. 2009, Penzes et al. 2011), whereas Parkinson disease is associated with decreased spine density in striatal MSNs (Zaja-Milatovic et al. 2005, Stephens et al. 2005). However, the molecular basis for these distinct synaptic properties are not well understood.
Differences in the localization, expression, and/or interactions of proteins that modulate postsynaptic signaling may contribute to the unique physiological properties and pathological susceptibilities of striatal and hippocampal neurons. For example, transgenic mice lacking postsynaptic density-95 (PSD-95), the prototypical postsynaptic scaffolding protein, have decreased spine density in striatal MSNs but increased spine density in CA1 hippocampal pyramidal neurons (Vickers et al. 2006). Total tissue levels of the alpha isoform of CaMKII are somewhat higher in hippocampus compared to striatum (Erondu & Kennedy 1985), whereas total levels of the actin- and CaMKII-binding protein, α-actinin-2, are higher in striatum compared to hippocampus (Wyszynski et al. 1998). However, to the best of our knowledge, there are no studies directly comparing interactions between signaling proteins in striatum and hippocampus.
We recently found that spinophilin targets protein phosphatase 1 (PP1) to CaMKII in adult striatum (Baucum et al. 2012). Here we report that the association of CaMKII with the spinophilin-PP1 complex is significantly greater in adult striatum compared to hippocampus. The enhanced striatal association was detected in an extrasynaptic, but not synaptic fraction. Moreover, extrasynaptic NMDAR GluN2B subunits are more robustly associated with both spinophilin and CaMKII in striatum compared to hippocampus. These differences in protein-protein interactions in specific subcellular compartments may contribute to the distinct physiological properties and/or pathological susceptibilities of striatal and hippocampal neurons.
Methods
Animals
Adult, male (1.8–7 month old) C57Bl6/J mice (Jackson Laboratories) were decapitated. Neostriatum (referred to as striatum) or hippocampus were dissected and either used fresh or frozen on dry ice and stored at −80°C until processed. To minimize postmortem differences, hippocampus and striatum were dissected from the same animals at the same time and processed in parallel. Total time from decapitation to homogenization or freezing is approximately 90 seconds. All animal protocols were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH, and were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Antibodies
CaMKII goat antibody was previously described (McNeill & Colbran 1995). Commercially available antibodies are listed in Table S1.
Tissue homogenization: total lysates and low-ionic strength Triton-soluble fraction
Fresh or frozen mouse striata or hippocampus were homogenized in 2 ml of a low ionic strength buffer (all values are w/v unless otherwise noted: 2 mM Tris-HCl pH 7.4, 2 mM EDTA, 2 mM EGTA, 1 mM DTT, 0.2 mM PMSF, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μM pepstatin, 20 μg/ml soybean trypsin inhibitor, 1 μM microcystin and 1% (v/v) Triton X-100) using a Teflon-glass tissue grinder (Wheaton) either by hand or with a motorized plunger. Total homogenates were adjusted to the same protein concentration in each experiment (0.84 – 1 mg/ml) as measured using the Bradford protein assay. Due to the labile nature of Thr286 phosphorylation, we only quantified Thr286 phosphorylation from freshly prepared striata or hippocampi homogenized in buffers containing additional phosphatase inhibitors (1 mM NaVO4 and 0.5 nM cypermethrin) and immediately mixed with 4X SDS-PAGE sample buffer (0.25 M Tris-HCl, 8% SDS, 40% glycerol (v/v), 0.032% bromophenol blue, 100 mM DTT). The remaining homogenate was incubated at 4°C for 30–60 min and then centrifuged at 9,000 × g for 10 min at 4°C. An aliquot of the supernatant (Triton-soluble fraction), was mixed with 4X SDS-PAGE sample buffer and the remaining Triton-soluble fraction was immunoprecipitated or incubated with GST spinophilin fusion protein (see below). We have previously shown that several PSD proteins are efficiently solubilized using this procedure (Baucum et al. 2010).
Tissue homogenization: subcellular fractionation studies
Subcellular fractions were prepared as previously described (Gustin et al. 2010). Briefly, fresh or frozen striatum or hippocampus were homogenized in an isotonic buffer (150 mM KCl, 50 mM Tris HCl pH 7.5, 1 mM DTT, 0.2 mM PMSF, 1 mM Benzamidine, 1 μM Pepstatin, 10 mg/l Leupeptin, 1 μM microcystin) without detergent and normalized to equal protein concentrations (0.34–0.6 mg/ml). The homogenate (1ml) was passed through a 22 gauge needle 2 times, incubated with rocking at 4°C for 30 minutes and then centrifuged at 100,000 × g for 1 hour. The pellet was resuspended in the isotonic buffer containing 1% (v/v) Triton X-100, triturated until homogenous, and then incubated with rocking at 4°C for 30 minutes. Lysates were then centrifuged at 18,403 × g and the supernatant (“Extrasynaptic” S2 fraction) was saved for immunoprecipitation (see below) or mixed with 4X SDS-PAGE sample buffer for direct loading on SDS-PAGE gels. The pellet was resuspended in isotonic buffer containing 1% Triton X-100 and 1% deoxycholate and sonicated (“Synaptic” S3 fraction) and then saved for immunoprecipitation (see below) or mixed with 4X SDS-PAGE sample buffer.
Immunoprecipitations and GST pulldowns
Immunoprecipitations were performed with goat spinophilin (2–3 μg) or goat CaMKII (1.8–4 μg) antibody as previously described (Baucum et al. 2010, Brown et al. 2008). Immunoprecipitations from fractionation experiments were performed using magnetic protein A/G beads (ThermoScientific). Pulldowns using GST-spinophilin fusion proteins (GSTSpN1 (amino acids 1–154), GSTSpN2 (amino acids 151–300), or GSTSpC1 (amino acids 665–817)) were performed as previously described (Baucum et al. 2012). A single spinophilin band is detected in immunoblots of total lysates, whereas immunoprecipitated spinophilin sometimes migrates as a doublet, presumably due to proteolysis during immunoprecipitation.
Immunoblots
Immunoblots were developed as previously described using either enhanced chemiluminescence and X-ray film or infra-red fluorescence and the Odyssey system (LiCor Biosciences) (Baucum et al. 2012).
Quantitation
Densitometry was performed using Image J (NIH) on images linearly adjusted for brightness and contrast. Normalization of signals in total lysates or specific subcellular fractions was performed by dividing the immunoblotted protein band density by the density of total protein stain (Ponceau S) (Gustin et al. 2010). For co-immunoprecipitations, levels of individual co-precipitating proteins were normalized to the amount of primary immunoprecipitated protein (e.g. CaMKII immunoreactivity in spinophilin immunoprecipitates was normalized to spinophilin immunoreactivity in the spinophilin immunoprecipitates). In co-immunoprecipitations from specific subcellular fractions, immunoreactivity of the co-precipitating protein was normalized to levels of the primary immunoprecipitated protein as well as the amount of the co-precipitated protein in the input of the corresponding subcellular fraction. For pulldowns, individual co-precipitating proteins were normalized to the amount of the spinophilin GST protein on the gel as measured by Ponceau S stain as well as the amount in the low ionic strength, Triton-soluble fraction.
To allow for comparison across multiple gels, a ratio was obtained by dividing the immunoreactivity in striatal samples by to immunoreactivity values in either the corresponding hippocampal fraction or the hippocampal S2 fraction (to compare S2 and S3 values together) analyzed on the same gel. An N of 1–3 animals were quantified per gel from 2–3 sets of experiments. All data were normalized to immunoreactivity in the hippocampal S2 fraction, transformed, and plotted on an antilog scale to allow for symmetrical comparisons of both increased and decreased ratios. The number of animals per analysis is shown on each graph.
Statistics
For comparisons of hippocampus and striatum within a fraction, an unpaired Student’s t-test was performed if the variances between the two groups were not significantly different. An unpaired t-test with Welch’s correction was applied if there was a significant difference in the variances. When data were evaluated for comparison of hippocampus and striatum from S2 and S3 fractions together, a two-way ANOVA followed by an uncorrected Fisher’s LSD post-hoc test was used.
Results
Differences in total striatal and hippocampal synaptic protein levels
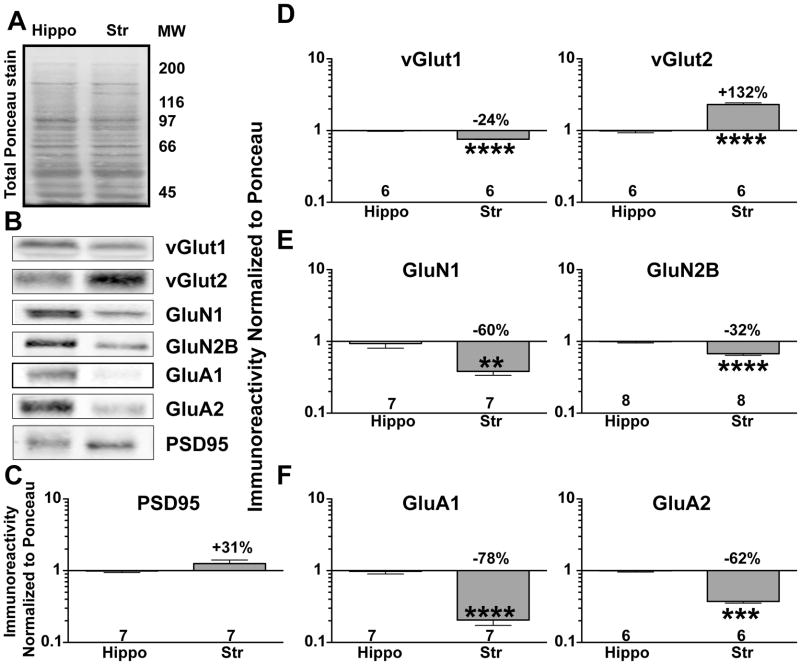
To begin to understand mechanisms underlying differences in synaptic regulation between striatum and hippocampus, we compared total tissue levels of several proteins that have been functionally implicated in excitatory synaptic modulation. Whole striatal and hippocampal lysates from adult mice were loaded at equal protein concentrations, as evidenced by approximately equal staining using Ponceau S (Fig 1A). Immunoblotting (Fig 1B) revealed that total levels of the postsynaptic density marker, PSD-95, were not significantly different between the two brain regions (Fig 1C). Levels of the presynaptic vGlut1 transporter were significantly lower in striatum compared to hippocampus (Fig 1D), whereas levels of vGlut2 were significantly higher in striatum compared to hippocampus (Fig 1D). Levels of the GluN1 and GluN2B NMDAR subunits were 30–60% lower in striatum compared to hippocampus (Fig 1E), whereas total levels of GluA1 and GluA2, subunits of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), were 60–80% lower in striatal lysates (Fig 1F).
Figure 1. Differential expression of excitatory, synaptic proteins in striatum and hippocampus.
A. Total hippocampal (Hippo) and striatal (Str) proteins stained using Ponceau S. MW -Molecular weight ladder.
B. Representative immunoblot of total hippocampal and striatal lysates for PSD-95, vGlut1, vGlut2, GluN1, GluN2B, GluA1, and GluA2.
C–F. Immunoreactivity for each protein was normalized to total protein loading (Ponceau S stain) and plotted as the striatal:hippocampal ratio on a Log10 scale (see Methods) C. PSD95.
D. vGlut1 and vGlut2. E. NMDAR GluN1 and GluN2B subunits. F. AMPAR GluA1 and GluA2 subunits. An unpaired Student’s t-test was performed between groups. **P<0.01, ***P<0.001, ****P<0.0001.
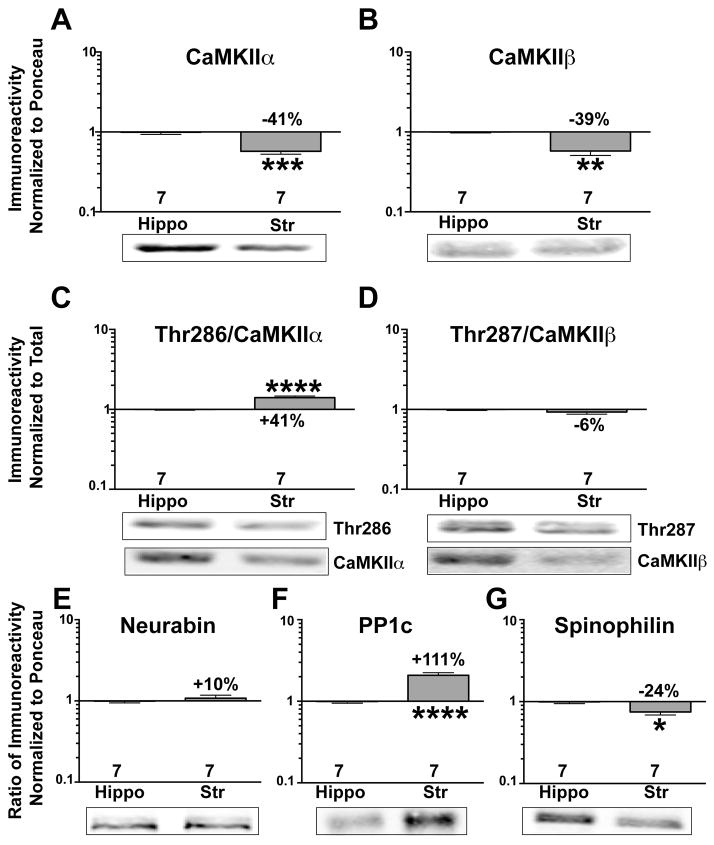
CaMKII is a key regulator of AMPARs and NMDARs (Sessoms-Sikes et al. 2005, Derkach et al. 1999, Lu et al. 2010, Hayashi et al. 2000). Total levels of CaMKIIα and CaMKIIβ were approximately 40% lower in striatum than in hippocampus (Fig 2A,B). Interestingly, levels of CaMKIIα phosphorylation at Thr286 (normalized to total CaMKIIα) were significantly higher in striatal, than in hippocampal, lysates (Fig 2C), but phosphorylation of CaMKIIβ at the equivalent residue (Thr287) was not significantly different (Fig 2D).
Figure 2. Differential levels and autophosphorylation of synaptic signaling and scaffolding proteins in adult striatum and hippocampus.
Representative immunoblots of hippocampal or striatal lysates from adult mice for CaMKIIα (A), CaMKIIβ (B), phospho-Thr286 (C) phospho-Thr287 (D), neurabin (E), PP1c (F), and spinophilin (G). Immunoreactivity was normalized to either total protein (Ponceau S) stain (A,B, E–G) or to total CaMKII levels (C, D), and plotted as the striatal:hippocampal ratio on a Log10 scale (see Methods). An unpaired Student’s t-test was performed between groups. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
PP1 activity is a key determinant of CaMKII autophosphorylation, glutamate receptor phosphorylation, and synaptic plasticity (Strack et al. 1997, Mullasseril et al. 2007, Farinelli et al. 2012, Hu et al. 2007, Blitzer et al. 1998), and PP1 targeting by neurabin and spinophilin is important for normal plasticity (Allen et al. 2006, Yan et al. 1999). While there was no difference in the total levels of neurabin between the two brain regions (Fig 2E), total PP1 catalytic subunit levels were ~2-fold higher in striatal compared to hippocampal lysates (Fig 2F). In contrast, striatal levels of a major synaptic PP1 targeting subunit, spinophilin, were ~25% lower (Fig 2G).
Differential association of CaMKII with spinophilin in striatum and hippocampus
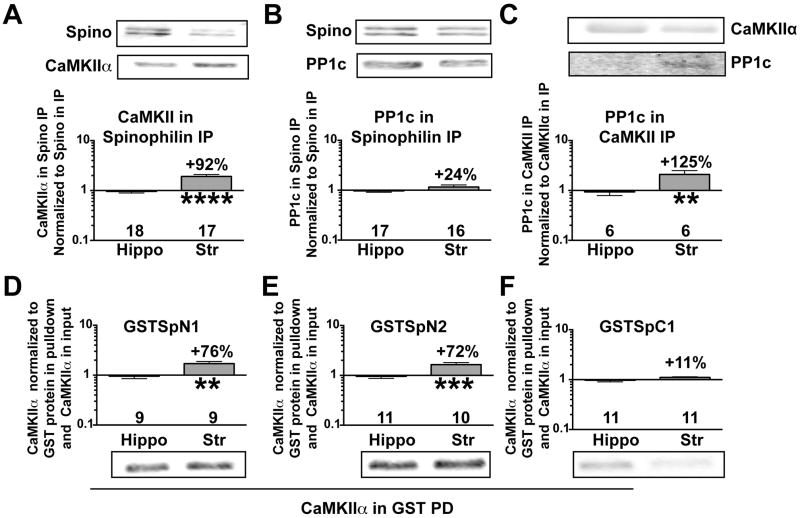
We recently found that CaMKII associated with spinophilin in a low ionic-strength, Triton-soluble fraction that largely solubilizes PSD proteins (Baucum et al. 2012, Baucum et al. 2010). Interestingly, CaMKII association with spinophilin was more robust in the striatal, compared to hippocampal, low-ionic strength, Triton-soluble fraction (Fig 3A). While the association of PP1 with spinophilin was not significantly different between brain regions (Fig 3B), there was a more robust association of PP1 with CaMKII in striatum compared to hippocampus (Fig 3C).
Figure 3. Differential association of spinophilin and PP1 with CaMKII in striatum and hippocampus.
A–C. Striatal or hippocampal low ionic strength Triton-soluble fractions were immunoprecipitated with either a spinophilin antibody and immunoblotted for spinophilin and CaMKII (A) or PP1 (B), or with a CaMKII antibody and immunoblotted for PP1 (C). D–F. Complexes isolated from striatal or hippocampal low ionic strength, Triton-soluble fractions using GSTSpN1, (E: amino acids 1–154), GSTSpN2 (F: amino acids 151–300), or GSTSpC1 (G: amino acids 665–817) and glutathione agarose were immunoblotted for CaMKIIα. Immunoreactivities were normalized to levels of GST protein isolated and to the levels of CaMKIIα in the input, and plotted as the striatum:hippocampus ratio on a Log10 scale (see Methods). Data were compared by an unpaired Student’s. **P<0.01, ***P<0.001, ****P<0.0001.
CaMKII directly and indirectly binds to two N-terminal regions and one C-terminal region in spinophilin (Baucum et al. 2012). Interestingly, GSTSpN1 (residues 1–154) (Fig 3D) and GSTSpN2 (residues 151–300) (Fig 3E), but not GSTSpC1 (residues 665–817) (Fig 3F), bound to more striatal CaMKII than hippocampal CaMKII after normalizing for the lower striatal CaMKII levels in the low ionic strength, Triton-soluble fraction.
Differential levels and interactions of proteins in the extrasynaptic and synaptic fractions
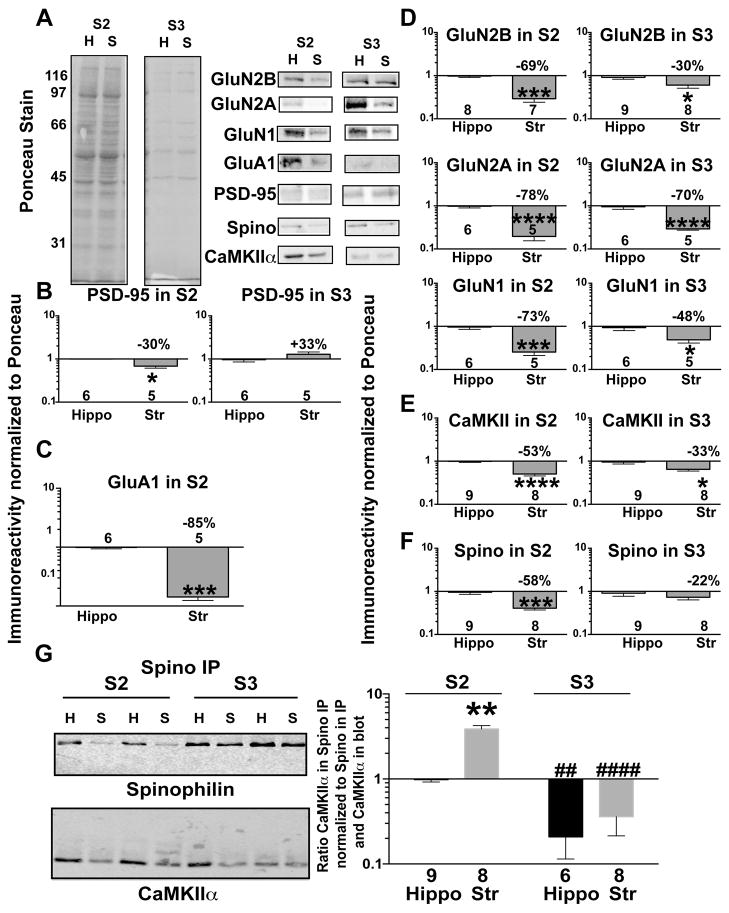
Glutamate receptor subunits and dendritic signaling/scaffolding proteins localize both synaptically and extrasynaptically, in some cases serving distinct functions (Gladding & Raymond 2011, Petralia et al. 2010). To better understand differences between striatum and hippocampus, we also compared protein levels in extrasynaptic (S2) and/or synaptic (S3) fractions isolated from the two brain regions (see Methods). PSD-enriched proteins such as PSD-95, GluN2B, GluN2A, and spinophilin were substantially enriched in synaptic fractions from both striatum and hippocampus, but were also detected in extrasynaptic fractions (Fig 4A, Supplemental figure 1). Levels of the canonical PSD marker, PSD-95, were modestly lower in the striatal extrasynaptic, but not striatal synaptic, fraction compared to the corresponding hippocampal fractions (Fig 4B). GluA1 was expressed at lower levels in the extrasynaptic fraction isolated from striatum compared to hippocampus (Fig 4C), with only weak detection in the synaptic fraction that could not be reliably quantified. GluN2A, GluN2B, and GluN1 levels were lower in both fractions isolated from striatum compared to hippocampus (Fig 4D). Striatal levels of CaMKII were significantly lower in both the extrasynaptic and synaptic fractions compared to hippocampal fractions (Fig 4E), whereas striatal levels of spinophilin were significantly lower only in the extrasynaptic fraction (Fig 4F).
Figure 4. Differential distribution and association of proteins between extrasynaptic (S2) and synaptic (S3) fractions isolated from striatum and hippocampus.
A. Representative Ponceau S stain and immunoblots for GluN2B, GluN2A, GluA1, PSD-95, Spinophilin (Spino) or CaMKIIα isolated from extrasynaptic (S2) and synaptic (S3) hippocampal or striatal lysates from adult mice. Immunoreactivities for glutamate receptors (B), CaMKIIα (C), Spino (D), and PSD-95 (E) was normalized to total protein (Ponceau S) stain, and plotted as the striatal:hippocampal ratio on a Log10 scale (see Methods). B–F: Data were compared by an unpaired Student’s t-test *P<0.05, ***P<0.001, ****P<0.0001. G: Spinophilin immune complexes isolated from striatal and hippocampal S2 and S3 fractions were immunoblotted in parallel for spinophilin and CaMKIIα. The ratio of CaMKIIα to spinophilin normalized to CaMKIIα in the Input was expressed relative to the ratio in the hippocampal S2 fraction and plotted on a Log10 scale. The two-way ANOVA results were: fractionation effect P<0.0001, brain region effect P=0.0129, Interaction P=0.2634. Post hoc analysis was by uncorrected Fisher’s LSD post hoc test. Comparison of striatum to hippocampus in same subcellular fraction: **P<0.01. Comparison to S2 fraction of samebrain region: ##P<0.01, ####P<0.0001
In order to investigate the subcellular localization of the spinophilin/CaMKII complex observed in striatum and hippocampus, we immunoprecipitated spinophilin from both synaptic and extrasynaptic fractions. There was a significantly more robust interaction between spinophilin and CaMKII in the extrasynaptic (S2) compared to synaptic (S3) fraction in both brain regions (Fig 4G). Moreover, significantly more CaMKII co-precipitated with spinophilin in striatal compared to hippocampal extrasynaptic fractions, while there was no difference in the association of spinophilin and CaMKII between the synaptic fractions (Fig 4G).
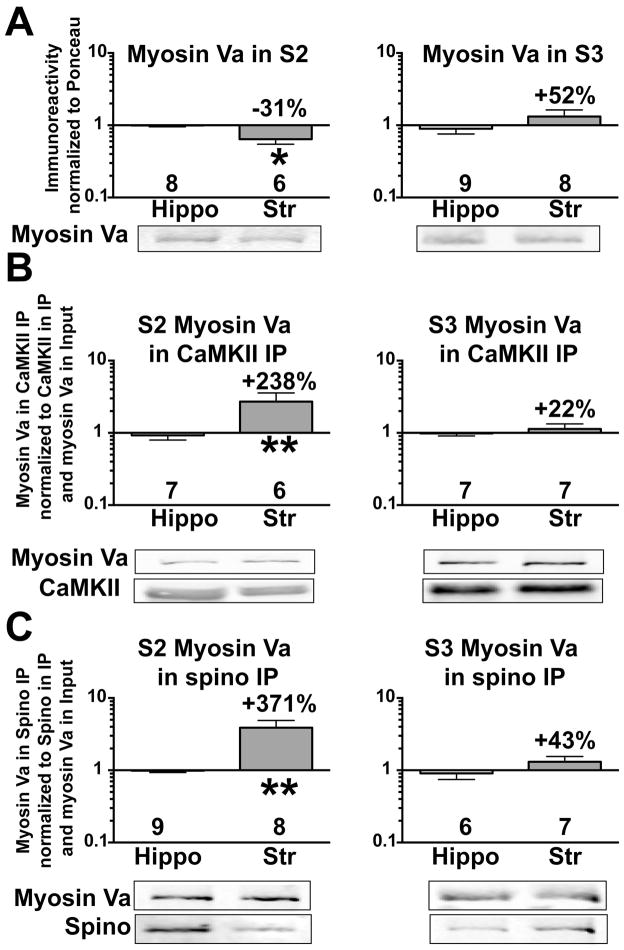
Enhanced striatal association of Myosin Va with CaMKII and spinophilin in the extrasynaptic fraction
Myosin Va interacts with CaMKII (Costa et al. 1999) as well as with N-terminal domain of spinophilin (Baucum et al. 2010). Myosin Va was detected in both subcellular fractions isolated from striatum or hippocampus, but was more enriched in the synaptic fraction (Supplemental Figure 2). While there were similar levels of myosin Va in synaptic fractions isolated from striatum and hippocampus, myosin Va levels in striatal extrasynaptic fractions were significantly lower than in hippocampal extrasynaptic fractions (Fig 5A). However, despite these lower levels, the interaction of myosin Va with both CaMKII (Fig 5B) and spinophilin (Fig 5C) was greater in striatal extrasynaptic, but not synaptic, fractions compared to the corresponding hippocampal fractions.
Figure 5. Differential association of myosin Va with CaMKII and spinophilin in the striatum and hippocampus.
Myosin Va immunoreactivities in extrasynaptic (S2) and synaptic (S3) hippocampal and striatal subcellular fractions were normalized to total protein (Ponceau S stain) and expressed as a ratio to the corresponding myosin Va immunoreactivity from hippocampus (A). Myosin Va immunoreactivity detected in S2 or S3 CaMKII immunoprecipitates was normalized to CaMKIIα immunoreactivity in the immunoprecipitate and myosin Va immunoreactivity in the input of the corresponding fraction (B). Myosin Va immunoreactivity detected in S2 or S3 spinophilin immunoprecipitates was normalized to spinophilin immunoreactivity in the immunoprecipitate and myosin Va immunoreactivity in the input of the corresponding fraction (C). All ratios are plotted on a Log10 scale (see Methods). An unpaired Student’s t-test was performed between groups. *P<0.05, **P<0.01.
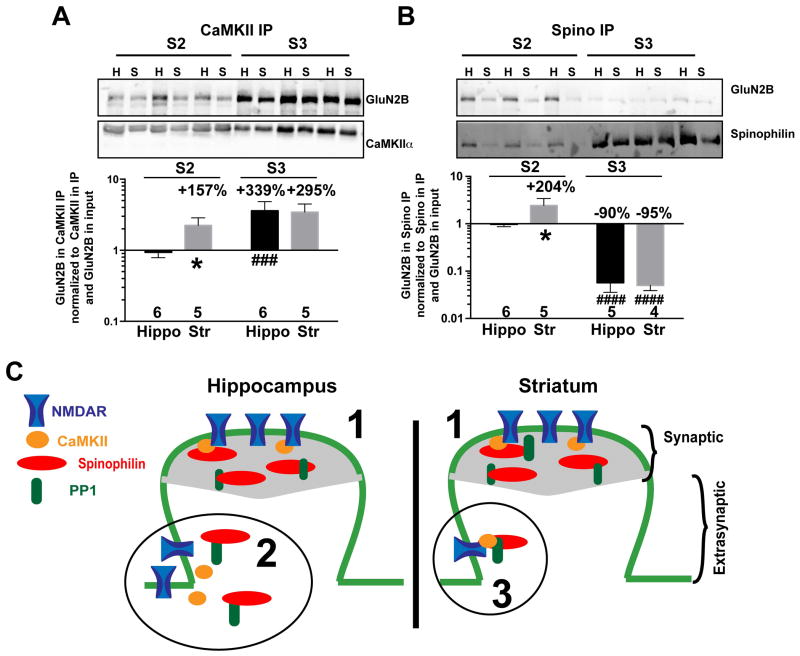
Enhanced association of GluN2B with spinophilin and CaMKII in the extrasynaptic fraction
Both myosin Va and CaMKII can associate with NMDARs (Husi et al. 2000, Strack & Colbran 1998, Bayer et al. 2006). Therefore, we compared the association of NMDARs with CaMKII in striatal and hippocampal subcellular fractions. The CaMKII-GluN2B complex was substantially enriched in synaptic fractions of both brain regions relative to the corresponding extrasynaptic fraction, consistent with an important role for GluN2B in subcellular targeting of CaMKII (Bayer et al. 2006). However, GluN2B-CaMKII association also was significantly more robust in the striatal extrasynaptic fraction compared to the hippocampal extrasynaptic fraction (Fig. 6A, Supplemental Figure 3A,B). Notably, NMDAR GluN1 and GluN2A subunits were also more robustly associated with CaMKII in striatal extrasynaptic S2 fractions relative to the hippocampal S2 fraction, whereas only GluN2A was more robustly associated with CaMKII in the synaptic S3 fraction from striatum relative to hippocampus (Supplemental figure 3C–F).
Figure 6. Differential association of GluN2B with synaptic and extrasynaptic spinophilin and CaMKII.
Immunoprecipitated CaMKII (A) or spinophilin (B) from S2 and S3 were evaluated on the same gel and quantified to compare levels of association in each fraction. Two-way ANOVA results: A: fractionation effect P=0.0021, brain region effect P=0.0789, Interaction P=0.1086. B: fractionation effect P<0.0001, Brain region effect P=0.2281, Interaction P=0.1140. Post hoc testing used the uncorrected Fisher’s LSD post hoc test. Comparison to corresponding hippocampal fraction: *P<0.05. Comparison to S2 fraction of corresponding brain region: ###P<0.001, ####P<0.0001. C. Model of differential expression and interactions in extrasynaptic and synaptic fractions isolated from hippocampus and striatum. 1. Equal expression and interaction of proteins in striatum and hippocampus synaptic fraction. 2. More spinophilin, NMDAR, and CaMKII expression in hippocampus compared to striatum extrasynaptic fraction. 3. Greater striatal association of spinophilin, CaMKII and GluN2B in extrasynaptic fraction.
Spinophilin plays a key role in PP1-dependent regulation of AMPAR and NMDAR currents as well as striatal LTD (Allen et al. 2006, Feng et al. 2000). However, to the best of our knowledge no one has evaluated the physical interactions between spinophilin and NMDARs ex vivo. We detected GluN2B in spinophilin immunoprecipitates. In contrast to the CaMKII-GluN2B complex (Fig. 6A), GluN2B association with spinophilin was enriched in the extrasynaptic fraction compared to the synaptic fraction (Fig. 6B). Moreover, GluN2B association with spinophilin also was more robust in striatal extrasynaptic fractions compared to hippocampal extrasynaptic fractions, whereas there was no significant difference between synaptic fractions from the two brain regions (Fig. 6B and supplemental Figure 3G,H).
Discussion
There is a general consensus that long-term plasticity of excitatory synaptic transmission is important for different forms of learning and memory. Extensive studies in hippocampus have identified molecular mechanisms and multiple proteins that contribute to control of synaptic plasticity. The precise subcellular localization and regulation of proteins such as NMDARs, AMPARs, CaMKII, and PP1 are important for normal synaptic plasticity. While these proteins are expressed across many brain regions, it is becoming increasingly apparent that there can be substantial differences in types of plasticity that can be induced using the same stimulation paradigms (see Introduction). Here we begin to explore biochemical mechanisms that may underlie such differences by demonstrating brain region-selective differences in the expression levels and interactions of key postsynaptic proteins in synaptic and extrasynaptic fractions. We suggest that such differences in postsynaptic signaling architecture contribute to the distinct physiological properties of hippocampal and striatal excitatory synapses.
Differential expression and distribution of excitatory synaptic proteins between brain regions
Currently available biochemical approaches do not allow for global analysis of protein levels and protein complexes in specific cell types. However, comparison of striatal and hippocampal homogenates represents an interesting model to begin to understand cell-type differences because ~95% of striatal neurons are γ-aminobutyric acid-containing MSNs (Huang et al. 1992, Kreitzer & Malenka 2008), whereas glutamatergic pyramidal neurons predominate in hippocampus. Excitatory inputs are critical in both cell types, and were the focus of our study, although we cannot exclude possible minor contributions to our biochemical analyses from proteins localized to additional types of synapses and/or neurons. Thus, while our results do not identify cell specific differences, the results give insight into more global, systems level, brain region specific differences in protein expression and interactions.
The levels of synaptic proteins in whole extracts of brain regions may reflect the overall density of excitatory synapses, the levels of these proteins at individual synapses, or a combination of both factors. Terminals expressing vGlut2 in the striatum originate from thalamic neurons, but vGlut2 is expressed at low levels at hippocampal synapses. In contrast, vGlut1 is expressed in hippocampal pyramidal neurons and corticostriatal neurons (Fremeau et al. 2004, Bellocchio et al. 1998, Kaneko et al. 2002). Consistent with this, total levels of vGlut2 were >2-fold higher in the striatum compared to the hippocampus (Fig. 1D). In contrast, total levels of vGlut1 were slightly lower in the striatum compared to the hippocampus (Fig. 1D).
The canonical postsynaptic density scaffolding protein PSD-95 localizes to both synaptic and extrasynaptic fractions (Petralia et al. 2010) and is critical for targeting AMPARs and NMDARs to modulate synaptic plasticity (Kopec et al. 2007, Ehrlich & Malinow 2004). While there was no significant difference in total lysate PSD-95 levels (Fig. 1), PSD-95 levels were significantly, if modestly, lower (~31%) in the striatal extrasynaptic fraction compared to hippocampus, with a trend for slightly higher PSD-95 levels in the striatal synaptic fraction (Fig. 4B). A lack of a robust difference in synaptic PSD-95 levels may indicate that the overall density of excitatory synapses is similar in these two brain regions, although it is also possible that differences in the amount of PSD-95 at individual synapses may compensate for differences in synaptic density. Total levels of all glutamate receptor subunits tested were 30–80% lower in striatal lysates compared to hippocampal lysates (Fig. 1), and this was reflected in significantly lower levels of all subunits in both the extrasynaptic and synaptic fractions (Fig. 3). Lower striatal NMDAR subunit levels may contribute to the relative difficulty in inducing NMDAR-dependent LTP in the striatum compared to hippocampus (Calabresi et al. 1992). Also, differences in NMDAR expression may underlie the predominance of NMDAR-independent forms of LTD, such as endocannabinoid-mediated LTD, in striatum compared to hippocampus (Lovinger 2010, Gerdeman et al. 2002). While striatal levels of GluN2B were lower in both fractions, the difference was much more pronounced in the extrasynaptic fraction (~69% lower striatal expression in the extrasynaptic vs. ~30% lower striatal expression in the synaptic fraction).
Differential levels, distribution, and association of synaptic signaling proteins
Total levels of CaMKIIα, CaMKIIβ, and spinophilin were significantly (24–41%) lower in striatal compared to hippocampus lysates. In contrast, total levels of PP1 catalytic subunits are more than 2-fold higher in striatum compared to hippocampus (Fig. 2). Interestingly, CaMKII levels were similarly lower in striatal synaptic and extrasynaptic fractions compared to the corresponding hippocampal fractions, whereas the significantly lower levels of spinophilin are restricted to the extrasynaptic fraction (Fig. 4E). Although our fractionation paradigm does not distinguish between pre- and postsynaptic proteins, immunohistochemical and electron microscopy studies suggest that spinophilin and CaMKII are highly enriched at postsynaptic sites (Ouimet et al. 1984, Muly et al. 2004). Together, these data suggest that the balance between postsynaptic CaMKII and PP1 signaling in synaptic and extrasynaptic fractions is quite different in these two brain regions.
To gain further insight into the nature of brain region selective differences in synaptic signaling, we also examined signaling protein complexes by co-immunoprecipitation. While this approach can fail to detect physiological protein-protein interactions or can detect interactions that are facilitated by tissue homogenization, depending on the extraction buffer and other conditions, we focused here on previously characterized interactions (see citations throughout). Initial studies using extracts solubilized in a low ionic strength buffer containing Triton X-100 found that relative levels of CaMKIIα associated with spinophilin were almost 2-fold higher in striatum compared to hippocampus (Fig. 3A). However, there was no difference in the levels of PP1 catalytic subunit associated with spinophilin, despite the substantially higher levels of striatal PP1 (Fig. 3B). As predicted from these changes in the spinophilin complex, the amount of PP1 associated with CaMKII immune complexes was also about 2-fold higher in the striatum compared to the hippocampus (Fig. 3C). We then immunoprecipitated proteins from different subcellular fractions isolated in an isotonic KCl buffer (see Methods). Interestingly, analysis of complexes isolated from the subcellular fractions revealed that there was a greater association of CaMKII and spinophilin in the extrasynaptic fraction compared to the synaptic fraction in both brain regions (Fig. 4G). Moreover, the enhanced striatal association of CaMKII is restricted to the extrasynaptic fraction (Fig. 4G). Unfortunately the low yields from isolated subcellular fractions precluded more complete analysis of these protein complexes for PP1 and other proteins.
The enhanced striatal association of CaMKII with spinophilin, and presumably PP1, in the extrasynaptic fraction may impact the dynamic control of phosphorylation of downstream targets of this complex, reminiscent of the coordinated targeting of protein kinase A and protein phosphatase 2B to AMPARs by AKAP79/150 (Logue & Scott 2010). However, it does not appear that the major CaMKIIα autophosphorylation site, Thr286, is a direct target for the PP1 that is associated with CaMKII via spinophilin because levels of Thr286 phosphorylation are significantly higher in the striatum than in the hippocampus (Fig. 2C). This is consistent with recent studies indicating that Thr286 may be protected from PP1-mediated dephosphorylation in PSDs (Mullasseril et al. 2007), perhaps because spinophilin can selectively inhibit PP1 activity towards certain substrates (Ragusa et al. 2010, Hsieh-Wilson et al. 1999, Terry-Lorenzo et al. 2000). Future studies will need to determine if differences in PP1 association with CaMKII modulate the phosphorylation of other sites on CaMKIIα or of other co-assembled proteins.
Mechanism of altered spinophilin/CaMKII interaction
CaMKII interacts both directly and indirectly with multiple domains in spinophilin (Baucum et al. 2012), making it difficult to uncover mechanism(s) underlying brain-region specific variations in the interaction. Although there was no difference in the ability of a C-terminal domain in spinophilin to bind CaMKII from hippocampal or striatal extracts (Fig. 3F), we found that N-terminal domains of spinophilin bound significantly more striatal CaMKII than hippocampal CaMKII (Fig. 3D,E). The increased interaction of striatal CaMKII with GSTSpN2 may be explained by higher levels of Thr286 phosphorylation (Fig. 2C), because autophosphorylation is required for a strong, direct interaction with this domain (Baucum et al. 2012). However, GSTSpN1 (containing residues 1–154) cannot directly bind purified CaMKII. Therefore, we speculate that binding of CaMKII in brain extracts to GSTSpN1 is mediated by other striatal proteins that can “bridge” an interaction of CaMKII with GSTSpN1. One such candidate is myosin Va, which can bind to both CaMKII and GSTSpN1 (Baucum et al. 2010, Costa et al. 1999), and regulates GluA1 trafficking to dendritic spines, and LTP, in a CaMKII-dependent manner (Correia et al. 2008). Interestingly, we found that the levels of myosin Va associated with both CaMKII and spinophilin were significantly higher in extrasynaptic, but not synaptic, fractions isolated from striatum compared to hippocampus (Fig. 5B,C). Taken together, these data suggest that myosin Va may be involved in enhancing the interaction of CaMKII with spinophilin in the extrasynaptic fraction. However, additional intermediates may be involved. For instance, α-actinin can also bind both spinophilin and CaMKII (Baucum et al. 2010, Walikonis et al. 2001) and some isoforms are more highly expressed in striatum compared to hippocampus (Wyszynski et al. 1998). Future studies need to compare the broader composition of these complexes in the two brain regions.
Differential interactions of NMDAR GluN2B subunits with spinophilin and CaMKII
As a first step toward identifying physiological targets of the CaMKII-spinophilin complex, we investigated its association of NMDAR GluN2B subunits in extrasynaptic and synaptic fractions. CaMKII interaction with GluN2B is critical for proper synaptic targeting of CaMKII, hippocampal LTP, and memory consolidation (Barria & Malinow 2005, Halt et al. 2012, Bayer et al. 2006, Strack & Colbran 1998). Moreover, CaMKII also modulates NMDARs (Sessoms-Sikes et al. 2005, Gustin et al. 2011). Consistent with these findings, levels of GluN2B associated with CaMKII were higher in synaptic fractions compared to extrasynaptic fractions from both brain regions (Fig. 6A). Notably, while similar amounts of GluN2B associated with synaptic CaMKII in the two brain regions, the association of GluN2B with extrasynaptic CaMKII appears to be stronger in the striatum compared to hippocampus (Fig. 6 and supplemental Fig. 3).
Biochemical studies have shown that the PDZ domain of spinophilin can associate with NMDAR GluN1 and GluN2 subunits in vitro (Kelker et al. 2007). Spinophilin has also been shown to facilitate PP1 regulation of NMDARs (Feng et al. 2000), but the association of native spinophilin with NMDARs in the brain has not been investigated. We demonstrate here for the first time that NMDAR GluN2B subunits are associated with spinophilin in the rodent brain. Similar to the association of CaMKII with spinophilin, the ratio of GluN2B to spinophilin within these complexes is substantially higher in the extrasynaptic fraction compared to the synaptic fraction in both striatum and hippocampus, despite the fact that spinophilin was relatively enriched in the total synaptic fraction (Supplemental Fig. 1). Moreover, more GluN2B was associated with extrasynaptic spinophilin in striatum compared to hippocampus (Fig. 6B and supplemental figure 3G,H), correlating with the enhanced CaMKII association with striatal extrasynaptic spinophilin. The mechanism(s) underlying the association of NMDAR subunits with spinophilin requires further investigation.
Taken together, these data indicate that synaptic and extrasynaptic signaling complexes are differentially assembled in different cell types, perhaps contributing to cell-specific regulation, activity, and roles of synaptic compared to extrasynaptic NMDARs (Papouin et al. 2012, Gladding & Raymond 2011, Kaufman et al. 2012). Moreover, these differences may play a role in unique changes in striatal neurons in neurological disorders, such as Parkinson and Huntington disease (HD). For example, the interaction of PP1 with spinophilin is increased following striatal dopamine depletion in a rat PD model (Brown et al. 2008). In addition, extrasynaptic GluN2B-containing NMDAR currents are selectively enhanced in cultured striatal MSNs isolated from a mouse model of HD compared to normal MSNs (Milnerwood et al. 2012). It will be interesting to investigate the impact of disease-related processes on the assembly of synaptic and extrasynaptic signaling complexes and their role in the resulting neurological deficits.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Qin Wang (University of Alabama at Birmingham) and Drs. Brian Wadzinski, Gregg Stanwood, Danny Winder, and Brian Shonesy, and Ms. Emily Anderson (Vanderbilt University School of Medicine) for critical reading of the manuscript. The authors would also like to thank members of the Colbran Laboratory for helpful comments and suggestions. RJC was supported by NIH Grant R01-MH063232 and by a Hobbs Discovery Grant from the Vanderbilt-Kennedy Center. AJB was supported by Grant K01NS073700 from NINDS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NIMH, or the NIH.
Abbreviations used
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CaMKII
calcium/calmodulin-dependent protein kinase II
- LTD
long-term depression
- LTP
long-term potentiation
- MSN
medium spiny neuron
- NMDAR
N-methyl-D-aspartate receptor
- PP1
protein phosphatase 1
- PSD
postsynaptic density
- vGlut
vesicular glutamate transporter
Footnotes
The authors have no conflicts of interest to declare.
Bibliography
- Allen PB, Zachariou V, Svenningsson P, et al. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience. 2006;140:897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Amso D, Davidson MC, Johnson SP, Glover G, Casey BJ. Contributions of the hippocampus and the striatum to simple association and frequency-based learning. Neuroimage. 2005;27:291–298. doi: 10.1016/j.neuroimage.2005.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ, Colbran RJ. Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach. Mol Cell Proteomics. 2010;9:1243–1259. doi: 10.1074/mcp.M900387-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Strack S, Colbran RJ. Age-Dependent Targeting of Protein Phosphatase 1 to Ca/Calmodulin-Dependent Protein Kinase II by Spinophilin in Mouse Striatum. PLoS One. 2012;7:e31554. doi: 10.1371/journal.pone.0031554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O’Leary H, Schulman H, De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Breck JT, Eichenbaum H. Striatal versus hippocampal representations during win-stay maze performance. J Neurophysiol. 2009;101:1575–1587. doi: 10.1152/jn.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science (New York, NY. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Brown AM, Baucum AJ, Bass MA, Colbran RJ. Association of protein phosphatase 1 gamma 1 with spinophilin suppresses phosphatase activity in a Parkinson disease model. The Journal of biological chemistry. 2008;283:14286–14294. doi: 10.1074/jbc.M801377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. The European journal of neuroscience. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Costa MC, Mani F, Santoro W, Jr, Espreafico EM, Larson RE. Brain myosin-V, a calmodulin-carrying myosin, binds to calmodulin-dependent protein kinase II and activates its kinase activity. The Journal of biological chemistry. 1999;274:15811–15819. doi: 10.1074/jbc.274.22.15811. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M, Heitz FD, Grewe BF, Tyagarajan SK, Helmchen F, Mansuy IM. Selective regulation of NR2B by protein phosphatase-1 for the control of the NMDA receptor in neuroprotection. PLoS One. 2012;7:e34047. doi: 10.1371/journal.pone.0034047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011;48:308–320. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gustin RM, Bichell TJ, Bubser M, et al. Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol Dis. 2010;39:283–291. doi: 10.1016/j.nbd.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin RM, Shonesy BC, Robinson SL, Rentz TJ, Baucum AJ, 2nd, Jalan-Sakrikar N, Winder DG, Stanwood GD, Colbran RJ. Loss of Thr286 phosphorylation disrupts synaptic CaMKIIalpha targeting, NMDAR activity and behavior in pre-adolescent mice. Molecular and cellular neurosciences. 2011;47:286–292. doi: 10.1016/j.mcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza RF, Zhou Y, et al. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science (New York, NY. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hsieh-Wilson LC, Allen PB, Watanabe T, Nairn AC, Greengard P. Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochemistry. 1999;38:4365–4373. doi: 10.1021/bi982900m. [DOI] [PubMed] [Google Scholar]
- Hu XD, Huang Q, Yang X, Xia H. Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. J Neurosci. 2007;27:4674–4686. doi: 10.1523/JNEUROSCI.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci U S A. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nature neuroscience. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci. 2010;30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kaufman AM, Milnerwood AJ, Sepers MD, et al. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci. 2012;32:3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker MS, Dancheck B, Ju T, Kessler RP, Hudak J, Nairn AC, Peti W. Structural basis for spinophilin-neurabin receptor interaction. Biochemistry. 2007;46:2333–2344. doi: 10.1021/bi602341c. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature reviews. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue JS, Scott JD. Organizing signal transduction through A-kinase anchoring proteins (AKAPs) FEBS J. 2010;277:4370–4375. doi: 10.1111/j.1742-4658.2010.07866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Isozaki K, Roche KW, Nicoll RA. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22266–22271. doi: 10.1073/pnas.1016289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McNeill RB, Colbran RJ. Interaction of autophosphorylated Ca2+/calmodulin-dependent protein kinase II with neuronal cytoskeletal proteins. Characterization of binding to a 190-kDa postsynaptic density protein. The Journal of biological chemistry. 1995;270:10043–10049. doi: 10.1074/jbc.270.17.10043. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Kaufman AM, Sepers MD, et al. Mitigation of augmented extrasynaptic NMDAR signaling and apoptosis in cortico-striatal co-cultures from Huntington’s disease mice. Neurobiology of disease. 2012;48:40–51. doi: 10.1016/j.nbd.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Mullasseril P, Dosemeci A, Lisman JE, Griffith LC. A structural mechanism for maintaining the ‘on-state’ of the CaMKII memory switch in the post-synaptic density. Journal of neurochemistry. 2007;103:357–364. doi: 10.1111/j.1471-4159.2007.04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Smith Y, Allen P, Greengard P. Subcellular distribution of spinophilin immunolabeling in primate prefrontal cortex: localization to and within dendritic spines. The Journal of comparative neurology. 2004;469:185–197. doi: 10.1002/cne.11001. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, McGuinness TL, Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, et al. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different Endogenous Coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nature neuroscience. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, Stephenson FA, Wenthold RJ. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa MJ, Dancheck B, Critton DA, Nairn AC, Page R, Peti W. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat Struct Mol Biol. 2010;17:459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. CaMKIIalpha enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Molecular and cellular neurosciences. 2005;29:139–147. doi: 10.1016/j.mcn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, et al. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. Journal of neurochemistry. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Terry-Lorenzo RT, Inoue M, Connor JH, Haystead TA, Armbruster BN, Gupta RP, Oliver CJ, Shenolikar S. Neurofilament-L is a protein phosphatase-1-binding protein associated with neuronal plasma membrane and post-synaptic density. The Journal of biological chemistry. 2000;275:2439–2446. doi: 10.1074/jbc.275.4.2439. [DOI] [PubMed] [Google Scholar]
- Vickers CA, Stephens B, Bowen J, Arbuthnott GW, Grant SG, Ingham CA. Neurone specific regulation of dendritic spines in vivo by post synaptic density 95 protein (PSD-95) Brain Res. 2006;1090:89–98. doi: 10.1016/j.brainres.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Kharazia V, Shanghvi R, Rao A, Beggs AH, Craig AM, Weinberg R, Sheng M. Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.