Abstract
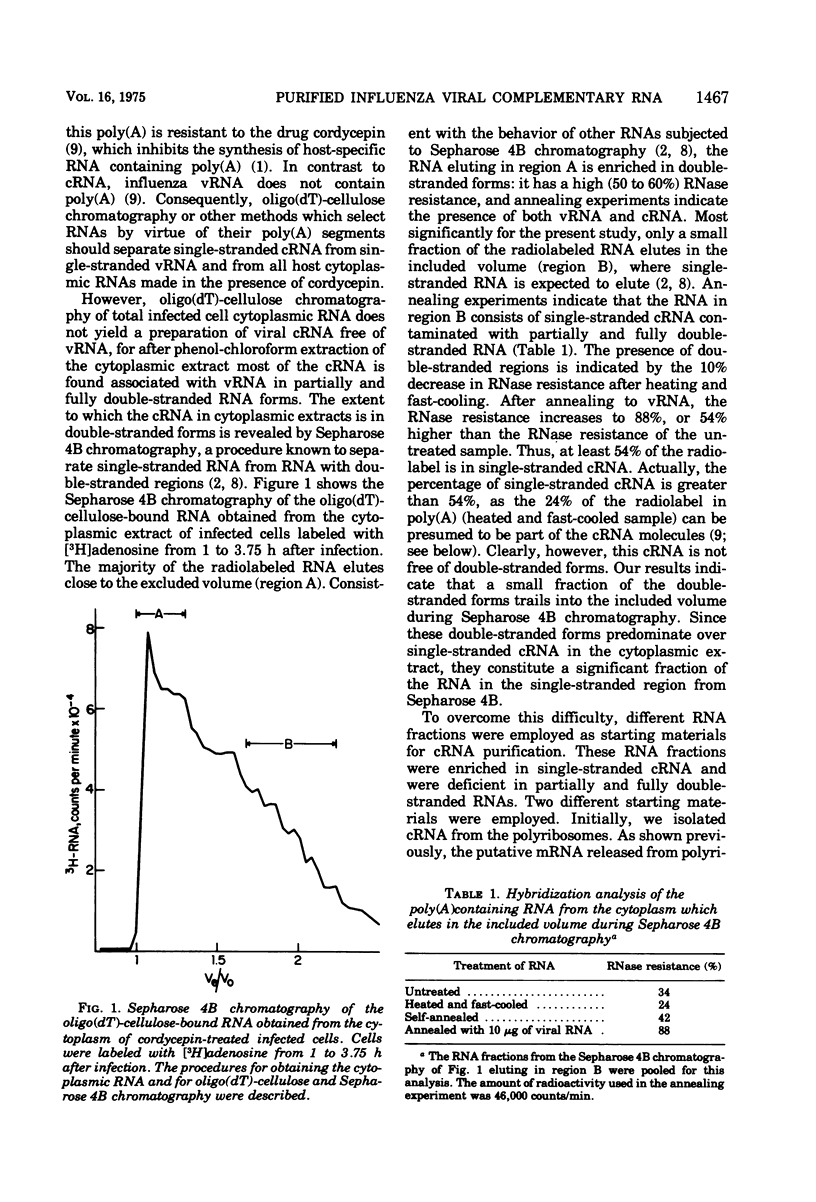
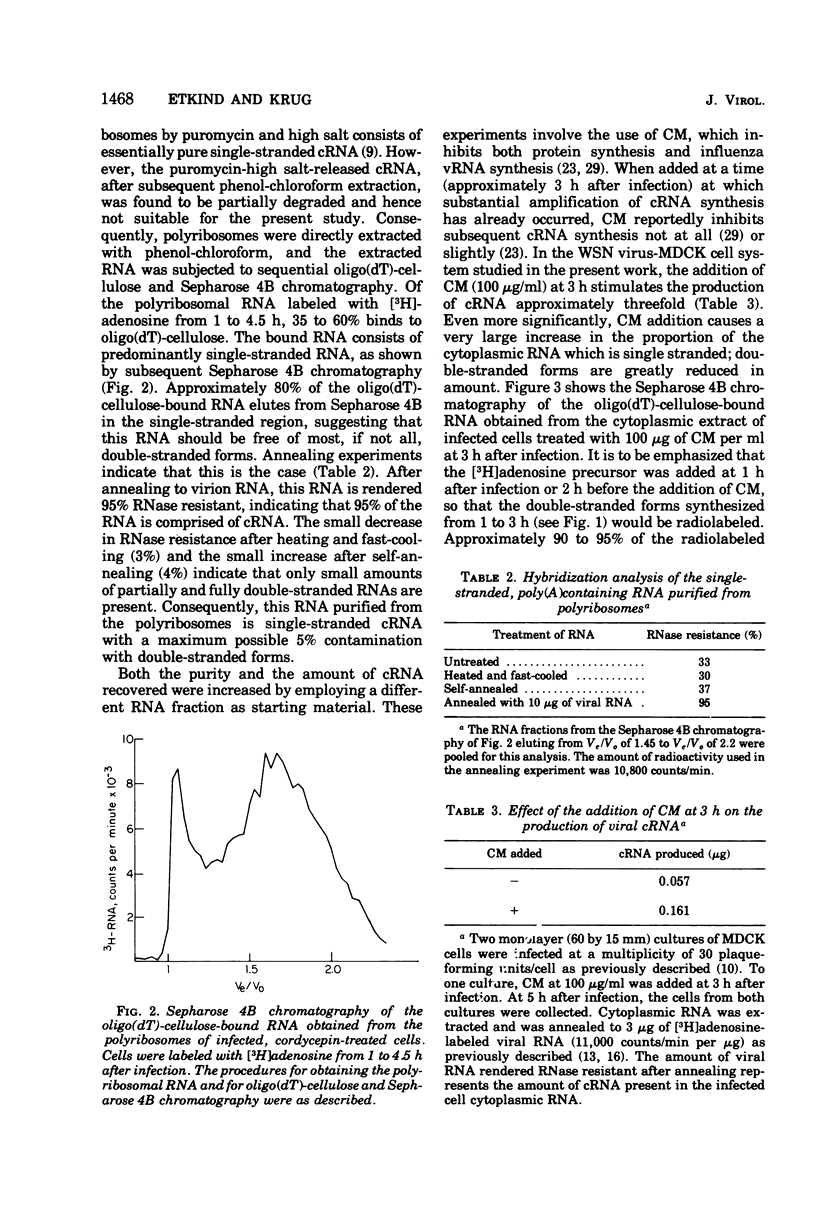
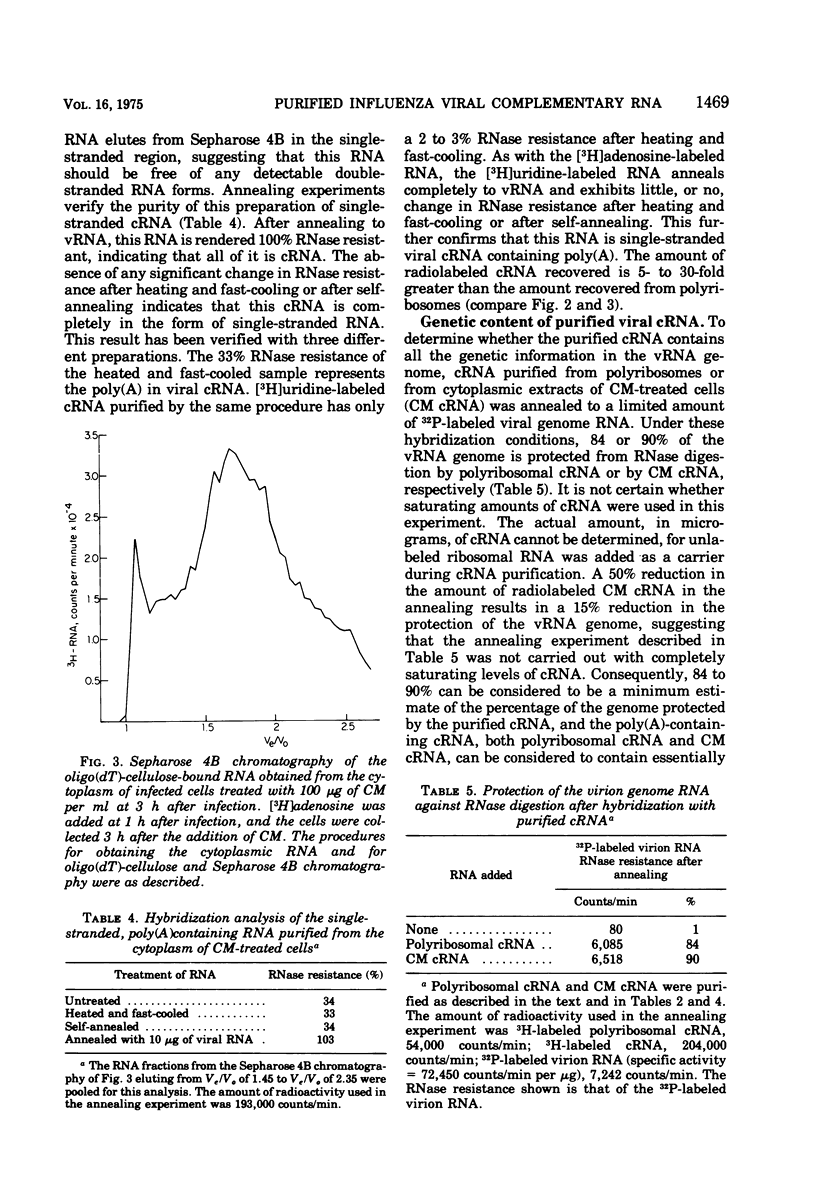
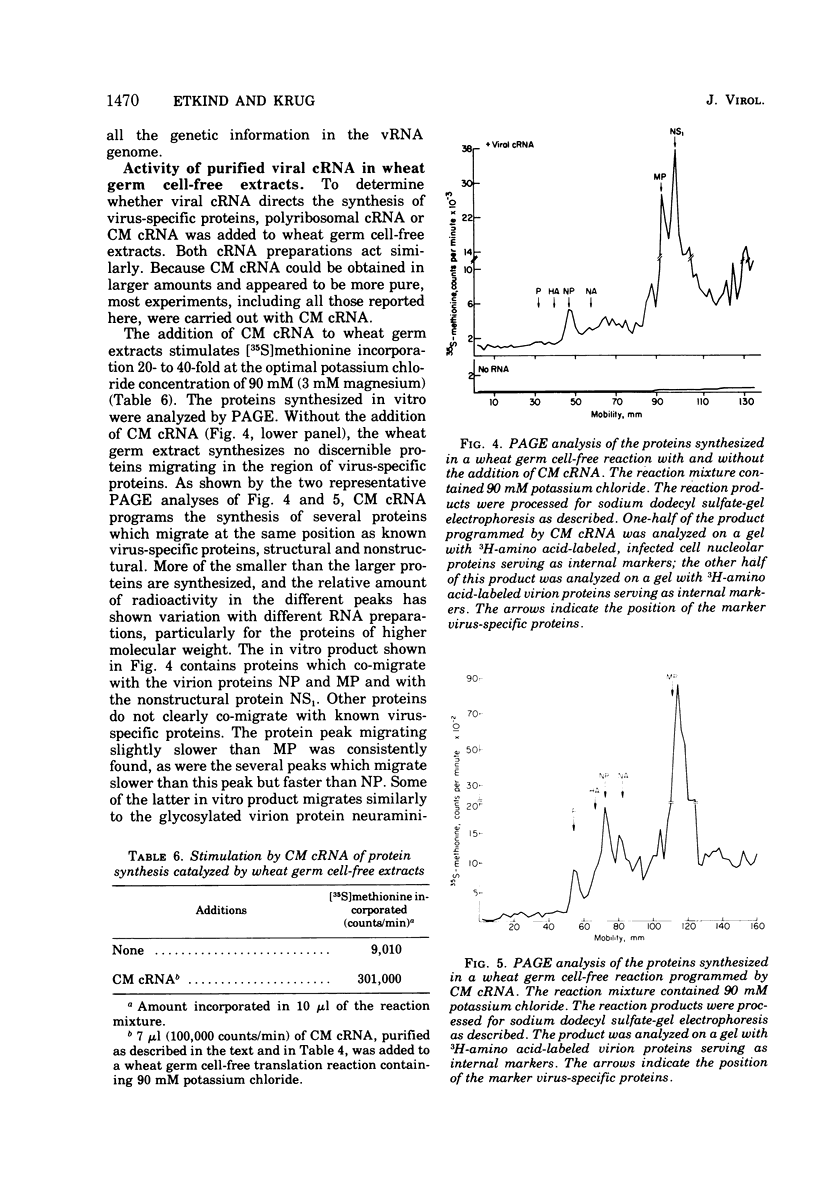
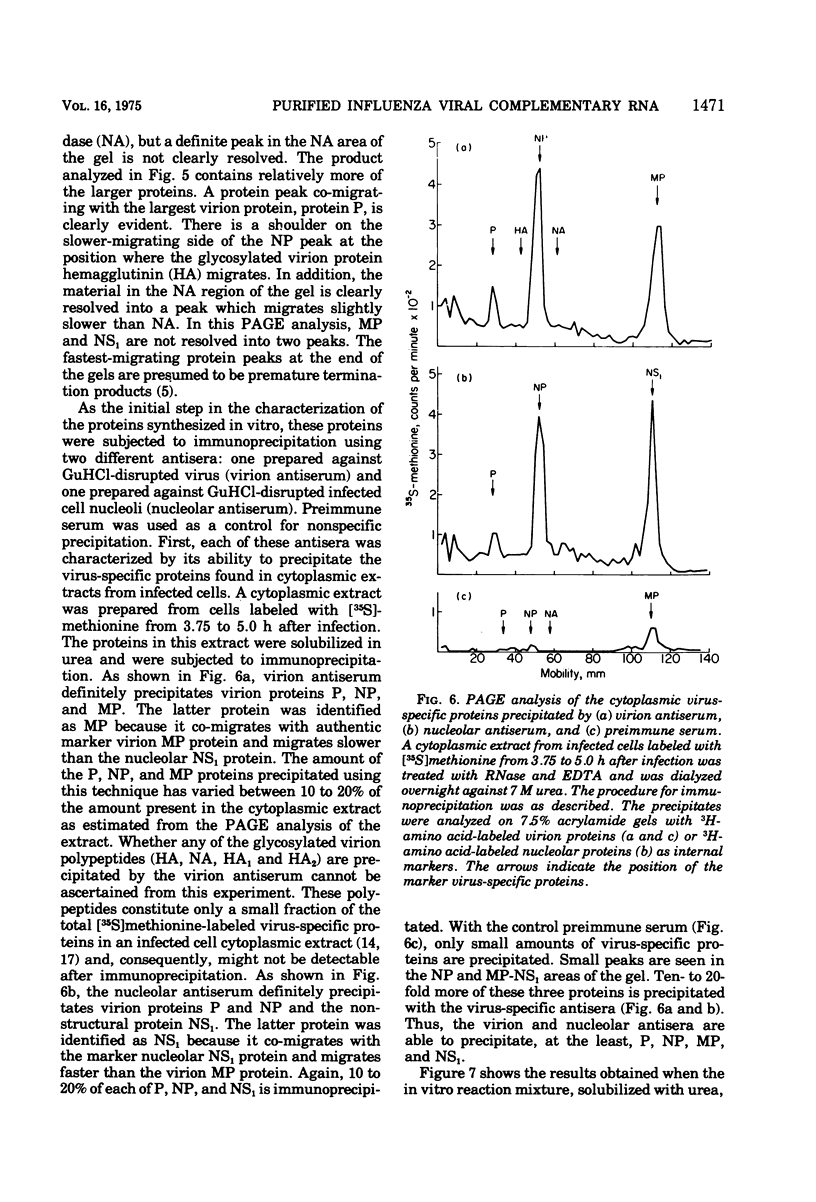
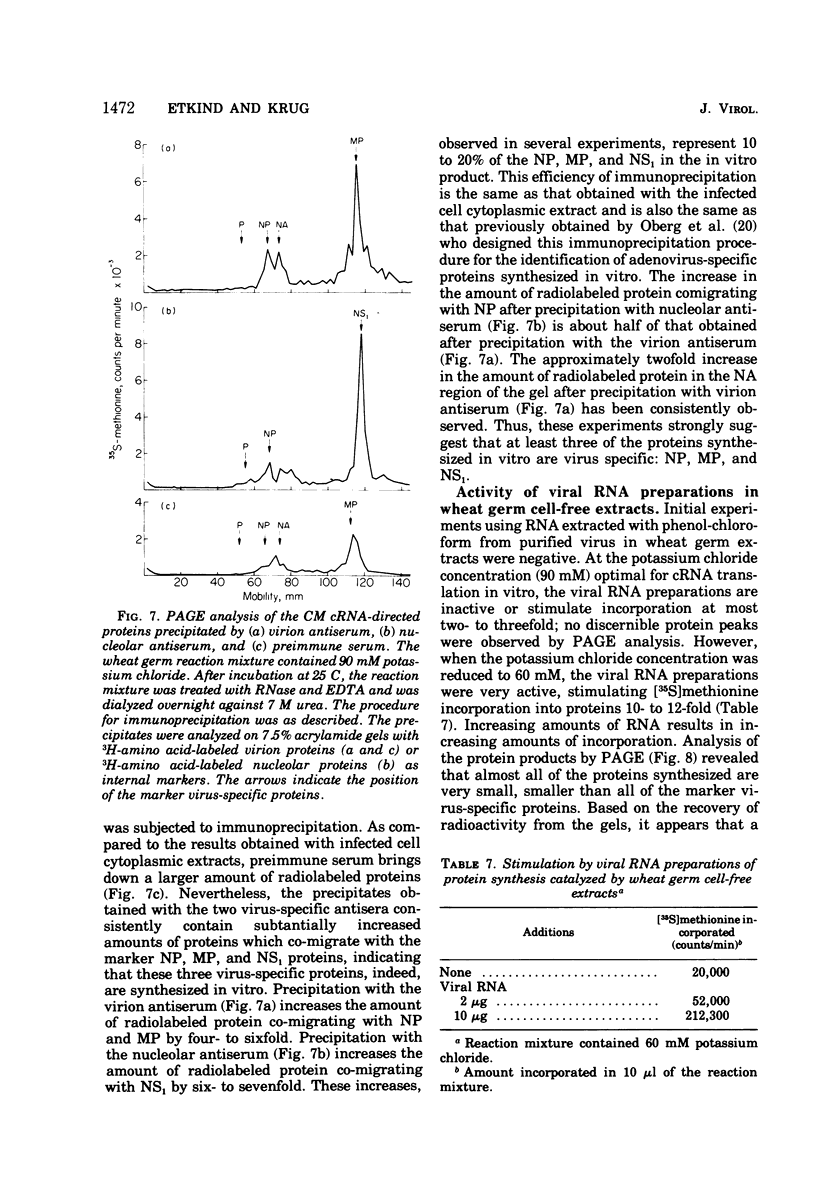
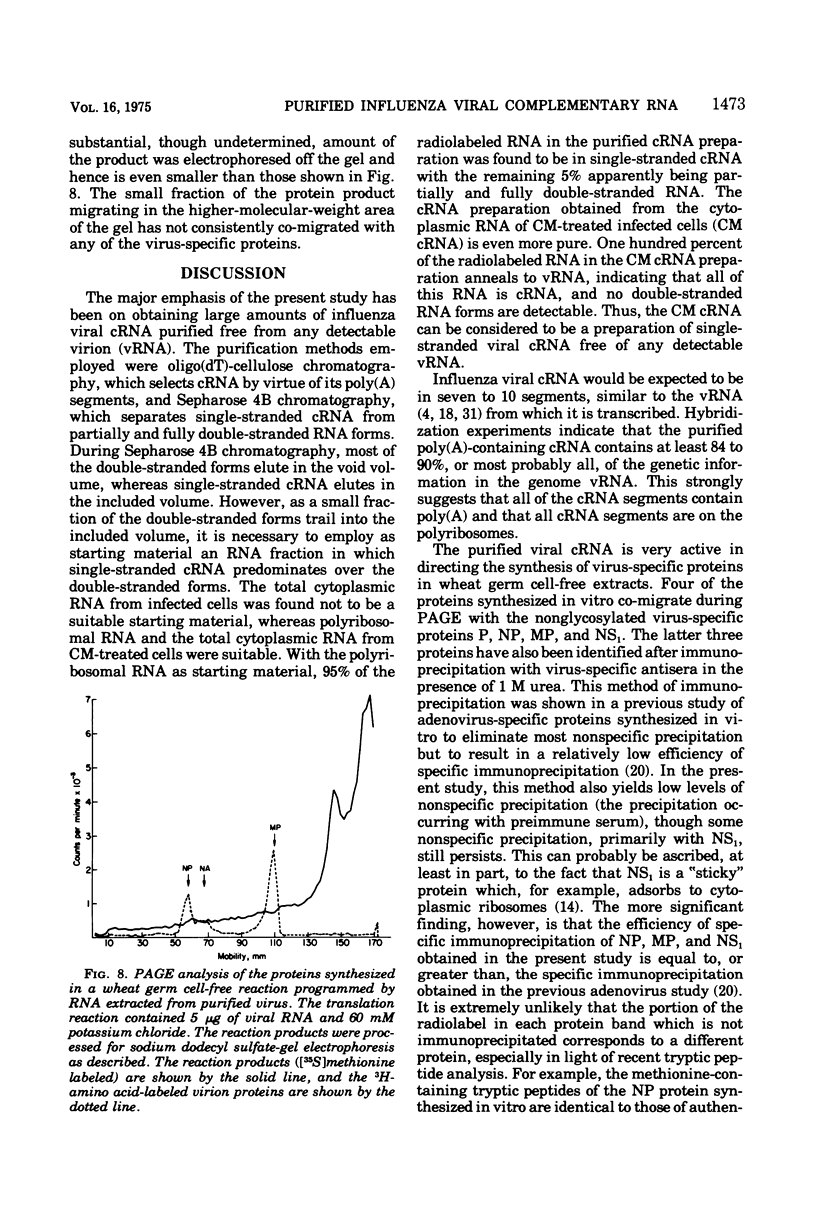
Influenza viral complementary RNA (cRNA) was purified free from any detectable virion-type RNA (vRNA), and its genetic content and activity in wheat germ cell-free extracts were examined. After phenol-chloroform extraction of cytoplasmic fractions from infected cells, poly(A)-containing viral cRNA is found in two forms: in single-stranded RNA and associated with vRNA in partially and fully double-stranded RNA. To purify single-stranded cRNA free of these double-stranded forms, it was necessary to employ, as starting material, RNA fractions in which cRNA was predominantly single stranded. Two RNA fractions were successfully employed as starting material: polyribosomal RNA and the total cytoplasmic RNA from infected cells treated with 100 mug of cycloheximide (CM) per ml at 3 h after infection. In WSN virus-infected canine kidney (MDCK) cells, the addition of CM at 3 h after infection stimulates the production of cRNA threefold and causes a very large increase in the proportion of the cytoplasmic cRNA which is single stranded; double-stranded RNA forms are greatly reduced in amount. Total cRNA was obtained by oligo(dT)-cellulose chromatography, and single-stranded cRNA was separated from double-stranded forms by Sepharose 4B chromatography. The cRNA preparation purified from polyribosomes consists of 95% single-stranded cRNA, with the remaining 5% apparently being double-stranded RNA forms. The cRNA preparation purified from CM-treated cells (CM cRNA) is even more pure: 100% of the radiolabeled RNA is single-stranded cRNA. Annealing experiments, in which a limited amount of 32P-labeled genome RNA was annealed to the cRNA, indicate that the purified cRNA contains at least 84 to 90% of the genetic information in the vRNA genome. Purified viral cRNA (CM cRNA) is very active in directing the synthesis of virus-specific proteins in wheat germ cell-free extracts.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. II. Nature of the in vitro polymerase product. J Virol. 1971 Jul;8(1):74–80. doi: 10.1128/jvi.8.1.74-80.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Gordon J. A. Replication of bacteriophage RNA: purification of the replicative intermediate by agarose column chromatography-1. Biochem Biophys Res Commun. 1966 May 25;23(4):422–428. doi: 10.1016/0006-291x(66)90744-3. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Virus protein synthesis in animal cell-free systems: nature of the products synthesized in resonse to ribonucleic acid of encephalomyocarditis virus. J Virol. 1971 Apr;7(4):438–447. doi: 10.1128/jvi.7.4.438-447.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Webster R. G. Cell-free translation of influenza virus messenger RNA. Virology. 1973 Dec;56(2):654–657. doi: 10.1016/0042-6822(73)90069-x. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Soeiro R. Studies on the intranuclear localization of influenza virus-specific proteins. Virology. 1975 Apr;64(2):378–387. doi: 10.1016/0042-6822(75)90114-2. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Content J., Leppla S. H. Characterization of the subunit structure of the ribonucleic acid genome of influenza virus. J Virol. 1971 Nov;8(5):701–707. doi: 10.1128/jvi.8.5.701-707.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K. An in vitro protein synthesizing system from mouse L fibroblasts infected with reovirus. Virology. 1971 Sep;45(3):724–733. doi: 10.1016/0042-6822(71)90186-3. [DOI] [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E., Miller H., Doyle M., Blatti S. RNA-dependent RNA polymerase activity in influenza virions. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1369–1371. doi: 10.1073/pnas.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. Isolation of influenza virus ribonucleoprotein from infected cells. Demonstration of the presence of negative-stranded RNA in viral RNP. Virology. 1971 Oct;46(1):149–160. doi: 10.1016/0042-6822(71)90014-6. [DOI] [PubMed] [Google Scholar]
- Pons M. W. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973 Jan;51(1):120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Complementary RNAs in paramyxovirions and paramyxovirus-infected cells. Nature. 1970 Dec 19;228(5277):1196–1197. doi: 10.1038/2281196a0. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Self-annealing of subgroup 2 myxovirus RNAs. Nature. 1970 Mar 7;225(5236):944–945. doi: 10.1038/225944a0. [DOI] [PubMed] [Google Scholar]
- Roy P., Repik P., Hefti E., Bishop D. H. Complementary RNA species isolated from vesicular stomatitis (HR strain) defective virions. J Virol. 1973 Jun;11(6):915–925. doi: 10.1128/jvi.11.6.915-925.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Becht H. Binding of ribonucleic acids to the RNP-antigen protein of influenza viruses. J Gen Virol. 1971 Jan;10(1):11–16. doi: 10.1099/0022-1317-10-1-11. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Synthesis in vivo of influenza virus plus and minus strand RNA and its preferential inhibition by antibiotics. Virology. 1970 Apr;40(4):989–996. doi: 10.1016/0042-6822(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Siegert W., Bauer G., Hofschneider P. H. Direct evidence for messenger activity of influenza virion RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2960–2963. doi: 10.1073/pnas.70.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Estimations of the molecular weight of the influenza virus genome. J Gen Virol. 1971 May;11(2):103–109. doi: 10.1099/0022-1317-11-2-103. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. RNA-dependent RNA polymerase activity of the influenza virus. Virology. 1971 Sep;45(3):793–796. doi: 10.1016/0042-6822(71)90197-8. [DOI] [PubMed] [Google Scholar]



